Synthesis of 2,6-trans- and 3,3,6-trisubstituted tetrahydropyran-4-ones from Maitland–Japp derived 2H-dihydropyran-4-ones: a total synthesis of diospongin B†
Received
31st May 2016
, Accepted 17th June 2016
First published on 20th June 2016
Abstract
6-Substituted-2H-dihydropyran-4-one products of the Maitland–Japp reaction have been converted into tetrahydropyrans containing uncommon substitution patterns. Treatment of 6-substituted-2H-dihydropyran-4-ones with carbon nucleophiles led to the formation of tetrahydropyran rings with the 2,6-trans-stereochemical arrangement. Reaction of the same 6-substituted-2H-dihydropyran-4-ones with L-Selectride led to the formation of 3,6-disubstituted tetrahydropyran rings, while trapping of the intermediate enolate with carbon electrophiles in turn led to the formation 3,3,6-trisubstituted tetrahydropyran rings. The relative stereochemical configuration of the new substituents was controlled by the stereoelectronic preference for pseudo-axial addition of the nucleophile and trapping of the enolate from the opposite face. Application of these methods led to a synthesis of the potent anti-osteoporotic diarylheptanoid natural product diospongin B.
Introduction
Substituted tetrahydropyran (THP) rings are present in a large number of biologically active natural products, and as such their synthesis has received much attention over the years.1,2 On inspection of these THP rings it is clear that some substitution patterns occur more often than others, and this has resulted in a greater amount of synthetic effort being directed towards their synthesis compared to the synthesis of other substitution patterns. The consequences of those efforts are that these common substitution patterns can now be accessed readily, while the more uncommon substitution patterns still require greater synthetic effort. For example, 2,6-cis-THP rings can be accessed by a wide variety of methods, including thermodynamically controlled oxy-Michael reactions,3,4 Diels–Alder reactions,5 Prins rearrangements,6,7 reduction of cyclic oxocarbenium ions,8 metal mediated cyclisations9 and the Maitland–Japp reaction.10 Conversely, construction of the 2,6-trans-THP ring is almost exclusively limited to either nucleophilic addition to cyclic hemiacetals via an oxocarbenium ion11,12 or kinetically controlled oxy-Michael reactions,3,4 though in the latter case the trans-selectivity is often only moderate.
A survey of THP-containing natural products shows that a sizable number do contain the 2,6-trans-THP ring, for example psymberin13 (an inhibitor of cancer cell proliferation), zincophorin14 (an antibiotic), aspergillide B15 (cytotoxicity against mouse lymphocytic leukemia cells) and diospongin B16 (anti-osteoporotic activity) (Fig. 1).
 |
| | Fig. 1 2,6-trans-Tetrahydropyran-containing natural products. | |
We recently reported the synthesis of substituted dihydropyran-4-ones (DHPs), by extension of the Maitland–Japp reaction,17 a method which is complementary to the Diels–Alder route popularised by Danishfesky.18 We then converted these DHPs into 2,6-cis-THPs.17 This strategy enabled us to complete syntheses of “Civet” and a fully functionalised model A-ring of lasonolide A. Given the dearth of methods for the construction of 2,6-trans-THP rings we turned our attention to the development of a new method for the selective synthesis of 2,6-trans-THPs. We envisaged that 2,6-trans-THPs could be formed from the conjugate addition of a carbon nucleophile to the double bond of Maitland–Japp DHPs such as 5. We rationalised that the stereoelectronic preference for axial addition of a nucleophile to the double bond would generate a 2,6-trans-THP with the opportunity to trap the resultant enolate, which would allow for further functionalisation of the THP-ring (Fig. 2).
 |
| | Fig. 2 Stereoelectronic preference for axial addition of nucleophiles leading to 2,6-trans-THPS. | |
Results and discussion
Synthesis of dihydropyran-4-ones
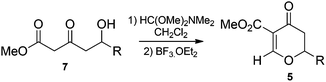
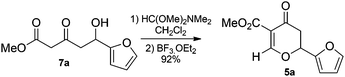
In order to investigate the formation of 2,6-trans-THPs we had to prepare DHPs 5. To this end we employed the conditions we had used for the synthesis of C2-substituted DHPs (an orthoamide or orthoester in toluene),17 however, when we used the dimethyl acetal of N,N-dimethylformamide and δ-hydroxy-β-ketoesters 7, complex mixtures of products resulted. Our initial results suggested that there was an inherent instability in the DHPs 5 that was not apparent in their C2-substituted counterparts, this was particularly noticeable during attempted isolation by chromatography on silica gel (2D TLC showed multiple interconverting spots). However, if the crude reaction mixture was exposed to a Gilman cuprate, it was possible to isolate some 2,6-trans THP – with the exception of 7a which gave a moderate isolated yield of DHP 5a. Following considerable investigation we realised that the Knoevenagel-like condensation of the orthoamide occurred but the oxy-Michael cyclisation to give the DHP did not. This issue could be rectified by performing the reaction with only one equivalent of orthoamide in CH2Cl2, rather than PhMe, followed by the addition of BF3·OEt2 to promote cyclisation, resulting in a 92% crude mass balance of 5a which could be used crude, without the need for purification (Scheme 1).
 |
| | Scheme 1 Formation of C2-unsubstituted DHPs. | |
These conditions proved general for the synthesis of a range of C2-unsubstituted, C6-substituted DHPs 5 (Table 1). In addition to the 2-furyl group 5a, other heteroaromatic substituents could be incorporated 5h, as well as phenyl 5b. n-Alkyl and branched alkyl substituents are readily tolerated 5c and 5d, along with alkene-containing side chains 5f and 5g. Perhaps the most encouraging, as it allows further elaboration of the C6-side chain, is the realisation that TIPS-protected alcohols can also be incorporated 5e.
Table 1 Synthesis of DHPs
With a range of DHPs to hand we were now in a position to study to formation of 2,6-trans-substituted THPs.
Conversion of dihydropyran-4-ones to 2,6-trans-tetrahydropyran-4-ones
When DHPs 5 were treated with a range of Gilman cuprates, Ph2CuLi, Me2CuLi and Bu2CuLi in the presence of TMSCl at −78 °C in THF, it was found that conjugate addition occurred smoothly to yield the 2,6-trans-THPs in a mixture of enol and keto-forms 8/9 (Table 2). Addition of Ph2CuLi generated the 2,6-trans-THPs exclusively as the enol tautomer 8. However, use of Me2CuLi and Bu2CuLi generated mixtures of enol–keto tautomers 8 and 9 of the 2,6-trans-THPs. For the purposes of characterisation, these tautomers were converted into enol acetates 10 by the action of Ac2O, pyridine and DMAP.
Table 2 Synthesis of 2,6-trans-THPs
We rationalise that 2,6-trans-THPs exist as a mixture of keto/enol tautomers because either the C2 or C6 substituent must be axial. The penalty for having an axial substituent may be partly relieved by enolisation as this allows for the formation of an intramolecular H-bond and the reduction of a 1,3-diaxial interaction for the axial group. Therefore, in order to definitively characterise the 2,6-trans-THP products the keto/enol mixture was treated with Ac2O, pyridine and DMAP to form enol acetates 10, where the 2,6-trans-THP stereochemical configuration was confirmed by analysis of the 1H NMR and NOE data (Fig. 3). In the representative case of 10h there was a strong NOE correlation between C2 methyl group and H6 of 2.3% and a NOE correlation between C2 methyl group and H5α of 1.86%.
 |
| | Fig. 3 NOE correlations confirming the 2,6-trans-stereochemical configuration of 10h. | |
Conversion of dihydropyran-4-ones to 3,6-disubstituted and 3,3,6-trisubstituted tetrahydropyran-4-ones
With the development of a successful strategy for the synthesis of 2,6-trans-THPs we sought to extend the scope for the conversion of DHPs 5 into THPs with other substitution patterns. We considered the possibility that 3,6-disubstituted-THPs could be accessed by the conjugate reduction of the C2–C3 double bond. When DHPs 5 were treated with L-Selectride at −78 °C and quenched, a range of 3,6-disubstituted THPs 11 were formed in good yields; the enol tautomer was the major product in all cases, with small amounts of the keto-tautomer present. In order to aid characterisation the product mixture was converted into the enol acetate 12 by the action of Ac2O, pyridine and DMAP (Table 3). In all cases studied we could not detect products from reduction of either the ketone or the ester carbonyl groups.
Table 3 Synthesis of 3,6-disubstituted-THPs
The addition of L-Selectride to DHPs 5 initially generated an enolate which was quenched upon workup to give 3,6-disubstituted THPs 11. We wondered if it would be possible to intercept the enolate with a carbon electrophile to form 3,3,6-trisubstituted THPs. Alkyl halides methyl iodide, allyl bromide and benzyl bromide were investigated (Table 4).
Table 4 Synthesis of 3,3,6-trisubstituted THPs
We reasoned that delivery of hydride would occur from the pseudo-axial trajectory and the electrophilic quenching would occur from the opposite face of the THP ring. This should deliver THP products with a quaternary stereocenter at C3, in which the R and R1 groups are cis to each other. No other diastereomer was detected in the 1H NMR of the crude reaction mixture. The THP products 13 were characterised, and the relative stereochemical configuration confirmed, by 1H NMR and NOE correlations. For example, in the representative case of 13i there was a clear NOE of 3.6% between H6 and H5α when H6 was irradiated. When H5α was irradiated a NOE to H6 of 2.26% was seen. There was a NOE of 3.16% between H5β and the benzyl CH2 group, indicating that these were both axial (Fig. 4). The protocol gave the desired functionalisation with the halide electrophiles but, to our disappointment, we were unable to intercept the enolate with aldehyde electrophiles, which probably reflects the inherent stability of the β-ketoester's enolate anion.
 |
| | Fig. 4 NOE correlations and coupling constants confirming the stereochemical configuration of 13i. | |
Synthesis of diospongin B
With procedures developed for the synthesis of highly substituted THP-rings, especially the less common and synthetically more challenging 2,6-trans-THP, we sought to demonstrate the utility of the approach by completing the total synthesis of the anti-osteoporotic 2,6-trans-THP-containing natural product diospongin B 2. Diospongin B is a diaryl heptanoid natural product which was isolated in 2003 from the rhizomes of Dioscorea spongiosa and was shown to exhibit potent inhibitory activity on bone resorption induced by parathyroid hormone.16 The activity of diospongin B is comparable to calcitonin, a drug currently used to treat osteoporosis, and this has led to a number of total syntheses being reported for it and its 2,6-cis-diastereomer, diospongin A.19
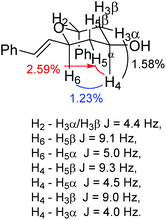
Our synthesis (Scheme 2) began with the Maitland–Japp formation of DHP 5g in 97% yield using the dimethylacetal of N,N-dimethyl formamide. Conjugate addition of Ph2CuLi to 5g yielded 2,6-trans-THP 8f in 91%. Microwave-mediated decarboxylation in wet DMF generated the desired tetrahydropyran-4-one,20 which was in turn reduced with L-Selectride to give THP 14 as the major diastereomer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with the correct relative stereochemical configuration for diospongin B. The stereochemical configuration of 14 was confirmed by H2 being coupled to both H3α and H3β with J = 4.4 Hz indicating its equatorial position, H6 was coupled to H5β J = 9.1 Hz and H5α J = 5.0 Hz, indicating its axial position while H4 was coupled to H5β J = 9.3 Hz, H5α J = 4.5 Hz, H3β J = 9.0 Hz and H3α J = 4.0 Hz indicating its axial orientation. Additionally, H2 only had NOE correlations to H3α of 1.33% and to H3β of 1.89%, H4 had NOE correlations to H6 of 1.23%, to H3α of 1.58% and to H5α of 2.59% (Fig. 5). The synthesis was completed by MOM-protection of the free hydroxyl in 60% yield, and Wacker oxidation of the double bond to give 15 in 70% yield.19f The final step was the removal of the MOM protecting group, which was achieved by the action of aqueous HCl19f and generated diospongin B 2 in 58% yield. Spectroscopic data for our sample of diospongin B 2 were identical to those reported in the literature.19
1) with the correct relative stereochemical configuration for diospongin B. The stereochemical configuration of 14 was confirmed by H2 being coupled to both H3α and H3β with J = 4.4 Hz indicating its equatorial position, H6 was coupled to H5β J = 9.1 Hz and H5α J = 5.0 Hz, indicating its axial position while H4 was coupled to H5β J = 9.3 Hz, H5α J = 4.5 Hz, H3β J = 9.0 Hz and H3α J = 4.0 Hz indicating its axial orientation. Additionally, H2 only had NOE correlations to H3α of 1.33% and to H3β of 1.89%, H4 had NOE correlations to H6 of 1.23%, to H3α of 1.58% and to H5α of 2.59% (Fig. 5). The synthesis was completed by MOM-protection of the free hydroxyl in 60% yield, and Wacker oxidation of the double bond to give 15 in 70% yield.19f The final step was the removal of the MOM protecting group, which was achieved by the action of aqueous HCl19f and generated diospongin B 2 in 58% yield. Spectroscopic data for our sample of diospongin B 2 were identical to those reported in the literature.19
 |
| | Scheme 2 Synthesis of Diospongin B. | |
 |
| | Fig. 5 NOE correlations and coupling constants confirming the stereochemical configuration of 14. | |
Conclusions
We have developed a modification of the Maitland–Japp reaction using orthoamides which provides access to a range of 6-substituted-2H-dihydropyran-4-ones in good yields. These 2H-dihydropyran-4-ones can be converted into tetrahydropyran products with uncommon substitution patterns which are found in a number of biologically active natural products. 2,6-trans-Tetrahydropyran-4-ones are obtained by the stereoselective addition of Gilman cuprates to 6-substituted-2H-dihydropyran-4-ones. Tetrahydropyrans with the 3,6-substitution pattern are accessed by the conjugate addition of L-Selectride, while 3,3,6-substitution pattern are obtained by trapping the enolate formed on addition of L-Selectride with a carbon electrophile. The utility of these procedures was demonstrated by their use in the total synthesis of diospongin B, a natural product with potent anti-osteoperotic activity. This work provides a new route to uncommon tetrahydropyran substitution patterns and may ease the synthesis of a significant number of natural products containing these units.
