Significant enhancement of photodetector performance by subtle changes in the side chains of dithienopyrrole-based polymers†
Liuyong Huab,
Wenqiang Qiao*a,
Ji Qiab,
Xiaoqin Zhangab,
Jinfeng Hanab and
Canglong Wangc
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: wqqiao@ciac.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cInstitute of Modern Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R. China
First published on 22nd February 2016
Abstract
A pair of analogous polymers based on diketopyrrolopyrrole (DPP) and dithienopyrrole (DTP) were designed to have a small structural difference in the side chain, PDDPDTP-C with an alkyl chain and PDPPDTP-P with an alkylphenyl chain. The two polymers have the same absorption and band gap but show different hole mobility, molecular stacking and film morphology. As a result, the PDPPDTP-P photodetector has a much higher responsivity (291 mA W−1 at 900 nm at −0.1 V) than the one based on PDDPDTP-C (36 mA W−1), and exhibits a specific detectivity over 1012 Jones in the spectral region of 350–950 nm.
Introduction
Polymer photodetectors (PPDs) have attracted considerable attention in the past few years because of the great advantages of low cost, flexibility, solution processing and high performance.1–6 The molecular design of conjugated polymers is of significant importance to realize the desirable performance of photodetectors. To achieve high performance, conjugated polymers should have broad absorption, suitable molecular energy level and appropriate mobility.7–14 Therefore, many strategies have been explored and used in molecular designs.15–20 The highest occupied molecular orbit (HOMO) and the lowest unoccupied molecular orbit (LUMO) levels of conjugated polymers can be finely tuned through the electron-withdrawing and donating groups in the main chain of conjugated polymers. For example, by adjusting the ratios of the electron-deficient diketopyrrolopyrrole and thienoisoindigo units in donor–acceptor polymers, specific detectivity of the corresponding photodetector could be improved over 1011 Jones within a broad spectral region of 300–1200 nm.21Molecular engineering at side chains is another effective way to improve the performance of some organic and polymer (opto)electronic devices.22–28 Pei et al. moved the branching point of flexible chains away from polymer backbone and found a large increase in the mobility, as a result of the reduced π–π stacking distance.29 Wang et al. replaced the pendent alkyl groups with oligo(ethylene glycol) for closer π–π stacking and improved the performance of polymer solar cells.30 In these cases, the π–π stacking distance is reduced as a result of reduction of the steric hindrance between the backbones and side chains. However, in case that steric hindrance is not apparent, it remains necessary to develop an alternative way to decrease the π–π stacking distance for better device performance.
Introduction of long alkyl chains at the side chains is a common approach to improvement of polymer solubility. Insertion of an aromatic moiety at the side chain could has recently been found to affect the device performance for unclear reasons.31–36 For example, Hou et al. replaced the alkoxy group with the alkylthienyl group on the benzodithiophene unit of donor–acceptor polymers and observed the red shift in absorption spectra, higher hole mobility, and greatly improved photovoltaic performance.37 In this case, incorporation of an aromatic unit into the side chains of conjugated polymers can alter the electronic energy levels and bandgaps. However, the exclusive effect of having an aromatic unit at the side chains on molecular stacking, film morphology and then device performance remains uncertain.
To probe such a “side-chain aromatic effect”, we designed a pair of analogous polymers based on diketopyrrolopyrrole (DPP) and dithienopyrrole (DTP): PDDPDTP-C with an alkyl chain and PDPPDTP-P with an alkylphenyl chain. This minor change of pendent groups allows the study of such an effect without disturbance to the energy levels of the conjugated main chains.
