A silica-based gel electrolyte system for improving the cycle performance of LiFePO4 batteries in an aqueous medium
J. L. Panab,
Y. Yinab,
Y. H. Wen*b,
S. L. Bai*a,
J. Chengb,
G. P. Caob and
Y. S. Yangab
aCollege of Science, Beijing University of Chemical Technology, Beijing, China 100029. E-mail: baisl@mail.buct.edu.cn
bResearch Institute of Chemical Defence, Beijing, China 100191. E-mail: yuehua_wen@126.com
First published on 22nd December 2015
Abstract
LiFePO4 based aqueous lithium batteries using aqueous electrolytes suffer from poor cycling performance. This is mainly caused by the Fe dissolution and Li loss generated from the effects of water. In this paper, fumed silica based gel electrolytes were prepared and optimized for improving the cycling performance of the LiFePO4 electrodes owing to superior stability and comparable ionic conductivity. It was manifested that the Zn/LiFePO4 cells using this homogenous gel electrolyte showed stable charge/discharge voltage profiles and excellent cycling performance at room temperature. The dissolution of Fe and the loss of Li in this electrolyte is significantly suppressed. These superior performances could endow this gel electrolyte as a promising alternative to aqueous electrolyte systems in the LiFePO4 batteries at room temperatures.
1. Introduction
Lithium transition metal phosphates with olivine structures have received a considerable amount of attention as promising alternative cathode materials for rechargeable lithium ion batteries due to their high energy density, low cost, safety, and chemical stability, which has resulted in the wide use of LiFePO4 as a cathode material.1–3 But, organic solvents may be toxic and/or flammable, and various attempts have therefore been made to switch from nonaqueous to aqueous-based systems.4–9 Manickam et al.10 carried out a study of the electrochemistry and its surface characterization of LiFePO4 in aqueous lithium hydroxide electrolyte with metallic zinc as the counter electrode. The Zn|LiOH|FePO4 battery provided only a discharge capacity of 65 mA h g−1. No cycling performance was reported. Huang et al.11 investigated the kinetics of electrode processes of LiFePO4 in saturated solution. Milica et al.12 synthesized the LiFePO4/C composite as a Li-ion intercalation material in aqueous solutions. In saturated aqueous solution, the initial discharge capacity reached 106 mA h g−1 and the capacity remained 80% of initial value upon 120 charging/discharging cycles. Luo et al.13 prepared LiFePO4 by a sol–gel process. By eliminating oxygen and adjusting the pH values of the electrolyte, the LiTi2(PO4)3/Li2SO4/LiFePO4 aqueous lithium-ion batteries exhibited good stability with capacity retention over 90% after 1000 cycles at a current rate of 6C in pH 13 aqueous electrolyte. But, at a low current rate of C/8, the capacity remained 85% of initial value upon only 50 cycles. Recently, a Zn–LiFePO4 hybrid secondary battery was proposed by Zhang et al. This battery combined the strong points of Zn–air battery, lithium-ion battery and redox-flow cell. This system can offer an output voltage about 1.2 V and satisfying rate performance above 20C. Also, no prolonged cycling performance was presented.It has been reported that prolonged exposure of LiFePO4 to oxygen or water results in Li loss and in increase in Fe(III) content.5,6,9 Porcher et al.5 investigated the stability of LiFePO4 in aqueous-based systems from an electrochemical perspective, and reported that the surface of LiFePO4 was changed upon contact with water and a Li3PO4 passive layer might be formed. Lee et al.14 found that the dissolution of Li and Fe ions in aqueous processing yields a depleted powder that has a lower rate capability for LiFePO4 cathodes.
It is well known that the valve regulated lead acid (VRLA) batteries with a gel electrolyte have excellent performance in several fields.10,15–18 Gel electrolyte is prepared by mixing a gelling agent with sulphuric acid solution. To improve cycling capability of LiFePO4 based electrodes in aqueous medium, recently we prepared a new gel electrolyte containing lithium and zinc salts to prevent the dissolution of Li and Fe ions by using fumed silica as a gelling agent. In addition, the gel electrolyte could play a positive role in preventing the generation of Zn dendrite.19 So, it was anticipated to improve the cycle performance of LiFePO4 cathodes. In order to explore the feasibility of using fumed silica based gel electrolyte in LiFePO4 batteries, in this article, the key factors affecting the performance of gel electrolytes were discussed and the composition was optimized. Further, the cyclic capability and the rate discharge performance of LiFePO4/Zn cells using fumed silica based gel electrolyte were investigated in comparison with those of aqueous electrolyte at room temperatures.