Experimental
General methods
Thin layer chromatography was performed on aluminium plates coated with Merck silica gel 60 F254. The plates were developed using ultraviolet light, acidic aqueous ceric ammonium molybdate, basic aqueous potassium permanganate or ethanolic anisaldehyde. Flash column chromatography was performed with the solvent systems indicated in the appropriate experimental procedure. The stationary phase was silica gel 60 (220–240 mesh), unless stated otherwise. Dichloromethane was distilled from calcium hydride; THF and Et2O were distilled from sodium–benzophenone ketyl radical; toluene was dried over sodium wire; hexane was distilled prior to use. All other solvents and reagents were used as received from commercial suppliers. 1H NMRs were recorded at ambient temperature at either 400 MHz or 500 MHZ and 13C NMRs were recorded at ambient temperature at either 100 MHz or 125 MHz. Mass spectrometry was performed using ES ionisation.
General procedure for the synthesis of 6-substituted-2H-dihydropyran-4-ones 5
N,N-Dimethylformamide dimethyl acetal (0.03 mL, 0.20 mmol) was added to a stirred solution of δ-hydroxy-β-ketoester 7 (0.2 mmol) in dry dichloromethane (2 mL) at room temperature. After stirring at this temperature for 45 minutes, BF3·OEt2 (0.03 mL, 0.20 mmol) was added. The reaction was stirred at room temperature and monitored by TLC (hexane–ethyl acetate). Upon completion the mixture was diluted with EtOAc (40.0 mL) and washed with sat. aq. NaHCO3 (10.0 mL). The aqueous layer was extracted with EtOAc (15.0 mL) and the combined organic extracts were washed with brine (10.0 mL), dried over MgSO4 and concentrated in vacuo to give the crude DHP 5. No further purification was carried out on the products and they were used crude in all subsequent reactions.
Methyl 2-(furan-2-yl)-4-oxo-3,4-dihydro-2H-pyran-5-carboxylate 5a.
δ-Hydroxy-β-ketoester 7a (0.698 g, 3.292 mmol), yielded 0.674 g (92%), light yellow oil. ν max/cm−1 2953, 1738, 1704, 1579, 1436, 1383, 1296, 1133, 1013, 816, 732 cm−1; δH (400 MHz, CDCl3) 8.27 (1H, s), 7.44 (1H, dd, J = 1.8, 0.6 Hz), 6.45 (1H, d, J = 3.3 Hz), 6.36 (1H, dd, J = 3.3, 1.8 Hz), 5.58 (1H, dd, J = 11.5, 4.3 Hz), 3.74 (3H, s), 3.07 (1H, dd, J = 16.6, 11.5 Hz) and 2.79 (1H, dd, J = 16.6, 4.3 Hz) ppm; δC (100 MHz, CDCl3) 186.8, 170.5, 170.4, 163.8, 148.7, 143.9, 110.8, 110.6, 74.7, 51.9 and 39.3 ppm; m/z (ESI+) 245 (M + Na)+, 223 (M + H)+, (Found 245.0423 (M + Na)+. C11H10NaO5 requires; 245.0426).
Methyl 4-oxo-2-phenyl-3,4-dihydro-2H-pyran-5-carboxylate 5b.
δ-Hydroxy-β-ketoester 7b (0.050 g, 0.204 mmol), yielded 0.045 g (96%), orange solid. ν max/cm−1 2955, 1738, 1661, 1572, 1372, 1290, 1244, 1146, 1047, 845, 761, 698, 500 cm−1; δH (400 MHz, CDCl3) 8.43 (1H, s), 7.43–7.36 (5H, m), 5.54 (1H, dd, J = 12.0, 4.0 Hz), 3.81 (3H, s), 2.96 (1H, dd, J = 16.0, 4.0 Hz) and 2.76 (1H, d, J = 16.0, 4.0 Hz) ppm; δC (100 MHz, CDCl3) 186.8, 171.3, 164.2, 136.9, 129.4, 129.0, 126.2, 111.2, 82.3, 52.1 and 43.1 ppm; m/z (ESI+) 255 (M + Na)+, 233 (M + H)+, (Found 255.0631 (M + Na)+. C13H12NaO4 requires; 255.0633).
Methyl 4-oxo-2-propyl-3,4-dihydro-2H-pyran-5-carboxylate 5c.
δ-Hydroxy-β-ketoester 7c (0.052 g, 0.276 mmol), yielded 0.049 g (91%), light yellow oil. ν max/cm−1 2958, 2874, 1741, 1700, 1582, 1435, 1380, 1300, 1147, 1074, 799, 506 cm−1; δH (400 MHz, C6D6) 7.97 (1H, s), 3.71–3.64 (1H, m), 3.52 (3H, s), 1.97 (1H, dd, J = 16.2, 4.1 Hz), 1.89 (1H, dd, J = 16.2, 12.4 Hz), 1.24–0.98 (2H, m), 0.98–0.86 (2H, m) and 0.64 (3H, t, J = 7.2 Hz) ppm; δC (100 MHz, C6D6) 187.6, 171.5, 164.5, 116.9, 81.3, 51.9, 41.6, 36.0, 17.9 and 13.7 ppm; m/z (ESI+) 221 (M + Na)+, 199 (M + H)+, (Found 221.0782 (M + Na)+. C10H14NaO4 requires; 221.0790).
Methyl 2-isopropyl-4-oxo-3,4-dihydro-2H-pyran-5-carboxylate 5d.
δ-Hydroxy-β-ketoester 7d (0.750 g, 3.780 mmol), yielded 0.713 g (88%), light yellow oil. ν max/cm−1 2962, 1741, 1699, 1633, 1583, 1435, 1383, 1295, 1122, 1047, 771, 593 cm−1; δH (400 MHz, CDCl3) 8.33 (1H, s), 4.28–4.22 (1H, m), 3.76 (3H, s), 2.55 (1H, dd, J = 16.3, 13.6 Hz), 2.46 (1H, dd, J = 16.3, 3.8 Hz), 2.05–1.97 (1H, m), 0.97 (3H, d, J = 6.8 Hz) and 0.99 (3H, d, J = 6.8 Hz) ppm; δC (100 MHz, CDCl3) 187.8, 171.8, 164.2, 110.6, 85.7, 51.9, 39.0, 31.7, 17.8 and 17.6 pm; m/z (ESI+) 221 (M + Na)+, 199 (M + H)+, (Found 221.0786 (M + Na)+. C10H14NaO4 requires; 221.0790).
Methyl 4-oxo-2-(((triisopropylsilyl)oxy)methyl)-3,4-dihydro-2H-pyran-5-carboxylate 5e.
δ-Hydroxy-β-ketoester 7e (0.033 g, 0.099 mmol), yielded 0.029 g (88%), light orange oil. ν max/cm−1 2953, 1738, 1704, 1579, 1436, 1383, 1296, 1133, 1013, 816, 732 cm−1; δH (400 MHz, C6D6) 7.99 (1H, s), 3.81–3.75 (1H, m), 3.49 (3H, s), 3.29–3.25 (1H, m), 2.42 (1H, dd, J = 16.2, 13.0 Hz), 2.07 (1H, dd, J = 16.2, 3.8 Hz) and 1.10–9.94 (21H, m) ppm; δC (100 MHz, C6D6) 187.4, 171.4, 164.3, 110.6, 81.5, 64.2, 52.0, 38.2, 17.9 and 11.9 ppm; m/z (ESI+) 365 (M + Na)+ (Found 365.1741 (M + Na)+. C17H30NaO5Si requires; 365.1760).
Methyl 4-oxo-2-(prop-1-en-1-yl)-3,4-dihydro-2H-pyran-5-carboxylate 5f.
δ-Hydroxy-β-ketoester 7f (0.300 g, 1.612 mmol), yielded 0.274 g (87%), light orange oil. ν max/cm−1 2952, 2919, 1740, 1701, 1579, 1435, 1380, 1297, 1135, 1053, 965 cm−1; δH (400 MHz, CDCl3) 8.01 (1H, s), 5.28 (1H, m), 5.08 (1H, m), 4.20 (1H, ddd, J = 8.6, 7.0, 7.0 Hz), 3.51 (3H, s), 2.08 (2H, m) and 1.31 (3H, d, J = 6.5 d) ppm; δC (100 MHz, CDCl3) 181.2, 169.9, 164.3, 131.5, 127.0, 111.5, 80.9, 51.3, 41.8 and 17.5 ppm; m/z (ESI+) 219 (M + Na)+ (Found 219.0629 (M + Na)+. C10H12NaO4 requires; 219.0628).
Methyl 4-oxo-2-styryl-3,4-dihydro-2H-pyran-5-carboxylate 5g.
δ-Hydroxy-β-ketoester 7g (0.106 g, 0.427 mmol), yielded 0.107 g (97%), orange solid. ν max/cm−1 2951, 1693, 1569, 1436, 1369, 1260, 1295, 1060, 966, 747, 692 cm−1; δH (400 MHz, CDCl3) 8.04 (1H, s), 7.10–7.03 (5H, m), 6.19 (1H, dd, J = 16.0, 1.1 Hz), 5.73 (1H, dd, J = 16.0, 6.8 Hz), 4.26 (1H, ddd, J = 9.1, 6.9, 6.8 Hz), 3.53 (3H, s) and 2.12 (2H, m) ppm; δC (100 MHz, CDCl3) 193.2, 184.9, 169.9, 164.9, 135.6, 134.3, 128.9, 127.1, 124.2, 111.8, 81.0, 51.5 and 42.0 ppm; m/z (ESI+) 281 (M + Na)+ (Found 281.0781 (M + Na)+. C15H14NaO4 requires; 281.0784).
Methyl 2-(2-methyloxazol-4-yl)-4-oxo-3,4-dihydro-2H-pyran-5-carboxylate 5h.
δ-Hydroxy-β-ketoester 7h (0.078 g, 0.343 mmol), yielded 0.062 g (77%), light yellow oil. ν max/cm−1 2953, 1738, 1704, 1579, 1436, 1383, 1296, 1133, 1013, 816, 732 cm−1; δH (400 MHz, CDCl3) 8.34 (1H, s), 7.61 (1H, s), 5.55 (1H, dd, J = 12.0, 4.0 Hz), 3.80 (3H, s), 3.10 (1H, dd, J = 16.7, 12.0 Hz), 2.81 (1H, dd, J = 16.7, 4.0 Hz) and 2.48 (3H, s) ppm; δC (100 MHz, CDCl3) 198.7, 167.7, 160.1, 137.2, 63.0, 52.6, 49.6, 45.5, 31.5, 30.1 and 14.2 ppm; m/z (ESI+) 260 (M + Na)+ (Found 260.0523 (M + Na)+. C11H11NNaO5 requires; 260.0535).
General procedure for the synthesis 2,6-trans-tetrahydropyran-4-ones 8/9
Addition of Ph2CuLi.
Phenyl lithium 1.9 M in dibutyl ether solution (0.58 mL, 0.90 mmol) was added to a suspension of copper iodide (86.3 mg, 0.45 mmol) in THF (3.00 mL) at 0 °C. The mixture was stirred at this temperature for 20 minutes then cooled to −78 °C. Addition of chlorotrimethylsilane (0.18 mL, 1.4 mmol) was followed by addition of DHP (0.28 mmol) in THF (2.00 mL) at −78 °C. The reaction mixture was stirred at this temperature for 30 minutes then at 0 °C for 1.5 hours. The reaction was quenched with sat. aq. NH4Cl (2.50 mL) and allowed to warm to rt with vigorous stirring. The mixture was diluted with sat. aq. NH4Cl (10.0 mL) and extracted with EtOAc (5 × 15.0 mL). The combined organic extracts were washed with H2O (15.0 mL) and brine (15.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) afforded the product as a mixture of enol/keto tautomers 8/9.
(2R*,6S*)-Methyl 4-hydroxy-2,6-diphenyl-5,6-dihydro-2H-pyran-3-carboxylate 8a.
Dihydropyran 5b (0.088 g, 0.379 mmol), yielded 0.082 g (70%), light brown oil. ν max/cm−1 (film) 2955, 2931, 2872, 1768, 1723, 1710, 1435, 1363, 1254, 1177, 1149, 1055, 875, 480 cm−1; δH (400 MHz, CDCl3) 12.4 (1H, s), 7.41–7.24 (10H, m), 5.8 (1H, s), 4.56 (1H, dd, J = 10.8, 4.0 Hz), 3.66 (3H, s), 2.73 (1H, dd, J = 18.1, 10.8 Hz), 2.59 (1H, dd, J = 18.1, 4.0 Hz) ppm; δC (100 MHz, CDCl3) 193.0, 171.2, 140.7, 128.6, 128.3, 128.0, 127.9, 126.0, 98.6, 73.3, 68.4, 51.8, 41.1, 35.6, 31.1 ppm; m/z (ESI+) 333 (M + Na)+ (Found 333.1108 (M + Na)+. C19H18NaO4 requires; 333.1103).
(2R*,6R*)-Methyl 4-hydroxy-2-phenyl-6-propyl-5,6-dihydro-2H-pyran-3-carboxylate 8b.
Dihydropyran 5c (0.045 g, 0.227 mmol), yielded 0.035 g (56%), oil. ν max/cm−1 (film) 2955, 2927, 2875, 1658, 1621, 1441, 1288, 1262, 1216, 1043, 775, 698 cm−1; δH (400 MHz, CDCl3) 12.29 (1H, s), 7.37–7.27 (5H, m), 5.59 (1H, s), 3.63 (3H, s), 3.47–3.41 (1H, m), 2.32 (1H, dd, J = 18.0, 10.0 Hz), 2.23 (1H, dd, J = 18.0, 4.2 Hz), 1.05–1.43 (2H, m), 1.37–1.28 (2H, m) and 0.72 (3H, t, J = 7.2 Hz) ppm; δC (100 MHz, CDCl3) 171.7, 171.2, 141.1, 128.5, 128.1, 127.8, 98.6, 72.2, 66.3, 51.7, 37.8, 35.0, 18.3 and 13.8 ppm; m/z (ESI+) 299 (M + Na)+ (Found 299.1255 (M + Na)+. C16H20NaO4 requires; 299.1254).
(2R*,6S*)-Methyl 4-hydroxy-6-isopropyl-2-phenyl-5,6-dihydro-2H-pyran-3-carboxylate 8c.