Experimental
Materials
The solvents for chemical syntheses were purified by distillation. The reactions were performed under an argon atmosphere. Unless otherwise stated, all of the chemicals were purchased from commercial sources and used as received. Synthetic procedures and characterizations of the monomers and polymers are given in ESI.†Characterizations
1H and 13C NMR spectra were measured on a Bruker Avance 400 NMR spectrometer. The element analysis was carried out on Vario EL Elementar Analysis Instrument. The UV-vis-NIR absorption spectra were taken on a Shimadzu UV-3600 spectrophotometer. The molecular weight of the polymers was measured by gel permeation chromatography using polystyrene standards and chloroform as eluent. Thermogravimetric analysis (TGA) was done on a Perkin-Elmer Pyris Diamond TG from 50 to 800 °C at a heating rate of 10 °C min−1 under a continuous nitrogen flow. Differential scanning calorimetry (DSC) was done on a TA-DSC Q100 from 20 °C to 280 °C with a heating/cooling rate of 10 °C min−1 under a nitrogen atmosphere. Electrochemical cyclic voltammetry (CV) was conducted on a CHI660b electrochemical workstation with Pt disk (2 mm diameter) as a working electrode, Pt plate as a counter electrode, and Ag/AgCl as a reference electrode in a solution of dry acetonitrile containing n-Bu4NPF6 (0.1 M). The polymer thin films for electrochemical measurements were coated from a chloroform solution, ca. 5 mg mL−1, onto a Pt disk electrode. Moreover, the CV of PC71BM was also carried out under the same condition. Ferrocene was used as an internal standard to calibrate the redox potentials. The out-of-plane grazing incidence X-ray diffraction (GIXD) was measured on a Bruker D8 Discover reflector with a step-scan rate of 0.05° per 5 s and the scattering angle 2-theta changing from 2° to 30°. Atomic force microscopy (AFM) was done on a SPA300HV instrument equipped with a SPI3800N controller (Seikoin Instruments, Japan) in tapping mode under ambient conditions using silicon cantilevers (Applied Nanostructures, nominal spring constant of 2.0 N m−1 and nominal resonance frequency of ∼75 kHz).Hole mobility
Hole mobility of the polymers was measured with the space charge limited current model (SCLC), using the hole-only device structure of ITO/PEDOT:PSS/polymer:PC71BM/MoO3/Ag. The current–voltage measurements were done in the range of 0–3 V and the results were fitted to a space charge limited form, according to the SCLC equation of J = (9/8)εrε0μ(V2/L3), where J is the current density in the space-charge limited region, L is the thickness of the active layer as measured using step profiler, ε0 is the permittivity of free space, εr is the dielectric constant of the polymer (assumed to be 3 for conjugated polymers), V is the voltage drop (V = Vappl − Vr − Vbi, where Vappl is the applied voltage, Vr is the voltage drop, and Vbi is the built-in voltage) and μ is the hole mobility.Fabrication of photodetectors
The photodetector devices with an active area of 0.16 cm2 were fabricated by spin coating PEDOT:PSS (Baytron P VP Al 4083) on pre-cleaned, patterned indium tin oxide (ITO) glass substrates. Solutions for spin coating of PDPPDTP-C and PDPPDTP-P blends contained 8 mg mL−1 of polymer and 16 mg mL−1 of PC71BM (ADS71BFA) in chlorobenzene. For device optimization, 5% of 1,8-diiodooctane (DIO) by volume was added as the additive in chlorobenzene. Spin coating was carried out at 1500 rpm under nitrogen atmosphere. The back electrode consisted of LiF (1 nm) and Al (100 nm) as deposited by evaporation under high vacuum (7 × 10−4 Pa).Characterization of photodetectors