2. Experimental
Commercial LiFePO4 powder was obtained from Tianjin (Tianjin Stl Energy Technology Co., Ltd) and used without further purification. The working electrode was fabricated by compressing a mixture of the active materials (LiFePO4), the conductive material (acetylene black), and the binder (polytetrafluoroethylene, PTFE) in a weight ratio of LiFePO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB
CB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTFE = 85
PTFE = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 onto a stainless steel grid by hydraulic machine at 20 MPa. The electrodes were made into the form of square typically (1 cm × 1 cm), and then dried at 120 °C for 1 h. The weight of active material is typically in the range 3–5 mg for each electrode sample. Li2SO4 + ZnSO4 aqueous electrolyte solution was prepared by dissolving Li2SO4 and ZnSO4 in distilled water. And then the pH was adjusted by H2SO4, HNO3 or LiOH. The fumed silica involved in this work were Aerosil 200 (produced by Degussa Co., Germany denoted as A200). The gel electrolyte was prepared by making water-based electrolyte (Li2SO4 and ZnSO4) firstly and then mixing fumed silica and Li2SO4 + ZnSO4 aqueous solutions. The mixture was dispersed in a homogenizer at a high stirring rate to form a colloidal solution. Electrochemical testing was performed after gelation of the colloidal solution.
5 onto a stainless steel grid by hydraulic machine at 20 MPa. The electrodes were made into the form of square typically (1 cm × 1 cm), and then dried at 120 °C for 1 h. The weight of active material is typically in the range 3–5 mg for each electrode sample. Li2SO4 + ZnSO4 aqueous electrolyte solution was prepared by dissolving Li2SO4 and ZnSO4 in distilled water. And then the pH was adjusted by H2SO4, HNO3 or LiOH. The fumed silica involved in this work were Aerosil 200 (produced by Degussa Co., Germany denoted as A200). The gel electrolyte was prepared by making water-based electrolyte (Li2SO4 and ZnSO4) firstly and then mixing fumed silica and Li2SO4 + ZnSO4 aqueous solutions. The mixture was dispersed in a homogenizer at a high stirring rate to form a colloidal solution. Electrochemical testing was performed after gelation of the colloidal solution.
Cyclic voltammograms, electrochemical impedance spectroscopy (EIS) and charge–discharge experiments were obtained by using a two-electrode beaker cell, which was assembled with a LiFePO4 cathode and a Zn anode. The active mass of the LiFePO4 in a piece of cathode is about 5 mg cm−2 while that of the zinc anode is excessive. CV curves were conducted using an electrochemical working station of CHI608D (Chenhua, Shanghai). The charge/discharge profiles, C-rate capability and cycling ability of cells were recorded on a LAND battery test system. The total ionic conductivity was tested by impedance with graphite blocking electrodes and the electronic conductivity of the electrode was tested by RTS-8 four probe tester (Guangzhou 4-probe technology Co., Ltd. China) the galvanostatic charge/discharge behaviour of Zn–LiFePO4 cells in aqueous and gel electrolytes were conducted over the range of 0.9–1.6 V. Electrochemical impedance measurements were taken at the open-circuit potential (OCP) of 1.2 V in the frequency range 20 KHz to 0.1 Hz with a Solarton instrument Model 1287 electrochemical interface, and the amplitude of the AC perturbation was 5 mV. All electrochemical measurements were performed at ambient temperature.
3. Results and discussion
3.1 Optimization of gel electrolyte
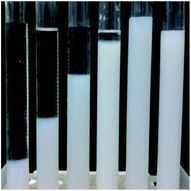 | ||
| Fig. 1 Effects of the fumed silica content on the stability of gel electrolyte. Silica content: (1) 1%; (2) 2.8%; (3) 3.2%; (4) 4.2%; (5) 4.5%; (6) 5.3%. | ||
Fig. 2 shows the CV curves and rate capability of the LiFePO4 electrode in 0.5 M Li2SO4 + 2 M ZnSO4 gel electrolytes prepared with different silica contents. With optimal dispersion, the response current of gel electrolytes prepared with 4.5 and 5.3 wt% silica are almost equal. However, when the silica content is increased to 6 wt%, a significant decrease in the oxidation and reduction peak current is presented. It can be more clearly seen that the LiFePO4 electrode in gel electrolytes with 4.5 and 5.3 wt% silica exhibits much better rate dischargeability than that in gel electrolytes with 6 wt% silica, which is likely related to the ionic conductivities of the different gel electrolytes.