Dihydropyran 5d (0.060 g, 0.303 mmol), yielded 0.060 g (73%), light yellow oil. ν max/cm−1 (film) 2924, 2852, 1946, 1739, 1661, 1365, 1268, 1222, 1060, 841 cm−1; δH (400 MHz, CDCl3) 12.30 (1H, s), 7.37–7.27 (5H, m), 5.62 (1H, s), 3.63 (3H, s), 3.10 (1H, dd, J = 10.8, 3.9 Hz), 2.36 (1H, dd, J = 18.0, 10.8 Hz), 2.23 (1H, dd, J = 18.0, 3.9 Hz), 1.64–1.56 (1H, m), 0.80 (3H, d, J = 6.8 Hz) and 0.77 (3H, d, J = 6.8 Hz) ppm; δC (100 MHz, CDCl3) 193.2, 141.5, 128.6, 128.0, 127.7, 98.5, 72.6, 71.6, 51.2, 41.3, 32.8, 32.3, 18.4 and 17.8 ppm; m/z (ESI+) 299 (M + Na)+ (Found 299.1245 (M + Na)+. C16H20NaO4 requires; 299.1254).
(2R*,6S*)-Methyl 4-hydroxy-2-phenyl-6-(((triisopropylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-3-carboxylate 8d.
Dihydropyran 5e (0.073 g, 0.213 mmol), yielded 0.043 g (48%), light yellow oil. ν max/cm−1 (film) 2941, 2861, 1660, 1623, 1442, 1280, 1264, 1215, 1095, 880, 681 cm−1; δH (400 MHz, C6D6) 12.90 (1H, s), 7.38–7.36 (2H, m), 7.18–7.08 (3H, m) 5.79 (1H, s), 3.65–3.60 (1H, m), 3.55–3.46 (2H, m), 3.05 (3H, s), 2.59 (1H, dd, J = 18.1, 10.8 Hz), 2.18 (1H, dd, J = 18.1, 2.8 Hz) and 1.01 (21H, m) ppm; δC (100 MHz, C6D6) 172.3, 171.3, 141.5, 128.9, 128.4, 127.9, 99.0, 73.1, 67.9, 66.3, 51.0, 31.4, 81.1 and 12.2 ppm; m/z (ESI+) 443 (M + Na)+ (Found 443.2209 (M + Na)+. C23H36NaO5Si requires; 443.2224).
(2R*,6S*)-Methyl 4-hydroxy-2-phenyl-6-((E)-prop-1-en-1-yl)-5,6-dihydro-2H-pyran-3-carboxylate 8e.
Dihydropyran 5f (0.055 g, 0.280 mmol), yielded 0.049 g (64%), light yellow oil. ν max/cm−1 (film) 3028, 2952, 2896, 1662, 1618, 1437, 1325, 1243, 1205, 1181, 1051, 958, 740, 690 cm−1; δH (400 MHz, CDCl3) 12.30 (1H, s), 7.40–7.27 (5H, m), 5.62 (1H, s), 5.56 (1H, d, J = 16.0, 4.0 Hz), 5.44 (1H, dd, J = 16.0, 4.0 Hz), 3.97 (1H, ddd, J = 12.0, 4.0, 4.0 Hz), 3.62 (3H, s), 2.47 (1H, dd, J = 16.0, 12.0 Hz), 2.32 (1H, dd, J = 16.0, 4.0 Hz) and 1.65 (1H, d, J = 4.0 Hz) ppm; δC (100 MHz, CDCl3) 171.1, 140.9, 130.4, 128.6, 128.5, 128.2, 127.9, 127.1, 98.6, 73.0, 67.1, 51.6, 34.4 and 17.9 ppm; m/z (ESI+) 297 (M + Na)+ (Found 297.1091 (M + Na)+. C16H18NaO4 requires; 297.1097).
(2R*,6S*)-Methyl 4-hydroxy-2-phenyl-6-((E)-styryl)-5,6-dihydro-2H-pyran-3-carboxylate 8f.
Dihydropyran 5g (0.988 g, 3.829 mmol), yielded 1.170 g (91%), light yellow solid. ν max/cm−1 (film) 3028, 2952, 2896, 1662, 1618, 1437, 1325, 1243, 1205, 1181, 1051, 958, 740, 690 cm−1; δH (400 MHz, CDCl3) 12.32 (1H, s), 7.46–7.20 (10H, m), 6.49 (1H, d, J = 16.1 Hz), 6.15 (1H, dd, J = 16.1, 5.7 Hz), 5.70 (1H, s), 4.24 (1H, ddd, J = 10.5, 5.7, 4.1 Hz), 3.64 (3H, s), 2.58 (1H, dd, J = 18.0, 10.5 Hz) and 2.46 (1H, dd, J = 18.0, 4.1 Hz) ppm; δC (100 MHz, CDCl3) 171.1, 170.9, 140.8, 136.4, 131.4, 128.6, 128.5, 128.3, 128.0, 127.9, 126.6, 115.4, 98.3, 73.0, 67.4, 51.8 and 34.5 ppm; m/z (ESI+) 359 (M + Na)+ (Found 359.1249 (M + Na)+. C21H20NaO4 requires; 359.1254).
Addition of Me2CuLi.
Methyl lithium 1.6 M in Et2O (0.56 ml, 0.72 mmol) was added to a suspension of copper iodide (0.069 g, 0.36 mmol) in THF (2.00 mL) at 0 °C. The mixture was stirred at this temperature for 20 minutes after which chlorotrimethylsilane (0.14 ml, 0.54 mmol) then DHP 5 (0.10 mmol) in THF (2.00 mL) were added at −78 °C. The reaction mixture was stirred at this temperature for 4.5 hours then sat. aq. NH4Cl (1.70 mL) was added to the mixture, which was stirred rapidly for 30 minutes at rt. The mixture was diluted with H2O (10.0 mL) extracted with Et2O (2 × 20.0 mL) and washed with H2O (20.0 mL) and brine (20.0 mL). The organic layer was dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded the products as an inseparable mixture of enol and ketone tautomers 8/9 which were then subjected to acylation. The THP mixture 8/9 (0.03 mmol), acetic anhydride (0.10 mL, 0.10 mmol) and DMAP (2 mg) were stirred in pyridine (0.47 mL) at 40 °C for 40 minutes. The mixture was cooled to rt, concentrated in vacuo and partitioned between Et2O (30.0 mL) and H2O (10.0 mL). The organic layer was washed with H2O (10.0 mL) and brine (10.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) gave products 10.
1) afforded the products as an inseparable mixture of enol and ketone tautomers 8/9 which were then subjected to acylation. The THP mixture 8/9 (0.03 mmol), acetic anhydride (0.10 mL, 0.10 mmol) and DMAP (2 mg) were stirred in pyridine (0.47 mL) at 40 °C for 40 minutes. The mixture was cooled to rt, concentrated in vacuo and partitioned between Et2O (30.0 mL) and H2O (10.0 mL). The organic layer was washed with H2O (10.0 mL) and brine (10.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) gave products 10.
(2R*,6S*)-Methyl 4-acetoxy-6-(furan-2-yl)-2-methyl-5,6-dihydro-2H-pyran-3-carboxylate 10g.
Dihydropyran 5a (0.028 g, 0.126 mmol), yielded 0.015 g (42% after 2 steps), light yellow oil. ν max/cm−1 (film) 2955, 2924, 2854, 1766, 1720, 1435, 1364, 1253, 1176, 1144, 1052, 742, 598 cm−1; δH (400 MHz, CDCl3) 7.41 (1H, s), 6.37–6.35 (2H, m), 5.04 (1H, dd, J = 9.2, 4.2 Hz), 4.85 (1H, q, J = 6.5 Hz), 3.74 (3H, s), 2.79 (1H, dd, J = 17.9, 9.2 Hz), 2.53 (1H, dd, J = 17.9, 4.2 Hz,), 2.22 (3H, s) and 1.48 (3H, d, J = 6.5 Hz) ppm; δC (100 MHz, CDCl3) 168.5, 164.0, 157.5, 152.6, 142.9, 121.6, 110.4, 108.0, 69.0, 63.4, 51.9, 32.6, 21.1 and 19.3 ppm; m/z (ESI+) 303 (M + Na)+ (Found 303.0827 (M + Na)+. C14H16NaO6 requires; 303.0845).
(2R*,6S*)-Methyl 4-acetoxy-2-methyl-6-phenyl-5,6-dihydro-2H-pyran-3-carboxylate 10h.
Dihydropyran 5b (0.254 g, 1.094 mmol), yielded 0.149 g (47% after 2 steps), light yellow oil. ν max/cm−1 (film) 2945, 2931, 1766, 1720, 1708, 1664, 1365, 1247, 1174, 1053, 758, 698 cm−1; δH (400 MHz, C6D6) 7.30–7.23 (2H, m), 7.17–7.16 (1H, m), 7.14–7.05 (2H, m), 5.13 (1H, q, J = 6.6 Hz), 4.83 (1H, dd, J = 9.7, 4.1 Hz), 3.26 (3H, s), 2.40 (1H, dd, J = 17.9, 9.7 Hz), 2.31 (1H, dd, J = 17.9, 4.1 Hz), 1.88 (3H, s) and 1.39 (3H, d, J = 6.6 Hz) ppm; NOE C2(Me) – H6 2.3% and C2(Me) – H5α 1.86%; δC (100 MHz, C6D6) 193.5, 167.8, 163.9, 154.1, 141.0, 128.6, 126.7, 121.7, 69.5, 68.8, 51.1, 36.9, 20.5 and 19.5 ppm; m/z (ESI+) 313 (M + Na)+ (Found 313.1040 (M + Na)+. C16H18NaO5 requires; 313.1052).
(2R*,6R*)-Methyl 4-acetoxy-2-methyl-6-propyl-5,6-dihydro-2H-pyran-3-carboxylate 10i.
Dihydropyran 5c (0.030 g, 0.151 mmol), yielded 0.015 g (40% after 2 steps), light yellow oil. ν max/cm−1 (film) 2955, 2931, 2872, 1768, 1723, 1710, 1435, 1363, 1254, 1177, 1149, 1055, 875, 480 cm−1; δH (400 MHz, C6D6) 5.02 (1H, q, J = 6.5 Hz), 3.73–3.67 (1H, m), 3.25 (3H, s), 2.09–2.02 (2H, m), 1.90 (3H, s), 1.50–1.39 (2H, m), 1.35 (3H, d, J = 6.5 Hz), 1.31–1.12 (2H, m) and 0.82 (3H, t, J = 7.2 Hz) ppm; δC (100 MHz, C6D6) 193.3, 168.0, 163.9, 154.4, 121.8, 68.9, 66.4, 51.0, 37.6, 35.4, 20.7, 18.8 and 14.0 ppm; m/z (ESI+) 279 (M + Na)+ (Found 279.1202 (M + Na)+. C13H20NaO5 requires; 279.1208).
(2R*,6S*)-Methyl 4-acetoxy-6-isopropyl-2-methyl-5,6-dihydro-2H-pyran-3-carboxylate 10j.
Dihydropyran 5d (0.713 g, 3.330 mmol), yielded 0.660 g (67% after 2 steps), light yellow oil. ν max/cm−1 (film) 2959, 2927, 2875, 1768, 1723, 1710, 1435, 1367, 1249, 1177, 1142, 1058, 875, 783 cm−1; δH (400 MHz, C6D6) 5.00 (1H, q, J = 6.5 Hz), 3.45–3.40 (1H, m), 3.24 (3H, s), 2.17 (1H, dd, J = 17.7, 10.1 Hz), 2.02 (1H, dd, J = 17.7, 3.4 Hz), 1.92 (3H, s), 1.60–1.53 (1H, m), 1.34 (3H, d, J = 6.5 Hz), 0.93 (3H, d, J = 6.5 Hz) and 0.73 (3H, d, J = 6.5 Hz) ppm; δC (100 MHz, C6D6) 193.5, 168.3, 164.1, 155.0, 121.8, 71.4, 69.0, 50.8, 33.0, 20.6, 19.3, 18.4 and 18.0 ppm; m/z (ESI+) 279 (M + Na)+ (Found 279.1199 (M + Na)+. C13H20NaO5 requires; 279.1208).
(2R*,6S*)-Methyl 4-acetoxy-2-methyl-6-(((triisopropylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-3-carboxylate 10k.
Dihydropyran 5e (0.057 g, 0.166 mmol), yielded 0.040 g (61% after 2 steps), light yellow oil. ν max/cm−1 (film) 2941, 2866, 1771, 1725, 1712, 1365, 1246, 1177, 1149, 1055, 880, 681 cm−1; δH (400 MHz, C6D6) 5.04 (1H, q, J = 6.5 Hz), 4.03–3.97 (1H, m), 3.76 (1H, dd, J = 10.2, 5.1 Hz), 3.63 (1H, dd, J = 10.2, 5.2 Hz), 3.23 (3H, s), 2.44 (1H, dd, J = 17.8, 10.0 Hz), 2.25 (1H, dd, J = 17.8, 3.8 Hz), 1.87 (3H, s), 1.39 (3H, d, J = 6.5 Hz) and 1.09 (21H, m) ppm; δC (100 MHz, C6D6) 168.0, 163.8, 154.4, 121.8, 69.1, 68.1, 66.4, 51.1, 32.0, 20.6, 19.4, 18.3 and 12.2 ppm; m/z (ESI+) 423 (M + Na)+ (Found 423.2160 (M + Na)+. C20H36NaO6Si requires; 423.2179).
(2R*,6S*)-Methyl 4-acetoxy-2-methyl-6-((E)-prop-1-en-1-yl)-5,6-dihydro-2H-pyran-3-carboxylate 10l.
Dihydropyran 5f (0.050 g, 0.255 mmol), yielded 0.019 g (29% after 2 steps), light yellow oil. ν max/cm−1 (film) 2952, 2930, 1767, 1721, 1664, 1436, 1366, 1245, 1212, 1176, 1046, 1050, 985 cm−1; δH (400 MHz, CDCl3) 5.78 (1H, dd, J = 16.0, 4.0 Hz), 5.52 (1H, dq, J = 16.0, 4.0 Hz), 4.85 (1H, q, J = 8.0 Hz), 4.37 (1H, ddd, J = 8.0, 4.0, 4.0 Hz), 3.72 (3H, s), 2.30 (2H, m), 2.20 (3H, s), 1.71 (2H, d, J = 4.0 Hz) and 1.42 (3H, d, J = 8.0 Hz) ppm; δC (100 MHz, CDCl3) 168.7, 164.1, 153.3, 130.2, 129.2, 121.3, 68.8, 67.9, 51.9, 34.8, 21.0, 19.6 and 17.9 ppm; m/z (ESI+) 277 (M + Na)+ (Found 277.1047 (M + Na)+. C13H18NaO5 requires; 277.1046).
