Synthesis of selenium nanoparticles with mesoporous silica drug-carrier shell for programmed responsive tumor targeted synergistic therapy†
Bo Yu,
Yang Zhou,
Meifang Song,
Yanan Xue,
Ning Cai,
Xiaogang Luo,
Sihui Long,
Han Zhang and
Faquan Yu*
Key Laboratory for Green Chemical Process of Ministry of Education, Hubei Key Laboratory for Novel Reactor and Green Chemistry Technology, School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430073, China. E-mail: fyu@wit.edu.cn; fyuwucn@gmail.com
First published on 17th December 2015
Abstract
A sophisticated delivery nanocarrier was designed to achieve programmed responsive tumor targeted synergistic therapy. By combining a mesoporous SiO2 drug-carrier shell and selenium nanoparticle therapy core while decorating with hidden folate, the size of nanocarrier was enlarged to about 380 nm which can enhance accumulation in tumor tissues by passive targeting due to the EPR effect. Subsequently, the stimulation of mild acid cleaved the citraconic anhydride-modified dextran, leading to the exposure of folate as well as targeting cellular uptake. In vitro results indicated the synergistic anticancer performance of the nanocarrier in combination with doxorubicin. This selenium nanoparticle based nanoplatform has the potential to avoid unexpected targeting and perform synergistic anticancer drug activity.
Although chemotherapy has been extensively employed in cancer treatment, the pressing challenge is how to maximize its therapy effect and minimize the damage to normal tissues.1 The dose of drug plays an important role in chemotherapy, where high dose leads to serious toxicity to normal tissue and the immune system, ultimately, influencing the patient's quality of life.2 Synergistic anticancer effect through co-administration of two anticancer agents offers a useful strategy to reduce drug dose.3 Recently nanocarriers have provided such a potential tool to construct this three-member system which contained the carrier and two drugs for the achievement of the effect.4,5 As known, the synergistic effect depends on the ratio of two drugs that reach cancer cells simultaneously. Nevertheless, it is difficult to integrate two drugs with different physicochemical properties into a nanocarrier and to maintain the expected release rate in the synergistic therapeutic optimal ratio.
Selenium is an essential dietary micronutrient, and it was found that selenium in the form of Selenium nanoparticle (SeNP) could kill cancer cells.6 Furthermore, SeNP was relatively a new member of drug nanocarriers7 and has been co-delivered with other compounds, such as 5-fluorouracil, ATP, peptide, ruthenium complex and sialic acid, to improve their biological activities.7,8 Thus, SeNP could be regarded as a promising synergistic anticancer nanocarrier with innate therapeutic effect. In this case, a three-member system of two synergistic drugs and a nanocarrier could degenerate to be a two-member system of one drug and SeNP, which circumvents the difficulty in optimizing the ratio of two drugs throughout the co-delivery system formulation. However, the reported SeNP provides the ambiguous space, which limits the drug loading. The latter is beneficial for the maximum of efficacy as well as positive clinical results.9 To date, little work has been done concerning this issue. Thus, there is a compelling need to design an improved SeNP with definite loading structure that could provide the capable loading capacity.
Decoration of nanocarriers with cancer-targeting ligands such as folic acid (FA), RGD and transferrin,10 presented a potential strategy for enhancing cancer therapy effect and avoiding unexpected side effect. However, the receptor distribution in tissues remains elusive,11 or both cancers and normal tissues contain the same kind of receptor,12 making the effective design of receptor-targeting function extremely challenging. Furthermore, as widely known, the enhanced permeability and retention (EPR) effect is used as the passive targeting tumor approach in cancer therapy.13,14 Compared to the extensive research in EPR based nanocarrier design, however, the clinical translation of nanocarrier-enabled drug delivery system absolutely was rare. Thus, the rational design of nanocarrier which is capable of targeting tumor probably by both passive (EPR effect) and active (cancer-targeting ligands–receptor interaction) mechanisms is meaningful to increase chemotherapy efficacy against cancer. Obviously, to hide the ligands until the decorated nanocarrier reached the targeting site is an alternative technique.
Herein, SeNP acted as the therapy core while mesoporous silica (mSiO2) was used as the shell, which provided exterior storage space for loading doxorubicin (Dox). After coating with folate-functionalized chitosan (FFC), the resultant nanoparticle was further coated by Dex-COOH via electrostatic interaction. Dex-COOH is citraconic anhydride-modified dextran, which was developed previously in our lab. The charge reversal of Dex-COOH could occur after mild acid stimulaiton in tumor microenvironment.15 Thus, the outmost layer of Dex-COOH ensures the negative surface charge and thus long circulation. In mild acid environment of tumor tissue, folate was subsequently exposed upon the degeneration of Dex-COOH, which achieved in situ targeting. Then Dox synergistically acts together with SeNP to realize low-dose therapy. In short, we integrated the SeNP carrier-based synergistic effect with hidden cancer-targeting ligands, charge reversal, pH-responsiveness, and high loading space into a multi-functionalized nanocarrier.