EQE measurements were carried out under ambient conditions using a setup from Beijing 7-Star Optical Instruments Co., Ltd. Incident light from a 250 W halogen lamp passing through two cascade monochromators was chopped at 25 Hz and focused on the active area of the device. The photocurrent signal was amplified by a low-noise current amplifier (DLPCA-200, Femto) and then examined with a lock-in amplifier (SR830, Stanford Research Systems). Keithley 236 Source Measure unit was employed to test the dark current density–voltage (J–V curve) characteristics of the devices.Results and discussion
Synthesis and characterization
The two analogous polymers, PDPPDTP-C and PDPPDTP-P with different types of side chains, were synthesized by the Stille cross-coupling reaction (Scheme S1†). They have good solubility in common solvents such as tetrahydrofuran (THF), chloroform (CF), and chlorobenzene (CB). The two polymers were well characterized by spectroscopic methods. GPC shows the number-average molecular weights (Mn) of PDDPDTP-C and PDDPDTP-P of 39.9 and 48.4 kDa, respectively (Table 1). The polymers are stable up 390 °C by thermogravimetric analysis and have no obvious thermal transitions by differential scanning calorimetry (Table 1 and ESI†).| Polymer | Mn (kDa)/PDI | Tda (°C) | λmaxb (nm) | Eopt.gc (eV) | HOMOd (eV) | LUMOd (eV) | EECge (eV) |
|---|---|---|---|---|---|---|---|
| a Onset temperature for 5% weight loss in nitrogen by TGA.b Film spin-cast from 10 mg mL−1 chlorobenzene solution on quartz substrate.c Optical bandgap estimated from the film absorption onset.d Calculated from ELUMO = −(φre + 4.43) (eV) and EHOMO = −(φox + 4.43) (eV).e Electrochemical band gap calculated from EHOMO − ELUMO. | |||||||
| PDPPDTP-C | 39.9/2.8 | 392 | 790/890 | 1.22 | −5.15 | −3.53 | 1.62 |
| PDPPDTP-P | 48.4/2.7 | 396 | 790/885 | 1.22 | −5.15 | −3.53 | 1.62 |
Optical and electrochemical properties
The UV-vis-NIR absorption spectra of the two polymer films are shown in Fig. 1a. The two polymers show almost identical absorption profiles, excepting that PDPPDTP-P exhibits double peaks at 790 and 890 nm, while PDPPDTP-C with a single peak and a shoulder peak at 790 and 865 nm, respectively. Accordingly, they have the same optical bandgap of 1.22 eV. | ||
| Fig. 1 (a) Absorption spectra of the polymer films spin-coated on quartz. (b) HOMO and LUMO energy levels of the two polymers relative to PC71BM. | ||
The energy levels of the highest (HOMO) and lowest unoccupied molecular orbital (LUMO) of the polymers are calculated from the onset values of electrochemical reduction and oxidation potentials (Table 1 and Fig. S3†). As expected, the two polymers have the same bandgap of 1.62 eV. As shown in Fig. 1b, the ΔLUMO between the two polymers and PC71BM appears as 0.37 eV, indicating the efficient charge separate from the polymers to PC71BM.38,39
Quantum calculations
To investigate the effect of the pendent phenyl group on the chain conformations and electronic structures of the polymer, density functional theory (DFT) calculations were performed with DMol3 code,40,41 and the generalized gradient approximation Perdew–Burke–Ernzerhof exchange-correlation functional were chosen.42 The optimized geometries and visualized LUMO and HOMO distributions of the trimer were shown in Fig. 2. In order to simplify the calculations, the alkyl chains on the trimer are replaced by the methyl groups. The insertion of the phenylene group in PDPPDTP-P does not increase the dihedral angle of polymer backbone in comparison with PDPPDTP-C and has little effect on the distributions of LUMO and HOMO along the conjugated main chains (Fig. S4, ESI†). Moreover, the phenylene group shows the dihedral angel of nearly 48° relative to the DTP unit, which is expected to enhance the intermolecular π–π interaction. | ||
| Fig. 2 DFT-optimized geometries and frontier molecular orbitals of DPP–DTP-P trimer. The long alkyl chains are replaced by methyl groups. | ||
Molecular stacking