 | ||
| Fig. 2 CV curves (A) and rate capability (B) of the LiFePO4 electrode in 0.5 M Li2SO4 + 2 M ZnSO4 gel electrolytes prepared with different silica contents: (a) 4.5 wt%; (b) 5.3 wt%; (c) 6.0 wt%. | ||
Since the dispersion of the fumed silica plays an important role in the electrochemical properties of LiFePO4 electrodes in gel electrolytes, CV and EIS were employed to evaluate the effect of agitation time on the electrochemical performance of the gels. CV results for gel electrolytes prepared under different agitation time are shown in Fig. 3A and B. Initially, the redox peak currents increase with increasing agitation time, reaching a maximum at about 120 min before decreasing. It can be concluded that a lower or higher agitation time will lead to a sharp decrease in the peak current of the LiFePO4 electrode.
The impedance spectra (performed at 1.2 V) for gel electrolyte prepared with different agitation times is shown in Fig. 4A. The results can be fitted well by the equivalent circuit included (see Fig. 4B). R1 represents the ohmic resistance, made up of the resistance of the electrolyte, and the resistance of the electrical connections to the electrode. R2 is the charge-transfer resistance of the rate-controlling electrochemical reaction of the lithium-ion intercalation and deintercalation process. CPE is the double-layer capacitance. It can be seen that the solution resistance (R1) and charge-transfer resistance (R2) of the gel reach a minimum with the optimal agitation time (120 min) (Fig. 4B). Therefore, the EIS results are consistent with the results obtained from the CV study.
| Sample | 1# | 2# | 3# | 4# | 5# | 6# |
| Li2SO4/M | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 |
| ZnSO4/M | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
Fig. 5 presents the CV curves of the LiFePO4 electrode in gel electrolytes with varying concentration of Li2SO4 and ZnSO4. As can be seen, compared with Li2SO4, a high concentration of ZnSO4 is more in favor of improving the kinetics of the LiFePO4 electrode. As a result, the corresponding peak currents of the gel electrolyte (3# and 6#) are much higher than the other gel electrolytes. And compared with 3# gel, 6# gel exhibits a lower oxidation peak potential, implying that the polarization of the LiFePO4 electrode is reduced further. This suggests that the 6# gel electrolyte system will provide the excellent kinetics and rate performance for the LiFePO4 electrode.
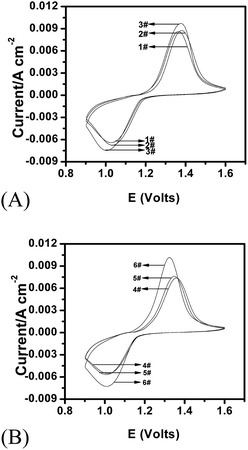 | ||
| Fig. 5 Cyclic voltammograms of the LiFePO4 electrode in gel electrolytes with varying concentration of Li2SO4 and ZnSO4 at the 5th cycle (v = 10 mV s−1). | ||
Fig. 6 shows the electrochemical impedance spectra of the gel electrolyte with varying concentration of Li2SO4 and ZnSO4 obtained at open circuit potential. The results can be fitted well by the equivalent circuit of Fig. 4B. The plots for the six gel compositions are similar and all of them exhibit a semicircle at high frequency and a long slant in the middle and low-frequency regions. This indicates that solid-state diffusion of lithium ion is a rate determining step. The slant for the 6# gel electrolyte is much shorter than the other gel electrolytes. It also can be seen from the fitting results shown in Table 2 that unequal concentration of Li2SO4 and ZnSO4 (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) generate the lowest solution resistance and charge-transfer resistance. This is generally consistent with rate capability testing below.
2) generate the lowest solution resistance and charge-transfer resistance. This is generally consistent with rate capability testing below.