To develop mesoporous silica-coated SeNP, we proposed a method illustrated in Fig. 1a. First, highly dispersed SeNP were prepared with polyvinylpyrrolidone (PVP, K30) as the capping agent following the protocol proposed in the experiment section.16 As shown in Fig. 1b (left), the size was found to be about 90 nm in diameter (by TEM) with negative surface charge (−4 mV, Fig. S1†). (3-Aminopropyl)-triethoxy-silan (APTES) was subsequently transferred into silica nanoshell by hydrolysis in aqueous medium, forming the core–shell composite SeNP@nSiO2 nanoparticle with the size of about 110 nm (Fig. 1b, middle). The coating layer was estimated to be 10 nm. In terms of the CTAB-templating condensation of tetraethyl orthosilicate (TEOS), the newly-formed silica layer with a thickness of about 125 nm was uniformly coated on the surface of SeNP@nSiO2 to obtain the SeNP@nSiO2@mSiO2 nanocarrier (Fig. 1b, right) with ζ potential value of −8.5 mV (Fig. S1†). The size distribution was analyzed by DLS as well, which was consistent with the TEM result (Fig. 1c).
The successful coating of silica intermediate nanoshell by APTES is crucial for the subsequent coating of mesoporous silica. When we utilized TEOS in place of APTES, a procedure in the silica-coated silver nanoparticles,17 the product was found to be a mixture of aggregated white pellet and red powder, or likely the mixture of silica and selenium. Probably, APTES tends to adsorb on the SeNP in terms of the interaction between negatively charged surface and amino groups. The result suggested that the strong interaction between organosilicone and SeNP was necessary for the successful coating. In addition, the solvent system is vital as well for the nanoshell coating. Black selenium nanorods instead of red nanoparticles (Fig. S2†) were yielded while ethanol/water mixture was employed in the previous procedure.17 Apparently, this procedure failed to form the coating structure. In fact, the mesoporous channels were opened only after the removal of the template SeNP@nSiO2@mSiO2 with solvent extraction by refluxing of NH4NO3/ethanol solution.17 The exterior mesoporous layer will provide spacious place for drug loading. The channel-opened SeNP@nSiO2@mSiO2 (meso-SeNP) has a smooth surface with a diameter of 350 nm (Fig. 2a). XRD pattern showed the peak at around 2.5° (2θ), indicating the ordered mesoporous structure was formed (Fig. 2b). Furthermore, the meso-SeNP was examined to be amorphous according to the XRD pattern (Fig. S3†). The EDX elemental analysis was acquired using a component attachment of TEM instrument. These peaks assigned to Si and Se (Fig. 2c), which further confirmed the successful coating in meso-SeNP.
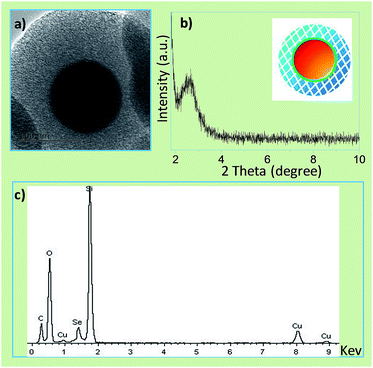 | ||
| Fig. 2 (a) TEM image, (b) XRD pattern and (c) EDS analysis of channel-opened SeNP@nSiO2@mSiO2 (meso-SeNP). | ||
Fig. 3a exhibited the process pertinent to the loading of Dox and the successive modification. By the virtue of the mesoporous space, the obtained Dox-loaded meso-SeNP(I) exhibited −21 mV of ζ potential. The coating of FFC on I, noted as II, turned the surface charge out to be +25 mV, an anticipated result owing to the abundant amine groups in chitosan. Through the electrostatic self-assembly of Dex-COOH on II, the ζ potential of the obtained product, III, was reversed into −17 mV (Fig. 3b), and the average diameter of nanoparticles changed from about 357.1 nm (II) to 379.8 nm (III) (Fig. S4†). The transformation track of ζ potential endorsed the successful coating of FFC and Dex-COOH (Fig. S5†). FT-IR further confirmed the coating (Fig. S6†). The characteristic peaks of silica, amide, and hydrogen bonding were consistent with the reported work.15,18,19 The TEM and SEM images of III appeared uniform in size with a statistical diameter of about 380 ± 11 nm (Fig. 3c and d). The architecture achieved on the nanoscale and negative surface charge could be beneficial for long-term circulation. On the other hand, for nanoparticles of 60–400 nm in diameter, tumor tissue will exhibit the enhanced permeability and retention (EPR) effect, which can enhance tumor uptake by passive targeting.20 The loaded amount of Dox in I was quantified. Experimentally, meso-SeNP exhibited 15.5-folded higher loading capacity than that of SeNP (Fig. S7†) in the identical loading process. This testified that the mesoporous structure coated on SeNP is significant in offering spacious reservoir for drug.