As shown in Fig. 3, from the out-of-plane gazing incident X-ray diffraction (GIXRD) the small and large 2θ zone of diffraction peaks can be regarded as the laminar packing and π–π stacking between polymer backbones, respectively.43–45 The laminar diffraction peaks of PDDPDTP-C and PDDPDTP-P are at 4.6° and 4.1°, corresponding to the d-spacing of 19.2 and 21.5 Å, respectively. The larger d-spacing of the PDDPDTP-P is probably caused by the rigid benzene ring at the side chain. However, the π–π diffraction peaks appear at 23.65° for PDDPDTP-C and at 24.65° for PDDPDTP-P, thus corresponding to the d-spacing of 3.76 and 3.61 Å, respectively. Therefore, the presence of the benzene ring at the side chains obviously reduces the π–π stacking distance, which is extremely beneficial to charge transport.Hole mobility
The hole mobility of polymer:PC71BM was estimated by the SCLC method, and the J–V curves are shown in Fig. S5.† The hole mobility of 3.26 × 10−3 and 5.76 × 10−3 cm2 V−1 s−1 were determined for PDPPDTP-C and PDPPDTP-P, respectively. The higher mobility of PDPPDTP-P can be attributed to the closer π–π stacking and better film morphology (see below), implying better performance for the device based on PDDPDTP-P.Photodetector performance
The BHJ photodetectors with the device configuration of ITO/PEDOT:PSS/polymer:PC71BM/LiF/Al were fabricated. The active layers consist of a polymer and PC71BM with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by weight. PC71BM was used in order to compensate the low absorption in the spectral region of 350–600 nm. The polymer active layers were spin-coated from a chlorobenzene solution at a speed of 1500 rpm. To optimize the film morphology, 1,8-diiodooctane (DIO) was later added as an additive, since DIO is known to promote the formation of nanoscale phase separation of polymer–PCBM blends.46,47
2 by weight. PC71BM was used in order to compensate the low absorption in the spectral region of 350–600 nm. The polymer active layers were spin-coated from a chlorobenzene solution at a speed of 1500 rpm. To optimize the film morphology, 1,8-diiodooctane (DIO) was later added as an additive, since DIO is known to promote the formation of nanoscale phase separation of polymer–PCBM blends.46,47
The device performance is mainly characterized by the specific detectivity (D*), which is governed by the dark current (Jd) and spectral responsivity (R).48 The D* of the devices based on PDDPDTP-C and PDDPDTP-P is in the order of 1011 Jones at 900 nm under −0.1 V bias, when the active layer was fabricated using chlorobenzene (Fig. 4, S8† and Table 2). However, when 5% DIO was added in the processing solvent, the devices based on the two polymers showed quite different performance. In comparison with the chlorobenzene-processed device, the device based on PDDPDTP-C showed only a slightly increase in the responsivity (from 27.1 to 36.1 mA W−1) and dark current (from 1.0 × 10−7 to 6.4 × 10−7 A cm−2), giving rise to a disappointing drop in specific detectivity (from 1.5 × 1011 to 8.0 × 1010 Jones). However, the effect of DIO additive on the device performance is much more pronounced for PDDPDTP-P (Fig. S9–S11†). All the key parameters improved, including about 6-fold increase in the responsivity (from 46.5 to 291 mA W−1 at 900 nm under −0.1 V bias), nearly 2-fold drop in the dark current (from 1.7 to 0.9 × 10−7 A cm−2 under −0.1 V bias) and finally one order of magnitude increase in specific detectivity (up to 1.7 × 1012 Jones at 900 nm under −0.1 V bias). The responsivity appears to be voltage-dependent (Fig. 4f and S10†), due to the field-induced charge injection and transport.49,50 In order to further prove this observation, another most used solvent, chloroform, was used as an alternative processing solvent. The chloroform-processed photodetector based on PDPPDTP-P also exhibits better performance (Fig. S12†), implying that the advantages of introducing phenyl may be independent of the solvent system. Therefore, the photodetector based on PDPPDTP-P is promising for UV-vis-NIR broadband detection.