 | ||
| Fig. 6 EIS for gel electrolyte prepared with varying concentration of Li2SO4 and ZnSO4 at open circuit potential. | ||
| Gel electrolytes | 1# | 2# | 3# |
| R1 | 2.49 | 2.54 | 2.85 |
| R2 | 17.08 | 10.81 | 5.03 |
| Gel electrolytes | 4# | 5# | 6# |
| R1 | 2.85 | 2.76 | 2.30 |
| R2 | 13.99 | 11.09 | 3.68 |
Fig. 7 shows the discharge specific capacities of the LiFePO4 electrode in different gel electrolytes at different charge/discharge current rates. In the current rate of 0.2–4C, the specific capacities of the electrodes in different gel electrolytes decrease with current rate increasing, but show different fading magnitudes. When the current rate is increased from 0.2C to 4C, the specific capacities of the electrodes in 1#, 2#, 3#, 4#, 5# and 6# gel electrolytes are decreased from 135.2, 136.6, 134.5, 136.1, 134.2 and 136.2 mA h g−1 to 60.2, 59.2, 63.1, 57.4, 59.9 and 64.8 mA h g−1, i.e., decreased by 55.5%, 56.7, 53.1%, 57.8%, 55.4% and 52.4%, respectively. The 6# gel electrolyte has the highest rate capabilities and then the 3# gel electrolyte, which is in agreement with the ac impedance results in Fig. 6 and Table 2. That is to say, the rate discharge ability decreases with the electrode impedance increasing. The above results indicate that the LiFePO4 electrode in 3# or in 6# gel electrolyte has higher specific capacity and discharge potential as well as better rate dischargeability, which is likely related to the Li+ ions activities and Zn2+ ions conductivities of the different gel electrolytes.
3.2 Gel batteries performance
The 6# gel electrolyte is employed to investigate the LiFePO4 batteries performance with comparison to aqueous electrolytes. The variation in electrode stability during cycling was first measured using cyclic voltammetry (CV). Herein, the cells were cycled at a scan rate of 1 mV s−1 under a voltage range of 0.9–1.6 V. Fig. 8(A and B) showed the cyclic voltammograms of the LiFePO4 cells using aqueous solution and gel electrolytes after the 1st and 110th cycle at room temperature. Similar to the previous results, one pair of major redox peaks were observed. In cyclic voltammograms, the peak currents and potential separations of the redox were known to indicate the kinetics, wherein a lower peak current and larger potential separation represented stronger electrode polarization. In the case of aqueous electrolytes, the peak currents decreased from 9.34 mA (the 1st cycle) to 7.47 mA (after the 110th cycle). In comparison, the peak currents for the gel electrolyte were reduced from 8.5 mA to 8.37 mA. A quantitative comparison of the above results confirmed that it was effective in preventing the Fe dissolution and Li loss from LiFePO4 by employing gel electrolytes, indicative of improving the cycle stability of LiFePO4 electrodes.The cycling performance of the Zn/LiFePO4 cells using aqueous and gel electrolytes at room temperature with various current rates were depicted in Fig. 9. As we can see from Fig. 9A, though both cells deliver similar initial discharge capacity and exhibit regular graded capacity change in the first 100 cycles, in the subsequent cycles the capacity in aqueous electrolyte drops more quickly than that in gel electrolyte, especially in higher current rates. The cycling data in Fig. 9A is also plotted as capacity retention versus time shown in Fig. 9B. The cell shows the same capacity fading tendency at both high and low current rates.
The capacity retention in the gel electrolyte is more than 75% after 180 cycles with various current rates, while in the aqueous electrolyte, it is only 60%. Since the battery performance is mainly dependent on the LiFePO4, it implies that the capacity fading of LiFePO4 in gel electrolytes is effectively prevented. The corresponding charge/discharge curves of the Zn/LiFePO4 cells using aqueous and gel electrolytes are presented in Fig. 9C. Both cells exhibited almost overlapped charge/discharge curves and the charge/discharge plateaus at about 1.24 V vs. Zn/Zn2+ and 1.13 V vs. Zn/Zn2+ respectively in the first cycle. But, in the 180th cycle, the capacity of LiFePO4 in the aqueous electrolyte is rather lower than that in the gel electrolyte.
In an effort to gain deep insight into the positive influence of the gel electrolyte on the cycling performance of LiFePO4 electrodes, the AC impedance spectra of the cells after the 1st and 180th cycle at room temperature were depicted in Fig. 10. It was shown that after 180 cycles, the cell impedance in the aqueous electrolyte increased more significantly than that in the gel electrolyte. This indicated that the capacity fading of LiFePO4 in aqueous electrolyte during cycling was closely associated with the continuous increase of cell resistance, which might result from the gradual dissolution of Fe and the loss of Li. This is demonstrated from Table 3, the content of dissolved Fe and Li loss in the aqueous electrolyte is nearly twice more than that in the gel electrolyte. Comparatively, although the initial cell impedance for the gel electrolyte was slightly higher than that of the aqueous electrolyte, the growth of cell impedance for the gel electrolyte was significantly retarded. This demonstrated that the use of fumed silica-based gel electrolyte may effectively alleviate the Fe dissolution and suppress the formation of a passive layer on the LiFePO4 surface during charge/discharge cycling.