As well known, the pH value of tumor extracellular, endosome and lysosome is at 6.5–7.2, 5.0–6.5 and 4.5–5.0, respectively.15 The pH 6.8 medium was investigated herein for the sake of simulation of the tumor extracellular environment. The surface charge was monitored for 12 h (Fig. 4a). No significant alteration was observed in pH 7.4. While in the pH 6.8 medium, the negative surface charge gradually turned into positive. The charge reversal behavior was believed to be from the dissociation of citraconic amide linkage in Dex-COOH as the previously report, releasing the positive amino group after the removal of negatively charged carboxylic group.15,21 The behavior would result in the decoating of Dex-COOH and eventually exposure of the hidden folate. In another respect, the pH-responsive release of Dox was observed (Fig. 4b). In the environment of pH 5.0, the drug release was significantly quicker than that of pH 6.8 and pH 7.4. This observation suggested that the nanocarriers displayed endosome-targeting release or low release in extracellular tumor. Upon the examination of nanoparticle via confocal microscopy (Fig. 4c), the red bright (white arrows) and dark area may represent the accumulation of body of loading-Dox nanoparticle and the decoated polymer, which captured the released Dox, respectively. Moreover, to confirm the fact that the tumor-extracellular acid stimulation can efficiently take off the Dex-COOH layer, TEM and DLS were performed on the observation of III after acid treatment. As shown in Fig. 4d, the morphology of III was turned to be much rough than that in Fig. 3c. The average diameter of nanoparticles was found to be about 427 nm (Fig. S8†). The light dark clusters surrounding the nanoparticles may come from the taken-off Dex-COOH. Noticeably, this result coincided with the phenomenon observed in confocal microscopy.
Further, we investigated the growth inhibition effects of III (Dox loading content was 1.7%, in Fig. S7†) on cancer cells in comparison with II. According to a previous report, HeLa was the folate receptor positive cell.22 The result revealed that III exhibited dose-dependent growth inhibition on HeLa cells and the cytotoxicity with IC50 0.198 (Dox) μg mL−1 after 24 h incubation, as shown in Fig. 4e. In contrast, the IC50 treated by II declined to be 0.138 (Dox) μg mL−1, which is 30% lower than that of III. Obviously, hidden folate (III) retarded the cytotoxicity or exposed folate (II) enhanced the ability. In contrast, this difference disappeared in the case of HepG2 (Fig. S9†). HepG2 was considered as a folate receptor negative cells.16 Folate is not the specific targeting moiety in this case. Therefore, whether hidden or exposed, folate is not correlated with the targeting-enhanced cytotoxicity. Interestingly, II and III almost exhibited the overlay inhibition curves, with the incubation time prolonged up to 72 h (Fig. S10†). The prolonged incubation time ensured the de-coating of Dex-COOH in III and the exposure of the hidden folate. Thus, the final effect in the proliferation inhibition would approach to that of II. These results suggested that hiding of folate is effective in the reduction of cytotoxicity and folate was exposed in targeting site.
The IC50 values of meso-SeNP and free Dox toward HeLa cells were 76.2 μg mL−1 and 2.0 μg mL−1 (Fig. S11†), respectively. Correspondingly, the IC50 values of III were 23.0 μg mL−1 (meso-SeNP) and 0.198 μg mL−1 (Dox). Dex-COOH showed no cytotoxicity even at 100 μg mL−1 of concentration (Fig. S11c†). These evidenced the obvious synergistic effect and indicated that III exhibited higher anticancer efficacy than that of free Dox and SeNP.