Addition of n-Bu2CuLi.
n-Butyl lithium 2.5 M in hexane solution (0.26 mL, 0.6 mmol) was added to a suspension of copper iodide (57.2 mg, 0.3 mmol) in THF (1.70 mL) at 0 °C. The mixture was stirred at this temperature for 20 minutes. After this time the mixture was cooled to −78 °C and chlorotrimethylsilane (0.12 mL, 0.9 mmol) was added, followed by addition of DHP 5 (0.2 mmol) in THF (1.80 mL) at −78 °C. The reaction mixture was stirred at −78 °C for 4 hours then quenched with sat. aq. NH4Cl (1.50 mL) and allowed to warm to rt with vigorous stirring. The mixture was diluted further with sat. aq. NH4Cl (10.0 mL) and extracted with EtOAc (5 × 15.0 mL). The combined organic extracts were washed with H2O (15.0 mL) and brine (15.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) afforded the products as an inseparable mixture of enol and ketone tautomers 8/9, which were then subjected to acylation. The THP mixture 8/9 (0.03 mmol), acetic anhydride (0.10 mL, 0.10 mmol) and DMAP (2 mg) were stirred in pyridine (0.47 mL) at 40 °C for 40 minutes. The mixture was cooled to rt, concentrated in vacuo and partitioned between Et2O (30.0 mL) and H2O (10.0 mL). The organic layer was washed with H2O (10.0 mL) and brine (10.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) gave products 10.
(2R*,6S*)-Methyl 4-acetoxy-2-butyl-6-phenyl-5,6-dihydro-2H-pyran-3-carboxylate 10m.
Dihydropyran 5g (0.100 g, 0.431 mmol), yielded 0.056 g (39% after 2 steps), light yellow oil. ν max/cm−1 (film) 2953, 2860, 1764, 1721, 1248, 1174, 1055, 698 cm−1; δH (400 MHz, CDCl3) 7.40–7.29 (5H, m), 4.92 (1H, dd, J = 9.0, 5.0 Hz), 4.79 (1H, d, J = 10.1 Hz), 3.75 (3H, s), 2.69–2.47 (2H, m), 2.21 (3H, s), 1.88–1.24 (6H, m), 0.89 (3H, t, J = 7.3 Hz) ppm; δC (100 MHz, CDCl3) 168.9, 164.1, 153.2, 141.0, 128.6, 128.0, 126.0, 121.2, 73.2, 68.4, 51.8, 36.0, 32.2, 28.3, 22.4, 21.0 and 14.0 ppm; m/z (ESI+) 355 (M + Na)+ (Found 355.1509 (M + Na)+. C19H24NaO5 requires; 355.1516).
(2R*,6R*)-Methyl 4-acetoxy-2-butyl-6-propyl-5,6-dihydro-2H-pyran-3-carboxylate 10n.
Dihydropyran 5c (0.070 g, 0.353 mmol), yielded 0.037 g (35% after 2 steps), light yellow oil. ν max/cm−1 (film) 2956, 2932, 2872, 1767, 1722, 1241, 1177, 1053, 900 cm−1; δH (400 MHz, CDCl3) 4.61 (1H, d, J = 10.3 Hz), 2.84 (1H, m), 3.71 (3H, s), 2.22–2.16 (2H, m), 2.18 (3H, s), 1.74–1.25 (10H, m), 0.95–0.88 (6H, m) ppm; δC (100 MHz, CDCl3) 168.6, 164.3, 153.9, 121.1, 77.3, 72.5, 66.2, 51.8, 37.7, 35.0, 32.0, 28.2, 22.3, 21.0, 18.8 and 14.1 ppm; m/z (ESI+) 321 (M + Na)+ (Found 321.1681 (M + Na)+. C16H26NaO5 requires; 321.1672).
(2R*,6S*)-Methyl 4-acetoxy-2-butyl-6-isopropyl-5,6-dihydro-2H-pyran-3-carboxylate 10o.
Dihydropyran 5d (0.037 g, 0.186 mmol) yielded 0.020 g (37% after 2 steps), light yellow oil. ν max/cm−1 (film) 2955, 2929, 1767, 1724, 1712, 1435, 1365, 1249, 1202, 1177, 1056, 490 cm−1; δH (400 MHz, C6D6) 4.89 (1H, d, J = 10.0 Hz), 3.43–3.38 (1H, m), 3.28 (3H, s), 2.15 (1H, dd, J = 17.7, 9.6 Hz), 2.07 (1H, dd, J = 17.7, 4.3 Hz), 1.92 (3H, s), 1.74–1.53 (2H, m), 1.48–1.39 (1H, m), 1.37–1.24 (4H, m), 0.96 (3H, d, J = 6.7 Hz), 0.88 (3H, t, J = 7.3 Hz) and 0.74 (3H, d, J = 6.8 Hz) ppm; δC (100 MHz, C6D6) 167.9, 164.0, 154.4, 121.4, 72.8, 71.4, 51.0, 33.0, 32.9, 32.2, 28.7, 22.6, 20.6, 18.6, 18.3 and 14.2 ppm; m/z (ESI+) 321 (M + Na)+ (Found 321.1669 (M + Na)+. C16H26NaO5 requires; 321.1672).
(2R*,6S*)-Methyl 4-acetoxy-2-butyl-6-(((triisopropylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-3-carboxylate 10p.
Dihydropyran 5e (0.056 g, 0.163 mmol), yielded 0.012 g (16% after 2 steps), light yellow oil. ν max/cm−1 (film) 2941, 2865, 1770, 1725, 1712, 1364, 1247, 1192, 1177, 1146, 1094, 1052, 881, 786, 681, 659 cm−1; δH (400 MHz, C6D6) 4.89 (1H, d, J = 9.6 Hz), 3.99–3.93 (1H, m), 3.75 (1H, dd, J = 10.3, 5.6 Hz), 3.61 (1H, dd, J = 10.3, 4.8 Hz), 3.27 (3H, s), 2.35 (1H, dd, J = 17.9, 10.0 Hz), 2.21 (1H, dd, J = 17.9, 3.9 Hz), 1.87 (3H, s), 1.73–1.59 (2H, m), 1.49–1.39 (2H, m), 1.38–1.23 (2H, m), 1.10 (21H, m) and 0.89 (3H, t, J = 7.3 Hz) ppm; δC (100 MHz, C6D6) 167.9, 163.9, 154.0, 121.5, 72.8, 67.9, 66.5, 51.0, 32.4, 31.8, 28.5, 22.6, 20.6, 18.2, 14.2 and 12.2 ppm; m/z (ESI+) 465 (M + Na)+ (Found 465.2625 (M + Na)+. C23H42NaO6Si requires; 465.2643).
General procedure for the synthesis of 3,6-disubstituted-tetrahydropyran-4-ones 11
A 1.0 M solution of L-Selectride in THF (0.04 mL, 0.04 mmol) was added to a stirred solution of DHP (0.04 mmol) in THF (1.00 mL) at −78 °C. The mixture was stirred for 1 hour at −78 °C then diluted with Et2O (10.0 mL) and quenched with sat. aq. NHCl4 (10.0 mL). The layers were separated and the aqueous layer was extracted with Et2O (10.0 mL). The combined organic extracts were washed with brine (20.0 mL), dried over MgSO4 and concentrated in vacuo. Purification by flash column chromatography (hexane–ethyl acetate) afforded the product as a mixture of enol/keto tautomers.
Methyl 6-(furan-2-yl)-4-hydroxy-5,6-dihydro-2H-pyran-3-carboxylate 11a.
Dihydropyran 5a (0.098 g, 0.439 mmol), yielded 0.043 g (44%) oil. ν max/cm−1 (film) 2954, 2927, 2868, 1737, 1667, 1627, 1442, 1275, 1066, 1011, 739, 598 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.81 (OH, s), 7.42 (1H, m), 7.38 (1H, m, keto) 6.37–6.33 (2H, m), 6.28–6.27 (2H, m, keto), 5.08 (1H, dd, J = 11.6, 2.9 Hz, keto), 4.88 (1H, dd, J = 10.0, 3.6 Hz, keto), 4.73 (1H, dd, J = 9.9, 3.9 Hz), 4.50 (2H, m), 4.44 (1H, d, J = 14.0), 4.37 (1H, d, J = 14.0 Hz), 3.77 (3H, s), 3.70 (3H, s, keto), 2.86 (1H, dd, J = 17.8, 9.9 Hz), 2.80 (1H, m, keto), 2.53 (1H, dd, J = 17.8, 3.8 Hz), 2.08 (1H, m, keto) ppm; δC (100 MHz, CDCl3): 170.4, 168.2, 152.7, 143.0, 110.4, 107.9, 106.2 (keto), 97.1, 72.9 (keto), 68.9, 67.7 (keto), 62.9, 51.6, 31.9 and 26.1 (keto) ppm; m/z (ESI+) 247 (M + Na)+. (Found 247.0575 (M + Na)+. C11H12NaO5 requires 247.0577).
Methyl 4-hydroxy-6-phenyl-5,6-dihydro-2H-pyran-3-carboxylate 11b.
Dihydropyran 5b (0.100 g, 0.427 mmol), yielded 0.074 g (74%). oil. ν max/cm−1 (film) 2952, 2922, 2852, 1664, 1622, 1445, 1269, 1209, 1066, 1027, 699 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.29 (OH, s), 7.43–7.29 (5H, m), 4.70 (1H, m, keto) 4.67 (1H, dd, J = 10.48, 3.71 Hz), 4.59 (1H, m, keto), 4.55–4.36 (2H, m, keto), 4.44 (1H, d, J = 14.0), 4.39 (1H, d, J = 14.0 Hz), 3.81 (3H, s), 3.80 (3H, s, keto), 3.02–2.68 (2H, m, keto) and 2.67–2.49 (2H, m) ppm; δC (100 MHz, CDCl3): 170.5, 168.8, 168.0 (keto), 140.8, 140.0 (keto), 128.9 (keto), 128.7, 128.5 (keto), 128.1, 125.9, 125.7 (keto), 97.2, 80.4 (keto), 75.6, 68.3 (keto), 63.5, 57.3 (keto), 53.0 (keto), 51.6, 49.5 (keto), 48.7 (keto) and 35.8 ppm; m/z (ESI+) 257 (M + Na)+. (Found 257.0782 (M + Na)+. C13H14NaO4 requires 257.0784).
Methyl 4-hydroxy-6-propyl-5,6-dihydro-2H-pyran-3-carboxylate 11c.
Dihydropyran 5c (0.100 g, 0.505 mmol), yielded 0.089 g, (89%). oil. ν max/cm−1 (film) 2957, 2871, 1666, 1626, 1441, 1213, 1074, 807 cm−1; 1H NMR (400 MHz, C6D6): δ 12.09 (OH, s), 4.50 (1H, d, J = 13.7), 4.40 (1H, m, keto), 4.11 (1H, d, J = 13.7 Hz), 4.05 (1H, m, keto), 3.22 (3H, s), 3.21 (3H, s, keto), 3.20 (1H, m), 3.05 (1H, m, keto), 2.12 (2H, m, keto), 2.08 (1H, m), 1.94 (1H, m), 1.47–0.96 (4H, m) and 0.79 (3H, t, J = 7.2 Hz) ppm; δC (100 MHz, C6D6): 200.8 (keto), 170.8, 170.0, 168.0 (keto), 97.5, 78.2 (keto), 73.5, 68.1 (keto), 63.2, 57.5 (keto), 51.6 (keto), 50.9, 47.7 (keto), 38.3 (keto), 37.9, 34.6, 18.7, 18.5 (keto), 14.1 and 14.0 (keto) ppm; m/z (ESI+) 223 (M + Na)+. (Found 223.0938 (M + Na)+. C10H16NaO4 requires 223.0941).
Methyl 4-hydroxy-6-(((triisopropylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-3-carboxylate 11e.
Dihydropyran 5e (0.095 g, 0.277 mmol), yielded 0.062 g, (65%) oil. ν max/cm−1 (film) 2924, 2865, 1774, 1729, 1709, 1148, 1057, 881, 787, 681 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.78 (OH, s), 4.69–4.66 (2H, m, keto), 4.43 (1H, d, J = 13.9), 4.22 (1H, d, J = 13.9 Hz), 3.93 (1H, m, keto), 3.88 (1H, m), 3.83–3.79 (2H, m), 3.76 (3H, s, keto), 3.75 (3H, s), 3.62–3.59 (2H, m, keto), 2.82 (1H, m, keto), 2.61–2.28 (2H, m), 2.31 (1H, m, keto) and 1.06–1.05 (21H, m) ppm; δC (100 MHz, CDCl3): 170.5, 169.5, 97.1, 79.2 (keto), 74.6, 68.2 (keto), 66.0, 63.2, 57.3 (keto), 52.4 (keto), 51.5, 44.2 (keto), 31.3, 18.8, 12.0 ppm; m/z (ESI+) 367 (M + Na)+. (Found 367.1905 (M + Na)+. C17H32NaO5Si requires 367.1911).
(E)-Methyl 4-hydroxy-6-styryl-5,6-dihydro-2H-pyran-3-carboxylate 11g.
Dihydropyran 5g (0.100 g, 0.387 mmol), yielded 0.051 g, (51%) oil. ν max/cm−1 (film) 2953, 2839, 1665, 1626, 1441, 1263, 1211, 1059, 965, 795, 746, 692 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.78 (OH, s), 7.41–7.23 (5H, m), 6.65 (1H, d, J = 16.0 Hz), 6.58 (1H, m, keto), 6.23 (1H, dd, J = 16.0, 6.0 Hz), 6.19 (1H, m, keto), 4.48 (1H, d, J = 13.9), 4.47 (1H, m, keto), 4.32 (1H, d, J = 13.9 Hz), 4.28 (1H, m), 4.26 (1H, m, keto), 3.79 (3H, s, keto), 3.77 (3H, s), 2.84 (1H, m, keto), 2.67 (1H, m, keto) and 2.69–2.38 (2H, m) ppm; δC (100 MHz, CDCl3): 170.5, 168.5, 136.4, 136.0 (keto), 132.3 (keto), 131.8, 128.7, 128.2, 128.1, 126.8 (keto), 126.7, 97.2, 74.0, 68.0 (keto), 62.9, 52.4 (keto), 51.6, 47.7 (keto) and 34.3 ppm; m/z (ESI+) 283 (M + Na)+. (Found 283.0934 (M + Na)+. C15H16NaO4 requires 283.0941).
General procedure for the acylation of 3,6-disubstituted-tetrahydropyran-4-ones 11, formation of enol acetates 12
The THP keto/enol 11 mixture (0.03 mmol), acetic anhydride (0.10 mL, 0.1 mmol) and DMAP (2 mg.) were stirred in pyridine (0.47 mL) at 40 °C for 40 minutes. The mixture was cooled to room temperature, concentrated in vacuo and partitioned between Et2O (30.0 mL) and H2O (10.0 mL). The organic layer was washed with H2O (10.0 mL) and brine (10.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) gave the product.
Methyl 4-acetoxy-6-(furan-2-yl)-5,6-dihydro-2H-pyran-3-carboxylate 12a.
Tetrahydropyran-4-one 11a (0.043 g, 0.191 mmol), yielded 0.029 g, (58%) oil. ν max/cm−1 (film) 2953, 2847, 1760, 1723, 1706, 1671, 1365, 1253, 1173, 1132, 1059, 1009, 743 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.41 (1H, s), 6.41 (1H, m), 6.35 (1H, m), 4.80 (1H, dd, J = 9.0, 4.1 Hz), 4.51–4.48 (2H, m), 3.72 (3H, s) 2.89 (1H, m), 2.55 (1H, m) and 2.52 (3H, s) ppm; δC (100 MHz, CDCl3): 168.4, 163.1, 153.5, 152.2, 143.0, 116.5, 110.4, 108.3, 68.7, 64.0, 51.8, 32.4 and 21.0 ppm; m/z (ESI+) 289 (M + Na)+. (Found 289.0692 (M + Na)+. C13H14NaO6 requires 289.0683).
Methyl 4-acetoxy-6-phenyl-5,6-dihydro-2H-pyran-3-carboxylate 12b.
Tetrahydropyran-4-one 11b (0.074 g, 0.316 mmol), yielded 0.059 g (68%) solid white. ν max/cm−1 (film) 2949, 2842, 1765, 1718, 1664, 1166, 1131, 1060, 743, 700 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.38–7.28 (5H, m), 6.69 (1H, d, J = 16.0 Hz), 4.67 (1H, m), 4.49 (1H, d, J = 16.0, 4.0 Hz), 3.74 (3H, s), 2.63 (1H, m), 2.47 (1H, m) and 2.24 (3H, s) ppm; δC (100 MHz, CDCl3): 168.4, 163.3, 154.1, 140.4, 128.7, 128.2, 125.9, 116.5, 75.5, 64.9, 51.7, 36.5 and 21.0 ppm; m/z (ESI+) 299 (M + Na)+. (Found 299.0894 (M + Na)+. C15H16NaO5 requires 299.0890).
Methyl 4-acetoxy-6-propyl-5,6-dihydro-2H-pyran-3-carboxylate 12c.
Tetrahydropyran-4-one 11c (0.090 g, 0.450 mmol), yielded 0.056 g (51%) oil. ν max/cm−1 (film) 2957, 2872, 1768, 1726, 1250, 1212, 1177, 1055 cm−1; 1H NMR (400 MHz, CDCl3): 4.53 (1H, dd, J = 15.0 Hz), 4.31 (1H, dd, J = 15.0 Hz), 3.70 (3H, s), 3.60 (1H, m), 2.30–2.14 (2H, m), 2.23 (3H, s) 1.64–1.50 (5H, m) and 0.93 (3H, t, J = 7.2 Hz) ppm; δC (100 MHz, CDCl3): 168.4, 163.5, 154.5, 116.3, 73.5, 64.6, 51.7, 37.4, 35.0, 21.0, 18.5 and 14.1 ppm; m/z (ESI+) 265 (M + Na)+. (Found 265.1052 (M + Na)+. C12H18NaO5 requires 265.1046).
Methyl 4-acetoxy-6-(((triisopropylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-3-carboxylate 12e.
Tetrahydropyran-4-one 11e (0.040 g, 0.116 mmol), yielded 0.029 g (65%) oil. ν max/cm−1 (film) 2924, 2865, 1774, 1729, 1709, 1148, 1057, 881, 787, 681 cm−1; 1H NMR (400 MHz, C6D6): 4.70 (1H, dd, J = 15.5, 2.0 Hz), 4.33 (1H, dd, J = 15.5, 3.4 Hz), 3.82 (1H, dd, J = 10.0, 5.0 Hz), 3.70 (1H, dd, J = 10.0, 5.0 Hz), 3.65 (1H, m), 3.30 (3H, s), 2.59 (1H, m), 2.27 (1H, m), 2.02 (3H, s) and 1.18–1.17 (21H, m) ppm; δC (100 MHz, C6D6): 167.7, 163.0, 154.9, 116.7, 74.7, 66.1, 64.8, 50.8, 32.0, 20.6, 18.1 and 12.2 ppm; m/z (ESI+) 409 (M + Na)+. (Found 409.2022 (M + Na)+. C19H34NaO6Si requires 409.2017).
(E)-Methyl 4-acetoxy-6-styryl-5,6-dihydro-2H-pyran-3-carboxylate 12g.
Tetrahydropyran-4-one 11g (0.015 g, 0.057 mmol), yielded 0.010 g (56%) oil. ν max/cm−1 (film) 3026, 2951, 2844, 1761, 1723, 1705, 1248, 1172, 1142, 1051, 748, 693 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.40–7.23 (5H, m), 6.68 (1H, d, J = 16.0 Hz), 6.23 (1H, dd, J = 16.0, 6.1 4.1 Hz), 4.63 (1H, d, J = 15.0 Hz), 4.43 (1H, d, J = 15.0 Hz), 4.33 (1H, m), 3.72 (3H, s) 2.52 (1H, m), 2.37 (1H, m) and 2.24 (3H, s) ppm; δC (100 MHz, CDCl3): 168.4, 163.3, 153.8, 136.3, 132.1, 128.7, 128.1, 127.6, 126.7, 116.5, 73.9, 64.3, 51.8, 34.9 and 21.0 ppm; m/z (ESI+) 325 (M + Na)+. (Found 325.1053 (M + Na)+. C17H18NaO5 requires 325.1046).
General procedure for the synthesis of 3,3,6-trisubstituted tetrahydropyran-4-ones 13
A 1.0 M solution of L-Selectride in THF (0.04 mL, 0.04 mmol) was added to a stirred solution of DHP (0.04 mmol) in THF (1.00 mL) at −78 °C. The mixture was stirred for 1 hour at this temperature before addition of the electrophile (0.4 mmol). The reaction mixture was stirred at room temperature until completion then diluted with Et2O (10.0 mL) and quenched with sat. aq. NHCl4 (10.0 mL). The layers were separated and aqueous layer was extracted with Et2O (10.0 mL). The combined organic extracts were washed with brine (20.0 mL), dried over MgSO4 and concentrated in vacuo. Purification by flash column chromatography (hexane–ethyl acetate) to afforded the product.
(3S*,6S*)-Methyl 3-methyl-4-oxo-6-phenyltetrahydro-2H-pyran-3-carboxylate 13a.
Dihydropyran 5b (0.100 g, 0.431 mmol), yielded 0.063 g (59%) oil. ν max/cm−1 (film) 3032, 2953, 2877, 1736, 1710, 1268, 1233, 1095, 1075, 699, 762 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.41–7.31 (5H, m), 4.89 (1H, dd, J = 9.5, 4.1 Hz), 4.29 (1H, d, J = 11.7 Hz), 3.94 (1H, d, J = 11.7 Hz), 3.79 (3H, s), 2.86 (1H, dd, J = 15.2, 9.5 Hz), 2.75 (1H, dd, J = 15.2, 4.1 Hz) and 1.54 (3H, s) ppm; δC (100 MHz, CDCl3): 204.9, 171.3, 139.8, 128.8, 128.4, 126.0, 79.5, 72.5, 58.6, 52.6, 45.1 and 18.6 ppm; m/z (ESI+) 271 (M + Na)+. (Found 271.0936 (M + Na)+. C14H16NaO4 requires 271.0941).
(3S*,6S*)-Methyl 3-methyl-4-oxo-6-((E)-styryl)tetrahydro-2H-pyran-3-carboxylate 13b.
Dihydropyran 5g (0.120 g, 0.465 mmol), yielded 0.068 g (53%) oil. ν max/cm−1 (film) 3026, 2952, 2875, 1733, 1713, 1232, 1264, 1114, 1088, 967, 748, 693 cm−1; 1H NMR (400 MHz, C6D6): δ 7.18–7.02 (5H, m), 6.44 (1H, d, J = 16.0 Hz), 5.94 (1H, dd, J = 16.0, 5.3 Hz), 4.19 (1H, d, J = 11.6 Hz), 4.06 (1H, ddd, J = 9.0, 5.3, 4.4 Hz), 3.66 (1H, d, J = 11.6 Hz), 3.36 (3H, s), 2.41 (1H, dd, J = 15.0, 4.4 Hz), 2.29 (1H, dd, J = 15.0, 9.0 Hz) and 1.31 (3H, s) ppm; δC (100 MHz, C6D6): 203.2, 171.9, 136.6, 132.2, 128.9, 128.1, 127.9, 126.9, 77.7, 72.1, 58.7, 52.0, 43.9 and 18.1 ppm; m/z (ESI+) 297 (M + Na)+. (Found 297.1095 (M + Na)+. C16H18NaO4 requires 297.1097).
(3S*,6S*)-Methyl 3-methyl-4-oxo-6-(((triisopropylsilyl)oxy)methyl)tetrahydro-2H-pyran-3-carboxylate 13c.
Dihydropyran 5d (0.097 g, 0.280 mmol), yielded 0.057 g (57%) oil. ν max/cm−1 (film) 2942, 2866, 1738, 1715, 1105, 881, 681, 659 cm−1; 1H NMR (400 MHz, C6D6): 4.13 (1H, d, J = 11.4 Hz), 3.73 (1H, d, J = 11.4 Hz), 3.58 (1H, dddd, J = 10.0, 3.8, 3.8, 3.5 Hz), 3.45 (1H, dd, J = 8.0, 3.8 Hz), 3.40 (1H, dd, J = 8.0, 3.8 Hz), 3.36 (3H, s), 2.59 (1H, dd, J = 15.1, 10.0 Hz), 2.25 (1H, dd, J = 15.1, 3.5 Hz), 1.43 (3H, s) and 1.05–104 (21H, m) ppm; δC (100 MHz, C6D6): 205.0, 171.1, 78.6, 73.0, 65.9, 58.8, 51.9, 40.2, 18.6, 18.1 and 12.2 ppm; m/z (ESI+) 381 (M + Na)+. (Found 381.2065 (M + Na)+. C18H34NaO5Si requires 381.2068).
(3S*,6R*)-Methyl 3-methyl-4-oxo-6-propyltetrahydro-2H-pyran-3-carboxylate 13d.
Dihydropyran 5c (0.100 g, 0.505 mmol), yielded 0.063 g (58%) oil. ν max/cm−1 (film) 2956, 2929, 2872, 1738, 1714, 1263, 1100, 782 cm−1; 1H NMR (400 MHz, C6D6): δ 4.08 (1H, d, J = 11.5 Hz), 3.62 (1H, d, J = 11.5 Hz), 3.38 (3H, s), 3.24 (1H, m), 2.12 (1H, dd, J = 15.0, 3.6 Hz), 1.99 (1H, dd, J = 15.0, 10.2 Hz), 1.35 (3H, s), 1.32–0.93 (4H, m) and 0.74 (3H, t, J = 7.3 Hz) ppm; δC (100 MHz, C6D6): 204.3, 171.9, 77.8, 72.2, 58.8, 51.8, 44.2, 37.7, 18.7, 18.5 and 14.0 ppm; m/z (ESI+) 237 (M + Na)+. (Found 237.1100 (M + Na)+. C11H18NaO4 requires 237.1097).
(3S*,6S*)-Methyl 3-allyl-4-oxo-6-phenyltetrahydro-2H-pyran-3-carboxylate 13e.
Dihydropyran 5b (0.100 g, 0.431 mmol), yielded 0.061 g (52%) oil. ν max/cm−1 (film) 3065, 2952, 2875, 1736, 1711, 1227, 1076, 763, 699 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.42–7.32 (5H, m), 5.80 (1H, m), 5.20 (1H, dd, J = 17.2, 4.4 Hz), 5.15 (1H, dd, J = 10.1, 4.4 Hz), 4.86 (1H, dd, J = 9.3, 4.6 Hz), 4.21 (1H, d, J = 11.8 Hz), 4.12 (1H, d, J = 11.8 Hz), 3.79 (3H, s) and 2.82–2.71 (4H, m) ppm; δC (100 MHz, CDCl3): 203.8, 170.2, 139.9, 132.1, 128.8, 128.4, 126.0, 119.9, 79.6, 70.1, 62.5, 52.5, 46.2 and 36.1 ppm; m/z (ESI+) 297 (M + Na)+. (Found 297.1101 (M + Na)+. C16H18NaO4 requires 297.1097).
(3S*,6S*)-Methyl 3-allyl-4-oxo-6-((E)-styryl)tetrahydro-2H-pyran-3-carboxylate 13f.
Dihydropyran 5f (0.100 g, 0.387 mmol), yielded 0.097 g (83%) oil. ν max/cm−1 (film) 2952, 1736, 1712, 1226, 1073, 1031, 966, 748, 693 cm−1; 1H NMR (400 MHz, C6D6): δ 7.19–7.02 (5H, m), 6.45 (1H, dd, J = 16.0, 1.2 Hz), 5.95 (1H, dd, J = 16.0, 5.4 Hz), 5.88 (1H, dddd, J = 17.1, 10.1, 7.5, 7.0 Hz), 5.05 (1H, dd, J = 17.1, 4.4 Hz), 4.99 (1H, dd, J = 10.1, 4.4 Hz), 4.16 (1H, d, J = 11.8 Hz), 4.03 (1H, dddd, J = 9.0, 5.4, 4.2, 1.2 Hz), 3.98 (1H, d, J = 11.8 Hz), 3.36 (3H, s), 2.69 (1H, dd, J = 13.8, 7.0 Hz), 2.52 (1H, dd, J = 13.8, 7.5 Hz), 2.41 (1H, dd, J = 14.7, 4.2 Hz) and 2.31 (1H, dd, J = 14.7, 9.0 Hz) ppm; δC (100 MHz, C6D6): 202.6, 170.2, 136.6, 133.0, 132.1, 128.9, 126.9, 119.4, 77.8, 69.8, 62.7, 51.9, 44.8 and 35.9 ppm; m/z (ESI+) 323 (M + Na)+. (Found 323.1242 (M + Na)+. C16H21NaO4 requires 323.1230).
(3S*,6S*)-Methyl 3-allyl-4-oxo-6-(((triisopropylsilyl)oxy)methyl)tetrahydro-2H-pyran-3-carboxylate 13g.
Dihydropyran 5e (0.096 g, 0.280 mmol), yielded 0.061 g (57%) oil. ν max/cm−1 (film) 2943, 2866, 1739, 1715, 1231, 1124, 1083, 881, 680, 660 cm−1; 1H NMR (400 MHz, C6D6): δ 6.05 (1H, dddd, J = 16.8, 9.9, 7.1, 7.0 Hz), 5.29 (1H, dd, J = 16.8, 1.8 Hz), 5.14 (1H, dd, J = 9.9, 1.8 Hz), 4.17 (1H, d, J = 11.6 Hz), 4.14 (1H, d, J = 11.6 Hz), 3.70 (1H, m), 3.50 (2H, m), 3.47 (3H, s), 2.90 (1H, dd, J = 13.6, 7.0 Hz), 2.79 (1H, dd, J = 13.6, 7.1 Hz), 2.73 (1H, dd, J = 15.0, 8.0 Hz) and 2.33 (1H, dd, J = 15.0, 3.2 Hz) and 1.16–1.14 (21H, m) ppm; δC (100 MHz, C6D6): 203.6, 170.1, 133.2, 119.4, 78.7, 70.3, 65.8, 62.7, 51.8, 41.0, 36.0, 18.1 and 12.2 ppm; m/z (ESI+) 407 (M + Na)+. (Found 407.2211 (M + Na)+. C20H36NaO5Si requires 407.2224).
(3S*,6R*)-Methyl 3-allyl-4-oxo-6-propyltetrahydro-2H-pyran-3-carboxylate 13h.
Dihydropyran 5c (0.100 g, 0.505 mmol), yielded 0.063 g (52%) oil. ν max/cm−1 (film) 2958, 2873, 1737, 1712, 1229, 1081, 922 cm−1; 1H NMR (400 MHz, CDCl3): δ 5.76 (1H, dddd, J = 17.4, 10.2, 7.3, 7.2 Hz), 5.18 (1H, dd, J = 17.4, 1.4 Hz), 5.10 (1H, dd, J = 10.2, 1.4 Hz), 4.05 (1H, d, J = 11.8 Hz), 4.01 (1H, d, J = 11.8 Hz), 3.74 (3H, s), 3.74 (1H, m), 2.69 (1H, dd, J = 13.8, 7.3 Hz), 2.64 (1H, dd, J = 13.8, 7.2 Hz), 2.45 (1H, dd, J = 14.8, 3.6 Hz), 2.36 (1H, J = 14.8, 10.0 Hz), 1.51–1.34 (4H, m, H-7) and 0.93 (1H, t, J = 7.0 Hz) ppm; δC (100 MHz, CDCl3): 204.6, 170.3, 132.3, 119.7, 78.1, 70.1, 62.5, 52.4, 45.2, 37.7, 36.0, 18.4 and 13.9 ppm; m/z (ESI+) 263 (M + Na)+. (Found 263.1264 (M + Na)+. C13H20NaO4 requires 263.1254).
(3S*,6S*)-Methyl 3-benzyl-4-oxo-6-phenyltetrahydro-2H-pyran-3-carboxylate 13i.
Dihydropyran 5b (0.050 g, 0.215 mmol), yielded 0.045 g (65%) oil. ν max/cm−1 (film) 2948, 2920, 1710, 1207, 1073, 767, 702, 597 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.41–7.24 (10H, m), 4.89 (1H, dd, J = 10.0, 3.9 Hz), 4.13 (1H, d, J = 12.4 Hz), 4.10 (1H, d, J = 12.4 Hz), 3.76 (3H, s), 3.40 (1H, d, J = 13.4 Hz), 3.33 (1H, d, J = 13.4 Hz), 2.96 (1H, dd, J = 15.1, 10.0 Hz) and 2.78 (1H, dd, J = 15.1, 3.9 Hz) ppm; NOE H6 – H5α 3.6%, H5α – H6 2.26%, H5β – benzyl–CH2 3.16%; δC (100 MHz, CDCl3): 203.9, 169.7, 140.1, 135.0, 130.8, 128.9, 128.6, 128.5, 127.3, 126.0, 80.0, 69.1, 63.9, 52.4, 46.0 and 37.0 ppm; m/z (ESI+) 347 (M + Na)+. (Found 347.1252 (M + Na)+. C20H20NaO4 requires 347.1254).
(3S*,6S*)-Methyl 3-benzyl-4-oxo-6-((E)-styryl)tetrahydro-2H-pyran-3-carboxylate 13j.
Dihydropyran 5g (0.096 g, 0.372 mmol), yielded 0.066 g (51%) oil. ν max/cm−1 (film) 2958, 2860, 1734, 1712, 1209, 1070, 742, 703, 597 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.42–7.19 (10H, m), 6.67 (1H, dd, J = 16.1, 1.0 Hz), 6.26 (1H, dd, J = 16.1, 5.7 Hz), 4.59 (1H, dddd, J = 8.6, 5.7, 4.5, 1.0 Hz), 4.11 (1H, d, J = 12.2 Hz), 4.05 (1H, d, J = 12.2 Hz), 3.73 (3H, s), 3.34 (1H, d, J = 13.5 Hz), 3.20 (1H, d, J = 13.5 Hz), 2.83 (1H, dd, J = 14.8, 8.6 Hz) and 2.73 (1H, dd, J = 14.8, 4.5 Hz) ppm; δC (100 MHz, CDCl3): 204.0, 169.8, 136.0, 135.1, 132.9, 130.7, 128.8, 128.5, 128.4, 127.4, 127.3, 126.8, 78.1, 68.7, 63.8, 52.5, 44.6 and 36.7 ppm; m/z (ESI+) 373 (M + Na)+. (Found 373.1401 (M + Na)+. C22H22NaO4 requires 373.1410).
(3S*,6S*)-Methyl 3-benzyl-4-oxo-6-(((triisopropylsilyl)oxy)methyl)tetrahydro-2H-pyran-3-carboxylate 13k.
Dihydropyran 5e (0.100 g, 0.292 mmol), yielded 0.078 g (62%) oil. ν max/cm−1 (film) 2942, 2865, 1736, 1713, 1121, 1074, 881, 682, 660 cm−1; 1H NMR (400 MHz, C6D6): δ 7.48–7.02 (5H, m), 4.15 (1H, d, J = 12.0 Hz), 3.97 (1H, d, J = 12.0 Hz), 3.64 (1H, m), 3.43 (2H, m), 3.34 (3H, s), 3.42 (1H, d, J = 13.0 Hz), 3.27 (1H, d, J = 13.0 Hz), 2.79 (1H, dd, J = 15.0, 10.6 Hz), 2.22 (1H, dd, J = 15.0, 3.4 Hz) and 1.08–1.00 (21H, m) ppm; δC (100 MHz, C6D6): 203.7, 169.6, 135.9, 131.2, 128.6, 128.1, 127.9, 127.3, 79.0, 69.2, 65.8, 64.2, 51.7, 40.5, 36.6, 18.1 and 12.2 ppm; m/z (ESI+) 457 (M + Na)+. (Found 457.2388 (M + Na)+. C24H38NaO5Si requires 457.2381).
(3S*,6R*)-Methyl 3-benzyl-4-oxo-6-propyltetrahydro-2H-pyran-3-carboxylate 13l.
Dihydropyran 5c (0.050 g, 0.252 mmol), yielded 0.045 g (62%) oil. ν max/cm−1 (film) 2956, 2932, 2872, 1734, 1711, 1262, 1206, 1077, 1016, 702 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.27–7.20 (5H, m), 3.99 (1H, d, J = 12.2 Hz), 3.94 (1H, d, J = 12.2 Hz), 3.81 (1H, m), 3.70 (3H, s), 3.31 (1H, d, J = 13.4 Hz), 3.21 (1H, d, J = 13.4 Hz), 2.56 (1H, dd, J = 15.0, 9.8 Hz), 2.48 (1H, dd, J = 15.0, 3.8 Hz), 1.58–1.37 (4H, m) and 0.95 (3H, t, J = 7.16 Hz) ppm; δC (100 MHz, CDCl3): 204.7, 169.8, 135.2, 130.7, 128.5, 127.7, 78.4, 69.0, 63.9, 52.3, 44.9, 37.7, 36.8, 18.3 and 14.0 ppm; m/z (ESI+) 313 (M + Na)+. (Found 313.1416 (M + Na)+. C17H22NaO4 requires 313.1410).
Synthesis of diospongin B 2
(E)-Methyl 5-hydroxy-3-oxo-7-phenylhept-6-enoate 7g.
Titanium tetraisopropoxide (11.48 mL, 38.80 mmol) was added to a stirred solution of cinnamaldehyde (4.88 g, 38.80 mmol) and diketene (5.36 mL, 69.60 mmol) in CH2Cl2 (104 mL) at −78 °C. After 5 minutes, methanol (6.24 mL, 154.0 mmol) was added and the mixture was stirred at −20 to −10 °C for 1.5 hours. The reaction mixture was diluted with Et2O (100.0 mL) and a 20% w/v citric acid solution (120.0 mL) was added. The layers were separated and the aqueous layer was extracted with Et2O (2 × 50 mL). The combined organic extracts were washed with brine (2 × 50 mL), dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) gave the product as an oil, isolated yield 7.33 g (76%). ν max/cm−1 (film) 3423, 3026, 2953, 1740, 1710, 1436, 1319, 1266, 1149, 1070, 967, 747, 694 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.28–7.05 (5H, m), 6.57 (1H, d, J = 16.0 Hz), (1H, dd, J = 16.0, 5.4 Hz), 4.62 (1H, m), 3.30 (3H, s), 3.10 (2H, s), 2.49 (1H, dd, J = 16.8, 8.9 Hz), 2.31 (1H, dd, J = 16.8, 3.6 Hz) ppm; δC (100 MHz, CDCl3): 201.9, 167.3, 137.2, 131.0, 130.2, 128.8, 127.8, 126.8, 68.4, 51.8, 49.7 and 49.6 ppm; m/z (ESI+) 271 (M + Na)+. (Found 271.0937 (M + Na)+. C14H16NaO4 requires 271.0941).
2) gave the product as an oil, isolated yield 7.33 g (76%). ν max/cm−1 (film) 3423, 3026, 2953, 1740, 1710, 1436, 1319, 1266, 1149, 1070, 967, 747, 694 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.28–7.05 (5H, m), 6.57 (1H, d, J = 16.0 Hz), (1H, dd, J = 16.0, 5.4 Hz), 4.62 (1H, m), 3.30 (3H, s), 3.10 (2H, s), 2.49 (1H, dd, J = 16.8, 8.9 Hz), 2.31 (1H, dd, J = 16.8, 3.6 Hz) ppm; δC (100 MHz, CDCl3): 201.9, 167.3, 137.2, 131.0, 130.2, 128.8, 127.8, 126.8, 68.4, 51.8, 49.7 and 49.6 ppm; m/z (ESI+) 271 (M + Na)+. (Found 271.0937 (M + Na)+. C14H16NaO4 requires 271.0941).
(2S*,4S*,6S*)-2-Phenyl-6-((E)-styryl)tetrahydro-2H-pyran-4-ol 14.
A solution of 8f (0.059 g, 0.175 mmol) in DMF (0.92 mL) and H2O (0.02 mL) was submitted to 200 W microwave radiation in a sealed tube at 160 °C for 10 minutes. The solution was cooled to rt and taken up in EtOAc (30.0 mL). The mixture was washed with H2O (2 × 20.0 mL). The aqueous layer was extracted with EtOAc (30.0 mL) and the combined organic extracts were washed with brine (20.0 mL), dried over MgSO4 and concentrated in vacuo to give 0.044 g (91%) of a dark yellow oil. ν max/cm−1 (film) 2978, 2881, 1721, 1230, 1047, 966, 753, 737, 695 cm−1; δH (400 MHz, CDCl3) 7.34–7.17 (10H, m), 6.53 (1H, dd, J = 16.3, 1.3 Hz), 6.23 (1H, dd, J = 16.3, 5.1 Hz), 5.12 (1H, dd, J = 7.7, 4.8 Hz), 4.82 (1H, ddd, J = 10.5, 5.2, 5.1 Hz), 2.78–2.68 (4H, m) ppm; NOE H2 – H3α 1.33%, H2 – H3β 1.89%, H4 – H6 1.23%, H4 – H3α 1.58%, H4 – H5α 2.59%; δC (100 MHz, CDCl3) 205.9, 140.5, 135.7, 133.5, 128.8, 128.7, 128.3, 128.2, 127.9, 126.7, 126.5, 73.6, 72.9, 47.8, 45.4 ppm; m/z (ESI+) 301 (M + Na)+. (Found 301.1196 (M + Na)+. C19H18NaO2 requires 301.1199). A 1.0 M solution of L-Selectride® in THF (0.73 mL) was added to a stirred solution of the crude decarboxylated product (0.079 g, 0.28 mmol) in THF (3.5 mL) at −78 °C. The mixture was stirred at this temperature for 15 minutes then warmed to room temperature. Upon completion, the mixture was diluted with Et2O (20.0 mL) and sat. aq. NH4Cl (20.0 mL) and the layers separated. The aqueous layer was washed with Et2O (20.0 mL) and the combined organic extracts were washed with brine (20.0 mL), dried over MgSO4 and concentrated in vacuo to give 14 as a light yellow oil, 0.052 g (66%), dr 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. ν max/cm−1 (film) 3374, 2925, 1448, 1364, 1052, 695 cm−1; δH (400 MHz, CDCl3) 7.47–7.22 (10H, m), 6.64 (1H, dd, J = 16.1, 1.1 Hz), 6.37 (1H, dd, J = 16.1, 5.8 Hz), 5.31 (1H, t, J = 4.4 Hz), 4.98 (1H, m, minor), 4.74 (1H, m, minor), 4.27 (1H, dddd, J = 9.1, 5.8, 5.0, 1.1 Hz), 4.19 (1H, m, minor), 4.07 (1H, dddd, J = 9.3, 9.0, 4.5, 4.0 Hz), 2.54 (1H, ddd, J = 13.5, 4.4, 4.0 Hz), 2.31 (1H, m, minor), 2.19 (1H, m, minor), 2.08 (1H, ddd, J = 12.6, 5.0, 4.5 Hz), 1.96 (1H, ddd, J = 13.5, 9.0, 4.4 Hz), 1.89 (1H, m, minor), 1.64 (1H, ddd, J = 12.6, 9.3, 9.1 Hz) and 1.64 (1H, bs) ppm; δC (100 MHz, CDCl3) 140.7, 136.8, 130.6, 129.9, 128.8, 128.7, 127.8, 127.3, 126.6, 126.4, 72.2, 70.6, 64.7, 40.5 and 37.0 ppm; m/z (ESI+) 303 (M + Na)+. (Found 303.1361 (M + Na)+. C19H20NaO2 requires 303.1356). Data in agreement with that previously reported.19f
1. ν max/cm−1 (film) 3374, 2925, 1448, 1364, 1052, 695 cm−1; δH (400 MHz, CDCl3) 7.47–7.22 (10H, m), 6.64 (1H, dd, J = 16.1, 1.1 Hz), 6.37 (1H, dd, J = 16.1, 5.8 Hz), 5.31 (1H, t, J = 4.4 Hz), 4.98 (1H, m, minor), 4.74 (1H, m, minor), 4.27 (1H, dddd, J = 9.1, 5.8, 5.0, 1.1 Hz), 4.19 (1H, m, minor), 4.07 (1H, dddd, J = 9.3, 9.0, 4.5, 4.0 Hz), 2.54 (1H, ddd, J = 13.5, 4.4, 4.0 Hz), 2.31 (1H, m, minor), 2.19 (1H, m, minor), 2.08 (1H, ddd, J = 12.6, 5.0, 4.5 Hz), 1.96 (1H, ddd, J = 13.5, 9.0, 4.4 Hz), 1.89 (1H, m, minor), 1.64 (1H, ddd, J = 12.6, 9.3, 9.1 Hz) and 1.64 (1H, bs) ppm; δC (100 MHz, CDCl3) 140.7, 136.8, 130.6, 129.9, 128.8, 128.7, 127.8, 127.3, 126.6, 126.4, 72.2, 70.6, 64.7, 40.5 and 37.0 ppm; m/z (ESI+) 303 (M + Na)+. (Found 303.1361 (M + Na)+. C19H20NaO2 requires 303.1356). Data in agreement with that previously reported.19f
2-((2S*,4S*,6S*)-4-(Methoxymethoxy)-6-phenyltetrahydro-2H-pyran-2-yl)-1-phenylethanone 15.
N,N-Diisopropylethylamine (0.6 mL, 3.36 mmol), MOMCl (0.34 mL, 4.48 mmol) and sodium iodide (0.1 g, 0.67 mmol) were added to a stirred solution of 14 (0.078 g, 0.28 mmol) in THF (5.0 mL) at room temperature. The mixture was heated at 50 °C for 10 hours, after which the solvent was removed in vacuo., the reaction mixture was diluted with H2O (20 mL) and extracted with EtOAc (20 mL). The extract was washed with brine and dried over MgSO4 and concentrated in vacuo to give the product 0.054 g (60%) as a light yellow oil. ν max/cm−1 (film) 2923, 2854, 1145, 1033, 695 cm−1; δH (400 MHz, CDCl3) 7.47–7.22 (10H, m), 6.62 (1H, d, J = 16.0 Hz), 6.38 (1H, dd, J = 16.0, 6.0 Hz), 5.29 (1H, t, J = 4.4 Hz), 4.74, (2H, s), 4.26 (1H, dddd, J = 9.2, 6.0, 4.6, 1.2 Hz), 3.96 (1H, dddd, J = 9.4, 9.2, 5.0, 4.0 Hz), 3.41, (3H, s), 2.54 (1H, dddd, J = 13.4, 4.4, 4.0, 1.5 Hz), 2.09 (1H, ddd, J = 12.8, 5.0, 4.6, 1.5 Hz), 2.03 (1H, ddd, J = 13.4, 9.4, 4.4 Hz), 1.67 (1H, ddd, J = 12.8, 9.2, 9.2 Hz) ppm; δC (100 MHz, CDCl3) 140.6, 136.9, 130.5, 129.9, 128.7, 128.6, 127.7, 127.3, 126.6, 126.4, 94.9, 72.3, 70.8, 69.8, 55.4, 37.9 and 34.6 ppm; m/z (ESI+) 347 (M + Na)+. (Found 347.1618 (M + Na)+. C21H24NaO3 requires 347.1618).
PdCl2 (0.004 g, 0.02 mmol) and CuCl (0.006 g, 0.06 mmol) were added to a stirred solution of crude MOM-protected alcohol (0.014 g, 0.04 mmol) in DMF (0.5 mL) and H2O (0.5 mL) at room temperature. The mixture was heated at 50 °C for 3 days under an oxygen atmosphere, after which the solvent was removed in vacuo to give 15 0.010 g (70%) as a light yellow oil. ν max/cm−1 (film) 2921, 1682, 1445, 1036, 690 cm−1; δH (400 MHz, CDCl3) 7.97 (2H, dd, J = 7.0, 1.0 Hz), 7.57 (1H, tt, J = 7.4, 1.2 Hz), 7.47 (2H, t, J = 7.3 Hz), 7.35–7.29 (5H, m), 5.16 (1H, t, J = 4.3 Hz), 4.70 (2H, s), 4.24 (1H, dddd, J = 9.3, 7.0, 5.9, 3.0 Hz), 3.91 (1H, dddd, J = 9.8, 9.3, 4.2, 4.1 Hz), 3.42 (1H, dd, J = 15.9, 7.0 Hz), 3.38, (3H, s), 3.18 (1H, dd, J = 15.9, 5.9 Hz), 2.52 (1H, ddd, J = 13.5, 4.3, 4.1 Hz), 2.08 (1H, ddd, J = 12.6, 4.2, 3.0 Hz), 1.98 (1H, ddd, J = 13.5, 9.8, 4.3 Hz), 1.67 (1H, ddd, J = 12.6, 9.3, 9.3 Hz) ppm; δC (100 MHz, CDCl3) 198.1, 140.4, 137.2, 133.2, 128.7, 128.3, 127.2, 126.4, 95.2, 72.4, 69.5, 67.5, 55.4, 44.4, 38.1, 34.7 and 30.0 ppm; m/z (ESI+) 363 (M + Na)+. (Found 363.1559 (M + Na)+. C21H24NaO4 requires 363.1567). Data in agreement with those previously reported.19f
Diospongin B 2
A solution of THP 15 (0.013 mg, 0.04 mmol) and 30% HCl (0.68 mL) was stirred in THF (2.1 mL) at room temperature for 2 hours, after which water was added and the mixture neutralized with NaHCO3 before being extracted with EtOAc (30.0 mL). The organic extract was washed with H2O (20.0 mL), dried over MgSO4 and concentrated in vacuo to give diospongin B, 0.006 g (58%) as a light yellow oil. ν max/cm−1 (film) 3375, 2916, 2846, 1557, 1411, 1129 cm−1; δH (400 MHz, CDCl3)) 7.98 (2H, dd, J = 8.0, 1.7 Hz), 7.58 (1H, tt, J = 7.1, 1.3 Hz), 7.47 (2H, td, J = 7.6, 2.2 Hz), 7.37–7.3 (5H, m), 5.19 (1H, t, J = 4.3 Hz), 4.23 (1H, dddd, J = 9.5, 7.1, 6.0, 3.0 Hz), 4.03 (1H, dddd, J = 9.9, 9.5, 5.5, 4.5 Hz), 3.46 (1H, dd, J = 15.8, 7.1 Hz), 3.18 (1H, dd, J = 15.8, 6.0 Hz), 2.52 (1H, ddd, J = 13.4, 5.5, 4.3 Hz), 2.06 (1H, ddd, J = 12.4, 4.5, 3.0 Hz), 1.92 (1H, ddd, J = 13.4, 9.9, 4.3 Hz), 1.51 (1H, ddd, J = 12.4, 9.5, 9.5 Hz) ppm; δC (100 MHz, CDCl3) 198.5, 140.4, 137.2, 133.3, 128.7, 128.6, 128.4, 127.2, 126.4, 72.5, 67.0, 64.3, 44.7, 40.3 and 36.8 ppm; m/z (ESI+) 319 (M + Na)+. (Found 319.1300 (M + Na)+. C19H20NaO3 requires 319.1305). Data in agreement with those previously reported.19
Acknowledgements
We thank the University of York (P. B. S.), the Malaysian Ministry of Higher Education (N. M. N.) and the EU ERASMUS+ programme (A. M. P) for funding.
Notes and references
- For reviews see:
(a) N. M. Nasir, K. Ermanis and P. A. Clarke, Org. Biomol. Chem., 2014, 12, 3323 RSC;
(b)
M. A. Perry, S. D. Rychnovsky and N. Sizemore, Synthesis of Saturated Oxygenated Heterocycles I, Topics in Heterocyclic Chemistry, ed. J. Cossy, Springer-Verlag, Berlin, Heidelberg, 2014, vol. 35, pp. 43–95 Search PubMed;
(c) P. A. Clarke and S. Santos, Eur. J. Org. Chem., 2006, 2015 Search PubMed.
- For recent examples see:
(a) T. Sakai, A. Fukuta, K. Nakamura, M. Nakano and Y. Mori, J. Org. Chem., 2016, 81, 3799 CrossRef CAS PubMed;
(b) J. Malassis, N. Bartlett, K. Hands, M. D. Selby and B. Linclau, J. Org. Chem., 2016, 81, 3818 CrossRef CAS PubMed;
(c) S.-I. Uesugi, T. Watanabe, T. Imaizumi, Y. Ota, K. Yoshida, H. Ebisu, T. Chinen, Y. Nagumo, M. Shibuya, N. Kanoh, T. Ussi and Y. Iwabuchi, J. Org. Chem., 2015, 80, 12333 CrossRef CAS PubMed.
- For reviews on oxy-Michael reactions and their selectivity:
(a) C. F. Nising and S. Bräse, Chem. Soc. Rev., 2008, 37, 1218 RSC;
(b) C. F. Nising and S. Bräse, Chem. Soc. Rev., 2012, 41, 988 RSC.
- For recent examples of selectivity issues in oxy-Michael reactions:
(a) L. Brewitz, J. Llaveria, A. Yada and A. Fürstner, Chem. – Eur. J., 2013, 19, 4532 CrossRef CAS PubMed;
(b) T. P. A. Hari, B. I. Wilke, J. A. Davey and C. N. Boddy, J. Org. Chem., 2016, 81, 415 CrossRef CAS PubMed;
(c) J. Uenishi, T. Iwamoto and J. Tanaka, Org. Lett., 2009, 11, 3262 CrossRef CAS PubMed;
(d) L. Ferrié, L. Boulard, F. Pradaux, S. Bouzbouz, S. Reymond, P. Capdevielle and J. Cossy, J. Org. Chem., 2008, 73, 1864 CrossRef PubMed.
-
(a) K. A. Jørgensen, Angew. Chem., Int. Ed., 2000, 39, 3558 CrossRef;
(b) J. S. Johnson and D. A. Evans, Acc. Chem. Res., 2000, 33, 325 CrossRef CAS PubMed;
(c) K. Gademann, D. E. Chavez and E. N. Jacobsen, Angew. Chem., Int. Ed., 2002, 41, 3059 CrossRef CAS.
- For a review on the application of the Prins reaction to THP synthesis see: C. Olier, M. Kaafarani, S. Gastaldi and M. P. Bertrand, Tetrahedron, 2010, 66, 413 CrossRef CAS.
- For recent developments see:
(a) R. J. Beattie, T. W. Hornsby, G. Craig, M. C. Galan and C. L. Willis, Chem. Sci., 2016, 7, 2743 RSC;
(b) S. J. Alvarez-Mendez, C. Garcia and V. S. Martin, Chem. Commun., 2016, 52, 3380 RSC;
(c) Z. Zhang, H. Xie, H. Li, L. Gao and Z. Song, Org. Lett., 2015, 17, 4706 CrossRef CAS PubMed;
(d) C. Bosset, P. Angibaund, I. Stanfield, L. Meerpoel, D. Berthelot, A. Guérinot and J. Cossy, J. Org. Chem., 2015, 80, 12509 CrossRef CAS PubMed.
-
(a) A. B. Smith III, T. Tomioka, C. A. Risatti, J. B. Sperry and C. Sfouggatakis, Org. Lett., 2008, 10, 4359 CrossRef PubMed;
(b) K. Tanaka, M. Watanabe, K. Ishibashi, H. Matsuyama, Y. Saikawa and M. Nakata, Org. Lett., 2010, 12, 1700 CrossRef CAS PubMed.
-
(a) S. S. Palimkar, J. Uenishi and H. Ii, J. Org. Chem., 2012, 77, 388 CrossRef CAS PubMed;
(b) J. Carpenter, A. B. Northrup, D. Chung, J. J. M. Wiener, S.-G. Kim and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2008, 47, 3568 CrossRef CAS PubMed;
(c) S. Y. Yun, E. C. Hansen, I. Volchkov, E. J. Cho, W. Y. Lo and D. Lee, Angew. Chem., Int. Ed., 2010, 49, 4261 CrossRef CAS PubMed.
-
(a) P. A. Clarke and K. Ermanis, Curr. Org. Chem., 2013, 17, 2025 CrossRef CAS;
(b) M. Iqbal, N. Mistry and P. A. Clarke, Tetrahedron, 2011, 67, 4960 CrossRef CAS;
(c) P. A. Clarke, P. B. Sellars and N. Mistry, Tetrahedron Lett., 2011, 52, 3654 CrossRef CAS;
(d) P. A. Clarke, S. Santos and W. H. C. Martin, Green Chem., 2007, 9, 438 RSC;
(e) P. A. Clarke, W. H. C. Martin, J. M. Hargreaves, C. Wilson and A. J. Blake, Org. Biomol. Chem., 2005, 3, 3551 RSC;
(f) P. A. Clarke, W. H. C. Martin, J. M. Hargreaves, C. Wilson and A. J. Blake, Chem. Commun., 2005, 1061 RSC;
(g) P. A. Clarke and W. H. C. Martin, Org. Lett., 2002, 4, 4527 CrossRef CAS PubMed.
-
(a) M. T. Yang and J. Woerpel, Org. Chem., 2009, 74, 545 CrossRef CAS PubMed;
(b) J.-F. Brazeau, A.-A. Guilbault, J. Kochuparampil, P. Mochirian and Y. Guindon, Org. Lett., 2010, 12, 36 CrossRef CAS PubMed;
(c) L. Ferrié, S. Reymond, P. Capdevielle and J. Cossy, Org. Lett., 2007, 9, 2461 CrossRef PubMed;
(d) I. Paterson, A. Steven and C. J. Luckhurst, Org. Biomol. Chem., 2004, 2, 3026 RSC.
- For a carbometallation approach: H. J. Edwards, S. Goggins and C. G. Frost, Molecules, 2015, 20, 6153 CrossRef CAS PubMed.
-
(a) R. H. Cichewicz, F. A. Valeriote and P. Crews, Org. Lett., 2004, 6, 195 CrossRef PubMed;
(b) G. R. Pettit, J. P. Xu, J. C. Chapuis, R. K. Pettit, L. P. Tackett, D. L. Doubek, J. N. Hooper and J. M. Schmidt, J. Med. Chem., 2004, 47, 1149 CrossRef CAS PubMed.
-
(a) U. Gräfe, W. Schade, M. Roth, L. Radics, M. Incze and K. Ujszaszy, J. Antibiot., 1984, 37, 836 CrossRef;
(b) H. A. Brooks, D. Gardner, J. P. Poyser and T. J. King, J. Antibiot., 1984, 37, 1501 CrossRef CAS PubMed.
- K. Kito, R. Ookura, S. Yoshida, M. Namikoshi, T. Ooi and T. Kusumi, Org. Lett., 2008, 10, 225 CrossRef CAS PubMed.
- J. Yin, K. Kouda, Y. Tezuka, Q. Le Tran, T. Miyahara, Y. Chen and S. Kadota, Planta Med., 2004, 70, 54 CrossRef CAS PubMed.
- P. A. Clarke, P. B. Sellars and N. M. Nasir, Org. Biomol. Chem., 2015, 13, 4743 Search PubMed.
- S. Danishefsky, Acc. Chem. Res., 1981, 14, 400 CrossRef CAS.
-
(a) K. B. Sawant and M. P. Jennings, J. Org. Chem., 2006, 71, 7911 CrossRef CAS PubMed;
(b) S. Chandrasekhar, T. Shyamsunder, S. J. Prakash, A. Prabhakar and B. Jagadeesh, Tetrahedron Lett., 2006, 47, 47 CrossRef CAS;
(c) C. Bressy, F. Allais and J. Cossy, Synlett, 2006, 3455 CAS;
(d) R. W. Bates and P. Song, Tetrahedron Lett., 2007, 63, 4497 CrossRef CAS;
(e) M.-A. Hiebel, B. Pelotier and O. Piva, Tetrahedron, 2007, 63, 7874 CrossRef CAS;
(f) N. Kawai, S. M. Hande and J. Uenishi, Tetrahedron, 2007, 63, 9049 CrossRef CAS;
(g) J. S. Yadav, B. Padmavani, B. V. S. Reddy, C. Venugopal and A. B. Rao, Synlett, 2007, 2045 CrossRef CAS;
(h) H. Wang, B. J. Shuhler and M. Xian, Synlett, 2008, 2651 Search PubMed;
(i) G. Sabitha, P. Padmaja and J. S. Yadav, Helv. Chim. Acta, 2008, 91, 2235 CrossRef CAS;
(j) K. Lee, K. H. Kim and J. Hong, Org. Lett., 2009, 11, 5202 CrossRef CAS PubMed;
(k) G. Kumaraswamy, G. Ramakrishna, P. Naresh, B. Jagadeesh and B. Sridhar, J. Org. Chem., 2009, 74, 8468 CrossRef CAS PubMed;
(l) J. D. More, Synthesis, 2010, 2419 CrossRef CAS;
(m) R. N. Kumar and H. M. Meshram, Tetrahedron Lett., 2011, 52, 1003 CrossRef CAS;
(n) T.-L. Ho, B. Tang, G. Ma and P. Xu, J. Chin. Chem. Soc., 2012, 59, 455 CrossRef CAS;
(o) E. Stefan, A. P. Nalin and R. E. Taylor, Tetrahedron, 2013, 69, 7706 CrossRef CAS PubMed;
(p) H. Yao, J. Ren and R. Tong, Chem. Commun., 2013, 49, 193 RSC;
(q) S. B. Meruva, R. Mekala, A. Raghunadh, K. Raghavendra, V. H. RaoDahanukar, T. V. Pratap, U. K. Syam Kumar and P. K. Dubey, Tetrahedron Lett., 2014, 55, 4739 CrossRef CAS;
(r) T. Rybak and D. G. Hall, Org. Lett., 2015, 17, 4165 CrossRef PubMed.
- P. A. Clarke, S. Santos, N. Mistry, L. Burroughs and A. C. Humphries, Org. Lett., 2011, 13, 624 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic data for all compounds synthesised. See DOI: 10.1039/c6ob01182a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *,
Nadiah Mad
Nasir
,
Philip B.
Sellars
,
Alejandra M.
Peter
,
Connor A.
Lawson
and
James L.
Burroughs
*,
Nadiah Mad
Nasir
,
Philip B.
Sellars
,
Alejandra M.
Peter
,
Connor A.
Lawson
and
James L.
Burroughs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3
CHCH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh
CHPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3
CHCH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with the correct relative stereochemical configuration for diospongin B. The stereochemical configuration of 14 was confirmed by H2 being coupled to both H3α and H3β with J = 4.4 Hz indicating its equatorial position, H6 was coupled to H5β J = 9.1 Hz and H5α J = 5.0 Hz, indicating its axial position while H4 was coupled to H5β J = 9.3 Hz, H5α J = 4.5 Hz, H3β J = 9.0 Hz and H3α J = 4.0 Hz indicating its axial orientation. Additionally, H2 only had NOE correlations to H3α of 1.33% and to H3β of 1.89%, H4 had NOE correlations to H6 of 1.23%, to H3α of 1.58% and to H5α of 2.59% (Fig. 5). The synthesis was completed by MOM-protection of the free hydroxyl in 60% yield, and Wacker oxidation of the double bond to give 15 in 70% yield.19f The final step was the removal of the MOM protecting group, which was achieved by the action of aqueous HCl19f and generated diospongin B 2 in 58% yield. Spectroscopic data for our sample of diospongin B 2 were identical to those reported in the literature.19
1) with the correct relative stereochemical configuration for diospongin B. The stereochemical configuration of 14 was confirmed by H2 being coupled to both H3α and H3β with J = 4.4 Hz indicating its equatorial position, H6 was coupled to H5β J = 9.1 Hz and H5α J = 5.0 Hz, indicating its axial position while H4 was coupled to H5β J = 9.3 Hz, H5α J = 4.5 Hz, H3β J = 9.0 Hz and H3α J = 4.0 Hz indicating its axial orientation. Additionally, H2 only had NOE correlations to H3α of 1.33% and to H3β of 1.89%, H4 had NOE correlations to H6 of 1.23%, to H3α of 1.58% and to H5α of 2.59% (Fig. 5). The synthesis was completed by MOM-protection of the free hydroxyl in 60% yield, and Wacker oxidation of the double bond to give 15 in 70% yield.19f The final step was the removal of the MOM protecting group, which was achieved by the action of aqueous HCl19f and generated diospongin B 2 in 58% yield. Spectroscopic data for our sample of diospongin B 2 were identical to those reported in the literature.19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded the products as an inseparable mixture of enol and ketone tautomers 8/9 which were then subjected to acylation. The THP mixture 8/9 (0.03 mmol), acetic anhydride (0.10 mL, 0.10 mmol) and DMAP (2 mg) were stirred in pyridine (0.47 mL) at 40 °C for 40 minutes. The mixture was cooled to rt, concentrated in vacuo and partitioned between Et2O (30.0 mL) and H2O (10.0 mL). The organic layer was washed with H2O (10.0 mL) and brine (10.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) gave products 10.
1) afforded the products as an inseparable mixture of enol and ketone tautomers 8/9 which were then subjected to acylation. The THP mixture 8/9 (0.03 mmol), acetic anhydride (0.10 mL, 0.10 mmol) and DMAP (2 mg) were stirred in pyridine (0.47 mL) at 40 °C for 40 minutes. The mixture was cooled to rt, concentrated in vacuo and partitioned between Et2O (30.0 mL) and H2O (10.0 mL). The organic layer was washed with H2O (10.0 mL) and brine (10.0 mL), then dried over MgSO4 and concentrated in vacuo. Flash column chromatography (hexane–ethyl acetate) gave products 10.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) gave the product as an oil, isolated yield 7.33 g (76%). ν max/cm−1 (film) 3423, 3026, 2953, 1740, 1710, 1436, 1319, 1266, 1149, 1070, 967, 747, 694 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.28–7.05 (5H, m), 6.57 (1H, d, J = 16.0 Hz), (1H, dd, J = 16.0, 5.4 Hz), 4.62 (1H, m), 3.30 (3H, s), 3.10 (2H, s), 2.49 (1H, dd, J = 16.8, 8.9 Hz), 2.31 (1H, dd, J = 16.8, 3.6 Hz) ppm; δC (100 MHz, CDCl3): 201.9, 167.3, 137.2, 131.0, 130.2, 128.8, 127.8, 126.8, 68.4, 51.8, 49.7 and 49.6 ppm; m/z (ESI+) 271 (M + Na)+. (Found 271.0937 (M + Na)+. C14H16NaO4 requires 271.0941).
2) gave the product as an oil, isolated yield 7.33 g (76%). ν max/cm−1 (film) 3423, 3026, 2953, 1740, 1710, 1436, 1319, 1266, 1149, 1070, 967, 747, 694 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.28–7.05 (5H, m), 6.57 (1H, d, J = 16.0 Hz), (1H, dd, J = 16.0, 5.4 Hz), 4.62 (1H, m), 3.30 (3H, s), 3.10 (2H, s), 2.49 (1H, dd, J = 16.8, 8.9 Hz), 2.31 (1H, dd, J = 16.8, 3.6 Hz) ppm; δC (100 MHz, CDCl3): 201.9, 167.3, 137.2, 131.0, 130.2, 128.8, 127.8, 126.8, 68.4, 51.8, 49.7 and 49.6 ppm; m/z (ESI+) 271 (M + Na)+. (Found 271.0937 (M + Na)+. C14H16NaO4 requires 271.0941).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. ν max/cm−1 (film) 3374, 2925, 1448, 1364, 1052, 695 cm−1; δH (400 MHz, CDCl3) 7.47–7.22 (10H, m), 6.64 (1H, dd, J = 16.1, 1.1 Hz), 6.37 (1H, dd, J = 16.1, 5.8 Hz), 5.31 (1H, t, J = 4.4 Hz), 4.98 (1H, m, minor), 4.74 (1H, m, minor), 4.27 (1H, dddd, J = 9.1, 5.8, 5.0, 1.1 Hz), 4.19 (1H, m, minor), 4.07 (1H, dddd, J = 9.3, 9.0, 4.5, 4.0 Hz), 2.54 (1H, ddd, J = 13.5, 4.4, 4.0 Hz), 2.31 (1H, m, minor), 2.19 (1H, m, minor), 2.08 (1H, ddd, J = 12.6, 5.0, 4.5 Hz), 1.96 (1H, ddd, J = 13.5, 9.0, 4.4 Hz), 1.89 (1H, m, minor), 1.64 (1H, ddd, J = 12.6, 9.3, 9.1 Hz) and 1.64 (1H, bs) ppm; δC (100 MHz, CDCl3) 140.7, 136.8, 130.6, 129.9, 128.8, 128.7, 127.8, 127.3, 126.6, 126.4, 72.2, 70.6, 64.7, 40.5 and 37.0 ppm; m/z (ESI+) 303 (M + Na)+. (Found 303.1361 (M + Na)+. C19H20NaO2 requires 303.1356). Data in agreement with that previously reported.19f
1. ν max/cm−1 (film) 3374, 2925, 1448, 1364, 1052, 695 cm−1; δH (400 MHz, CDCl3) 7.47–7.22 (10H, m), 6.64 (1H, dd, J = 16.1, 1.1 Hz), 6.37 (1H, dd, J = 16.1, 5.8 Hz), 5.31 (1H, t, J = 4.4 Hz), 4.98 (1H, m, minor), 4.74 (1H, m, minor), 4.27 (1H, dddd, J = 9.1, 5.8, 5.0, 1.1 Hz), 4.19 (1H, m, minor), 4.07 (1H, dddd, J = 9.3, 9.0, 4.5, 4.0 Hz), 2.54 (1H, ddd, J = 13.5, 4.4, 4.0 Hz), 2.31 (1H, m, minor), 2.19 (1H, m, minor), 2.08 (1H, ddd, J = 12.6, 5.0, 4.5 Hz), 1.96 (1H, ddd, J = 13.5, 9.0, 4.4 Hz), 1.89 (1H, m, minor), 1.64 (1H, ddd, J = 12.6, 9.3, 9.1 Hz) and 1.64 (1H, bs) ppm; δC (100 MHz, CDCl3) 140.7, 136.8, 130.6, 129.9, 128.8, 128.7, 127.8, 127.3, 126.6, 126.4, 72.2, 70.6, 64.7, 40.5 and 37.0 ppm; m/z (ESI+) 303 (M + Na)+. (Found 303.1361 (M + Na)+. C19H20NaO2 requires 303.1356). Data in agreement with that previously reported.19f