 | ||
| Fig. 10 AC impedance spectra of the cells using gel electrolyte (A) and aqueous solution based electrolyte (B) after the 1st and 180th charge/discharge cycle at room temperature. | ||
| Fe | Li | |
|---|---|---|
| a The volume of aqueous and gel electrolytes was 10 ml. | ||
| Aqueous electrolytes/ppm | 48.4 | 6.1 |
| Gel electrolytes/ppm | 21 | 2.8 |
4. Conclusion
Fumed silica based gel electrolytes were prepared and optimized in aqueous medium of pH 5. The Zn/LiFePO4 cells using homogenous gel electrolytes demonstrated stable charge/discharge voltage profiles and excellent cycling performance, though the rate capability is somewhat lowered. Moreover, fumed silica based gel electrolyte alleviated the growth of cell impedance and the Fe dissolution, lithium loss problems. The superior cycling performances could endow this class of fumed silica based gel electrolyte a very promising alternative to state of the art liquid electrolyte system in the LiFePO4 lithium batteries.Acknowledgements
The acknowledgements come at the end of an article after the conclusions and before the notes and references.Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- Y. Wang, J. Wang, J. Yang and Y. Nuli, Adv. Funct. Mater., 2006, 16, 2135–2140 CrossRef CAS.
- J. H. Lee, U. Paik, V. A. Hackley and Y. M. Choi, J. Electrochem. Soc., 2005, 152, A1763 CrossRef CAS.
- W. Porcher, P. Moreau, B. Lestriez, S. Jouanneau and D. Guyomard, Electrochem. Solid-State Lett., 2008, 11, A4 CrossRef CAS.
- J. F. d. r. Martin, A. Yamada, G. Kobayashi, S.-i. Nishimura, R. Kanno, D. Guyomard and N. Dupré, Electrochem. Solid-State Lett., 2008, 11, A12 CrossRef CAS.
- J. H. Lee, J. S. Kim, Y. C. Kim, D. S. Zang and U. Paik, Ultramicroscopy, 2008, 108, 1256–1259 CrossRef CAS PubMed.
- W. Porcher, B. Lestriez, S. Jouanneau and D. Guyomard, J. Electrochem. Soc., 2009, 156, A133 CrossRef CAS.
- Y. W. Y. Denis, K. Donoue, T. Kadohata, T. Murata, S. Matsuta and S. Fujitani, J. Electrochem. Soc., 2008, 155, A526–A530 CrossRef.
- M. Manickam, P. Singh, S. Thurgate and K. Prince, J. Power Sources, 2006, 158, 646–649 CrossRef CAS.
- K. L. Huang, S. Yang, S. Q. Liu and H. B. Wang, Acta Phys.-Chim. Sin., 2007, 23, 129–133 CAS.
- M. Vujković, I. Stojković, N. Cvjetićanin and S. Mentus, Electrochim. Acta, 2013, 92, 248–256 CrossRef.
- J. Y. Luo, W. J. Cui, P. He and Y. Y. Xia, Nat. Chem., 2010, 2, 760–765 CrossRef CAS PubMed.
- J. H. Lee, H. H. Kim, G. S. Kim, D. S. Zang, Y. M. Choi, H. Kim, D. K. Yi, W. M. Sigmund and U. Paik, J. Phys. Chem. C, 2010, 114, 4466–4472 CAS.
- S. Hyub Oh, M. Kim, J. Bok Lee and H. L. Lee, Bull. Korean Chem. Soc., 2002, 23, 75–80 CrossRef.
- S. K. Martha, B. Hariprakash, S. A. Gaffoor and A. K. Shukla, Bull. Mater. Sci., 2003, 26, 465–469 CrossRef CAS.
- J. C. Hernández, M. L. Soria, M. González, E. García-Quismondo, A. Muñoz and F. Trinidad, J. Power Sources, 2006, 162, 851–863 CrossRef.
- H. Zhang, X. Wu, T. Yang, S. Liang and X. Yang, Chem. Commun., 2013, 49, 9977–9979 RSC.
- B. Qin, Z. Liu, G. Ding, Y. Duan, C. Zhang and G. Cui, Electrochim. Acta, 2014, 141, 167–172 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |