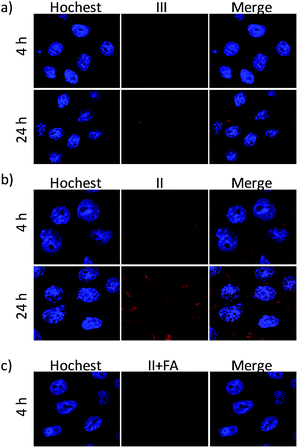
To further demonstrate whether the obtained nanoparticle can be efficiently internalized by HeLa cells, experiments on the intracellular study of Dox-loaded nanoparticle were performed on HeLa cells after treatment for different time. The cellular distribution of Dox was examined by CLSM analysis. As shown in Fig. 5a, cells incubated with III showed Dox red fluorescence in the cytoplasm after 4 h of incubation with III, indicating successful internalization of III. With further incubation for 24 h, the Dox fluorescence intensity inside the cells increased and the accumulation of Dox in nuclei became more evident than that after 4 h of incubation. This phenomenon implied that nanoparticle of III transferred to the mild acid cytoplasm, and the dissociation of anhydride-modified dextran would cause de-coating of Dex-COOH and FFC, which led to the release of Dox from the III. Thus, more red fluorescent dots were observed in the nuclei of Hela cells for longer incubation time. Interestingly, as showed in Fig. 5b, II without the coating layer of Dex-COOH showed the faster internalization of nanoparticle and stronger accumulation of Dox in cell nuclei than those of III. Therefore, the direct folate decoration was a key factor in determining the internalization speed of nanoplatform. The growth inhibition effect on HeLa cells was elevated as the internalization speed increased (Fig. 4e) in 24 h incubation. This result also suggested that the covered Dex-COOH layer supplied hidden folate method and resulted in avoiding unexpected target.
To confirm the role of folate in the cell uptake, we also examined the cellular uptake of II in HeLa cells blocked with FA. Comparing the CLSM result as showed in Fig. 5b and c, the uptake intensity of II decreased obviously after pre-incubated with 2 mg mL−1 FA. Those results revealed that FA surface decoration efficiently enhanced the cellular uptake of nanoplatform, and the hidden FA capped nanoplatform with the tumor environment responsiveness could be a potential targeting carrier for anticancer drugs.
Conclusions
In conclusion, we took the advantage of the cancer cell-killing properties of SeNP and the drug loading capacity of mesoporous SiO2 to construct the hidden cancer-targeting nanocarrier to achieve enhanced anticancer activity of DOX. The mesoporous silica was proven to offer a spacious reservoir for high drug loading capacity as the coating shell on SeNP. Folate was hidden until the nanocarrier reached the targeting site to diminish the systemic adverse effect and enhance the targeting efficiency. Dex-COOH exhibited pH-responsive release. The behavior would result in the decoating of Dex-COOH and eventually exposure of the hidden folate in targeting site. This sophisticated nanocarrier presented a programmed release to avoid unexpected target, which dramatically enhanced the anticancer effect of Dox at low dose.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21571147), as well as by the Program of Hubei Provincial Department of Education, China (B2013214), by Innovative Team Program of Natural Science Foundation of Hubei Province (2014CFA011), by Innovative Team Incubation Program in High-tech Industry of Wuhan City (2014070504020244), by Hubei Collaborative Innovation Center for Down-Streaming Products in Ethylene Project and Process Intensification, and by the Scientific Research Foundation of Wuhan Institute of Technology (K201459 and 10125032).Notes and references
- J. Zhang, Y. Li, F. F. An, X. Zhang, X. Chen and C. S. Lee, Nano Lett., 2015, 15, 313–318 CrossRef CAS PubMed.
- Y. K. Lee, J. Choi, W. Wang, S. Lee, T. H. Nam, W. S. Choi, C. J. Kim, J. K. Lee, S. H. Kim, S. S. Kang and D. Khang, ACS Nano, 2013, 7, 8484–8497 CrossRef CAS PubMed.
- Q. Du, B. Hu, H. M. An, K. P. Shen, L. Xu, S. Deng and M. M. Wei, Oncol. Rep., 2013, 29, 1851–1858 CAS.
- L. Qiu, T. Chen, I. Ocsoy, E. Yasun, C. Wu, G. Zhu, M. You, D. Han, J. Jiang, R. Yu and W. Tan, Nano Lett., 2015, 15, 457–463 CrossRef CAS PubMed.
- L. Feng, L. Wu and X. Qu, Adv. Mater., 2013, 25, 168–186 CrossRef CAS PubMed.
- S. Verma, F. W. Hoffmann, M. Kumar, Z. Huang, K. Roe, E. Nguyen-Wu, A. S. Hashimoto and P. R. Hoffmann, J. Immunol., 2011, 186, 2127–2137 CrossRef CAS PubMed.
- A. Nasrolahi Shirazi, R. K. Tiwari, D. Oh, B. Sullivan, A. Kumar, Y. A. Beni and K. Parang, Mol. Pharm., 2014, 11, 3631–3641 CrossRef CAS PubMed.
- D. Sun, Y. Liu, Q. Yu, D. Liu, Y. Zhou and J. Liu, J. Inorg. Biochem., 2015, 150, 90–99 CrossRef CAS PubMed.
- Y.-J. Cheng, G.-F. Luo, J.-Y. Zhu, X.-D. Xu, X. Zeng, D.-B. Cheng, Y.-M. Li, Y. Wu, X.-Z. Zhang, R.-X. Zhuo and F. He, ACS Appl. Mater. Interfaces, 2015, 7, 9078–9087 CAS.
- J. A. Loureiro, B. Gomes, G. Fricker, I. Cardoso, C. A. Ribeiro, C. Gaiteiro, M. A. Coelho, M. D. Pereira and S. Rocha, Colloids Surf., B, 2015, 134, 213–219 CrossRef CAS PubMed.
- M. Uhlen, L. Fagerberg, B. M. Hallstrom, C. Lindskog, P. Oksvold, A. Mardinoglu, A. Sivertsson, C. Kampf, E. Sjostedt, A. Asplund, I. Olsson, K. Edlund, E. Lundberg, S. Navani, C. A. Szigyarto, J. Odeberg, D. Djureinovic, J. O. Takanen, S. Hober, T. Alm, P. H. Edqvist, H. Berling, H. Tegel, J. Mulder, J. Rockberg, P. Nilsson, J. M. Schwenk, M. Hamsten, K. von Feilitzen, M. Forsberg, L. Persson, F. Johansson, M. Zwahlen, G. von Heijne, J. Nielsen and F. Ponten, Science, 2015, 347, 1260419 CrossRef PubMed.
- H. Elnakat and M. Ratnam, Adv. Drug Delivery Rev., 2004, 56, 1067–1084 CrossRef CAS PubMed.
- Z. Zhen, W. Tang, Y.-J. Chuang, T. Todd, W. Zhang, X. Lin, G. Niu, G. Liu, L. Wang, Z. Pan, X. Chen and J. Xie, ACS Nano, 2014, 8, 6004–6013 CrossRef CAS PubMed.
- W. Rao, H. Wang, J. Han, S. Zhao, J. Dumbleton, P. Agarwal, W. Zhang, G. Zhao, J. Yu, D. L. Zynger, X. Lu and X. He, ACS Nano, 2015, 9, 5725–5740 CrossRef CAS PubMed.
- H. Zhang, Y. Xue, J. Huang, X. Xia, M. Song, K. Wen, X. Zhang, X. Luo, N. Cai, S. Long and F. Yu, J. Mater. Sci., 2015, 50, 2429–2442 CrossRef CAS.
- B. Yu, X. Li, W. Zheng, Y. Feng, Y.-S. Wong and T. Chen, J. Mater. Chem. B, 2014, 2, 5409–5418 RSC.
- J. Yang, F. Zhang, Y. Chen, S. Qian, P. Hu, W. Li, Y. Deng, Y. Fang, L. Han, M. Luqman and D. Zhao, Chem. Commun., 2011, 47, 11618–11620 RSC.
- L. Pan, Q. He, J. Liu, Y. Chen, M. Ma, L. Zhang and J. Shi, J. Am. Chem. Soc., 2012, 134, 5722–5725 CrossRef CAS PubMed.
- C. V. Durgadas, C. P. Sharma and K. Sreenivasan, Analyst, 2011, 136, 933–940 RSC.
- D. P. O'Neal, L. R. Hirsch, N. J. Halas, J. D. Payne and J. L. West, Cancer Lett., 2004, 209, 171–176 CrossRef PubMed.
- T. Zhou, X. Zhou and D. Xing, Biomaterials, 2014, 35, 4185–4194 CrossRef CAS PubMed.
- S. A. Kularatne, V. Deshmukh, M. Gymnopoulos, S. L. Biroc, J. Xia, S. Srinagesh, Y. Sun, N. Zou, M. Shimazu, J. Pinkstaff, S. Ensari, N. Knudsen, A. Manibusan, J. Y. Axup, C. H. Kim, V. V. Smider, T. Javahishvili and P. G. Schultz, Angew. Chem., Int. Ed. Engl., 2013, 52, 12101–12104 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experiment details; zeta potential analysis; XRD; FT-IR; HNMR; MTT results. See DOI: 10.1039/c5ra21460b |
| This journal is © The Royal Society of Chemistry 2016 |