| Polymer | Solventa | Rb (mA W−1) | Jdb (A cm−2) | D*b (Jones) |
|---|---|---|---|---|
| a Solvent used to form the polymer films. CB: chlorobenzene, DIO: 1,8-diiodooctane.b Responsivity, dark current density and specific detectivity at 900 nm under −0.1 V bias. | ||||
| PDPPDTP-C | CB | 27.1 | 1.0 × 10−7 | 1.5 × 1011 |
| CB + 5% DIO | 36.1 | 6.4 × 10−7 | 8.0 × 1010 | |
| PDPPDTP-P | CB | 46.5 | 1.7 × 10−7 | 1.9 × 1011 |
| CB + 5% DIO | 291.3 | 0.9 × 10−7 | 1.7 × 1012 | |
Morphology study
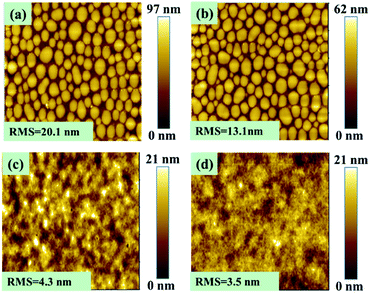
Within the general BHJ structure, some significant morphological differences could appear, such as domain size and interpenetration between domains, which can significantly affect the device performance.51–53 To find out the morphological difference between these two analogous polymers, the topography images of the polymer–PC71BM films cast without and with 5% DIO were revealed by AFM (Fig. 5). Without DIO in the processing solvent, the large PC71BM aggregates appear in the films of both polymer blends (Fig. 5a and b), which prevents intercalation of PC71BM into polymer domains and thus results in poor responsivity. With DIO added in the processing solvent, smaller domains with improved polymer/PC71BM interpenetration network were observed (Fig. 5c and d). Moreover, the film morphology of PDDPDTP-P appears to be better than PDDPDTP-C with or without using DIO, clearly indicating the unique effect brought by the phenylene unit in PDDPDTP-P on film forming and phase separation or maximization of charge separation and minimization of charge recombination. Therefore, the phenylene unit adjacent to the planar DTP moiety in PDDPDTP-P not only helps enhance the π–π interaction between the main chains and but also improves the film morphology for better charge transport and excition separation, resulting in significant enhancement of photodetector performance.Conclusion
This work confirms that the molecular stacking and film property can be optimized effectively by inserting a small aromatic unit at the side chain, ideally adjacent to the backbone, of conjugated polymers. A subtle modification by insertion of the phenylene unit at the side chain of DPP–DTP donor–acceptor polymers effectively reduces the π–π stacking distance and promotes better film-forming property and morphology, resulting in a significant 6-fold increase in the responsivity (from 46.5 to 291 mA W−1 at 900 nm under −0.1 V bias) and nearly 2-fold drop in the dark current (from 1.7 to 0.9 × 10−7 A cm−2 under −0.1 V bias). The photodetector based on PDDPDTP-P polymer shows specific detectivity of about 1012 Jones in the spectral region of 350–950 nm.Acknowledgements
This work was supported by National Natural Science Foundation of China (21474105).Notes and references
- X. Gong, M. Tong, Y. Xia, W. Cai, J. S. Moon, Y. Cao, Y. G. Yu, C. L. Shieh, B. Nilsson and A. J. Heeger, Science, 2009, 325, 1665–1667 CrossRef CAS PubMed.
- E. Saracco, B. Bouthinon, J. M. Verilhac, C. Celle, N. Chevalier, D. Mariolle, O. Dhez and J.-P. Simonato, Adv. Mater., 2013, 25, 6534–6538 CrossRef CAS PubMed.
- Y. Yao, Y. Liang, V. Shrotriya, S. Xiao, L. Yu and Y. Yang, Adv. Mater., 2007, 19, 3979–3983 CrossRef CAS.
- H. Dong, H. Zhu, Q. Meng, X. Gong and W. Hu, Chem. Soc. Rev., 2012, 41, 1754–1808 RSC.
- R. Dong, C. Bi, Q. Dong, F. Guo, Y. Yuan, Y. Fang, Z. Xiao and J. Huang, Adv. Opt. Mater., 2014, 2, 549–554 CrossRef CAS.
- E. Perzon, F. Zhang, M. Andersson, W. Mammo, Q. Inganäs and M. Andersson, Adv. Mater., 2007, 19, 3308–3311 CrossRef CAS.
- J. Qi, X. Zhou, D. Yang, W. Qiao, D. Ma and Z. Y. Wang, Adv. Funct. Mater., 2014, 24, 7605–7612 CrossRef CAS.
- L. Zhang, T. Yang, L. Shen, Y. Fang, L. Dang, N. Zhou, X. Guo, Z. Hong, Y. Yang, H. Wu, J. Huang and Y. Liang, Adv. Mater., 2015, 27, 6496–6503 CrossRef CAS PubMed.
- G. Qian, J. Qi and Z. Y. Wang, J. Mater. Chem., 2012, 22, 12867–12873 RSC.
- K. H. Hendriks, W. Li, M. M. Wienk and R. A. Janssen, J. Am. Chem. Soc., 2014, 136, 12130–12136 CrossRef CAS PubMed.
- K. R. Graham, C. Cabanetos, J. P. Jahnke, M. N. Idso, A. El Labban, G. O. Ngongang Ndjawa, T. Heumueller, K. Vandewal, A. Salleo, B. F. Chmelka, A. Amassian, B. M. Beaujuge and M. D. McGehee, J. Am. Chem. Soc., 2014, 136, 9608–9618 CrossRef CAS PubMed.
- M. Wang, H. Wang, T. Yokoyama, X. Liu, Y. Huang, Y. Zhang, T. Q. Nguyen, S. Aramaki and G. C. Bazan, J. Am. Chem. Soc., 2014, 136, 12576–12579 CrossRef CAS PubMed.
- J. W. Jo, S. Bae, F. Liu, T. P. Russell and W. H. Jo, Adv. Funct. Mater., 2015, 25, 120–125 CrossRef CAS.
- T. Lei, J. Y. Wang and J. Pei, Chem. Mater., 2014, 26, 594–603 CrossRef CAS.
- H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. S. Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Wienk, R. A. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272–3275 CrossRef CAS PubMed.
- J. C. Bijleveld, V. S. Gevaerts, D. Di Nuzzo, M. Turbiez, S. G. Mathijssen, D. M. de Leeuw, M. M. Wienk and R. A. Janssen, Adv. Mater., 2010, 22, E242–E246 CrossRef CAS PubMed.
- W. Li, W. S. Roelofs, M. Turbiez, M. M. Wienk and R. A. Janssen, Adv. Mater., 2014, 26, 3304–3309 CrossRef CAS PubMed.
- J. Lee, S. B. Jo, M. Kim, H. G. Kim, J. Shin, H. Kim and K. Cho, Adv. Mater., 2014, 26, 6706–6714 CrossRef CAS PubMed.
- Z. Zhu, D. Waller, R. Gaudiana, M. Morana, D. Muhlbacher, M. Scharber and C. Brabec, Macromolecules, 2007, 40, 1981–1986 CrossRef CAS.
- N. Blouin, A. Michaud and M. A. Leclerc, Adv. Mater., 2007, 19, 2295–2300 CrossRef CAS.
- J. Qi, J. Han, X. Zhou, D. Yang, J. Zhang, W. Qiao, D. Ma and Z. Y. Wang, Macromolecules, 2015, 48, 3941–3948 CrossRef CAS.
- J. Lee, A. Han, J. Hong, J. Seo, J. Oh and C. Yang, Adv. Funct. Mater., 2012, 22, 4128–4138 CrossRef CAS.
- I. Kang, H. Yun, D. Chung, S. Kwon and Y. Kim, J. Am. Chem. Soc., 2013, 135, 14896–14899 CrossRef CAS PubMed.
- J. Lee, A. Han, J. Kim, Y. Kim, J. Oh and C. Yang, J. Am. Chem. Soc., 2012, 134, 20713–20721 CrossRef CAS PubMed.
- C. Kanimozhi, N. Yaacobi-Gross, K. Chou, A. Amassian, T. D. Anthopoulos and S. Patil, J. Am. Chem. Soc., 2012, 134, 16532–16535 CrossRef CAS PubMed.
- K. R. Graham, C. Cabanetos, J. P. Jahnke, M. N. Idso, A. El Labban, G. O. Ngongang Ndjawa, T. Heumueller, K. Vandewal, A. Salleo, B. F. Chmelka, A. Amassian, P. M. Beaujuge and M. D. McGehee, J. Am. Chem. Soc., 2014, 136, 9608–9618 CrossRef CAS PubMed.
- N. Cho, C. W. Schlenker, K. M. Knesting, P. Koelsch, H. L. Yip, D. S. Ginger and A. K. Y. Jen, Adv. Energy Mater., 2014, 4, 1301857–1301862 Search PubMed.
- Y. Liang, D. Feng, Y. Wu, S. T. Tsai, G. Li, C. Ray and L. Yu, J. Am. Chem. Soc., 2009, 131, 7792–7799 CrossRef CAS PubMed.
- T. Lei, J. H. Dou and J. Pei, Adv. Mater., 2012, 24, 6457–6461 CrossRef CAS PubMed.
- B. Meng, H. Song, X. Chen, Z. Xie, J. Liu and L. Wang, Macromolecules, 2015, 48, 4357–4363 CrossRef CAS.
- K. Ye, S. Zhang, L. Huo, M. Zhang and J. Hou, Acc. Chem. Res., 2014, 47, 1595–1603 CrossRef PubMed.
- L. Dou, J. Gao, E. Richard, J. You, C. C. Chen, C. Cha, K. Y. He, G. Li and Y. Yang, J. Am. Chem. Soc., 2012, 134, 10071–10079 CrossRef CAS PubMed.
- Z. Li, F. Wu, H. Lv, D. Yang, Z. Chen, X. Zhao and X. Yang, Adv. Mater., 2015, 27, 6999–7003 CrossRef CAS PubMed.
- S. Zhang, L. Ye, Q. Wang, Z. Li, X. Guo, L. Huo, H. Fan and J. Hou, J. Phys. Chem. C, 2013, 117, 9550–9557 CAS.
- Y. Wu, Z. Li, W. Ma, Y. Huang, L. Huo, X. Guo, M. Zhang, H. Ade and J. Hou, Adv. Mater., 2013, 25, 3449–3455 CrossRef CAS PubMed.
- H. J. Son, L. Lu, W. Chen, T. Xu, T. Zheng, B. Carsten, J. Strzalka, S. B. Darling, L. X. L. Chen and L. Yu, Adv. Mater., 2013, 25, 838–843 CrossRef CAS PubMed.
- L. Huo, S. Zhang, X. Guo, F. Xu, Y. Li and J. Hou, Angew. Chem., Int. Ed., 2011, 50, 9697–9702 CrossRef CAS PubMed.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- L. Lu, T. Xu, W. Chen, L. E. Landry and L. Yu, Nat. Photonics, 2014, 8, 716–722 CrossRef CAS.
- B. Delley, J. Chem. Phys., 1990, 92, 508–517 CrossRef CAS.
- B. Delley, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- T. L. Nelson, T. M. Young, J. Liu, S. P. Mishra, J. A. Belot, C. L. Balliet, A. E. Javier, T. Kowalewski and R. D. McCullough, Adv. Mater., 2010, 22, 4617–4621 CrossRef CAS PubMed.
- M. Brinkmann, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1218–1233 CrossRef CAS.
- T. J. Prosa, M. J. Winokur and R. D. McCullough, Macromolecules, 1996, 29, 3654–3656 CrossRef CAS.
- G. Li, C. Kang, X. Gong, J. Zhang, C. Li, Y. Chen, H. Dong, W. Hu, F. Li and Z. Bo, Macromolecules, 2014, 47, 4645–4652 CrossRef CAS.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS PubMed.
- By assuming the dark current as the major contributor to shot noise, the specific detectivity (D*) is expressed as: D* = R/(2qJd)1/2 = (Jph/Jlight)/(2qJd)1/2 (Jones), where R is the responsivity as specific value of photocurrent (Jph) to incident-light intensity (Jlight), q is the absolute value of electron charge (1.6 × 10−19 C), and Jd is the dark current density (A cm−2).
- P. E. Keivanidis, P. K. H. Ho, R. H. Friend and N. C. Greenham, Adv. Funct. Mater., 2010, 20, 3895–3903 CrossRef CAS.
- J. D. Zimmerman, V. V. Diev, K. Hanson, R. R. Lunt, E. K. Yu, M. E. Thompson and S. R. Forrest, Adv. Mater., 2010, 22, 2780–2783 CrossRef CAS PubMed.
- J. J. van Franeker, G. H. Heintges, C. Schaefer, G. Portale, W. Li, M. M. Wienk, P. van der Schoot and R. A. Janssen, J. Am. Chem. Soc., 2015, 137, 11783–11794 CrossRef CAS PubMed.
- S. J. Lou, J. M. Szarko, T. Xu, L. Yu, T. J. Marks and L. X. Chen, J. Am. Chem. Soc., 2011, 133, 20661–20663 CrossRef CAS PubMed.
- B. A. Collins, Z. Li, J. R. Tumbleston, E. Gann, C. R. McNeill and H. Ade, Adv. Energy Mater., 2013, 3, 65–74 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic characterizations of the monomers and polymers, TGA, DSC and CV data, device characteristics. See DOI: 10.1039/c5ra27224f |
| This journal is © The Royal Society of Chemistry 2016 |