Nanoflower-like CoS-decorated 3D porous carbon skeleton derived from rose for a high performance nonenzymatic glucose sensor
Pingping Qua,
Zheni Gonga,
Haoyan Chenga,
Wei Xionga,
Xu Wua,
Pei Peia,
Ruofei Zhaoa,
Yan Zeng*b and
Zhihong Zhu*a
aInstitute of Nano-science and Nano-technology, College of Physical Science and Technology, Central China Normal University, Wuhan, 430079, P. R. China. E-mail: zhzhu@phy.ccnu.edu.cn
bCollege of Chemistry, Central China Normal University, Wuhan, Hubei 430079, China. E-mail: zyan@mail.ccnu.edu.cn
First published on 2nd December 2015
Abstract
Novel carbon materials derived from biological species attract a great deal of attention due to their characteristic ordered and porous microstructures. In this work, a nanoflower-like CoS-decorated 3D porous carbon skeleton from rose was firstly synthesized through a facial one-pot solvothermal synthetic process and innovatively used to fabricate a high performance nonenzymatic glucose biosensor. The nanoflower-like structure of CoS provided abundant highly electrocatalytic active sites and the carbon skeleton of rose featured plenty of interconnected channels, which could enhance mass diffusion and electron transfer. The prepared sensor exhibited good electrocatalytic performance toward glucose with a linear range from 10 μM to 960 μM, a low detection limit of 2 μM and a high sensitivity of 697 μA mM−1 cm−2 at the potential of +0.45 V. Overall, this work highlights the great promise offered by this novel nanocomposite material in terms of its application in nonenzymatic sensing.
1. Introduction
The increasing demand for the detection of glucose in clinical diagnosis, environment and food industries calls for the rapid development of glucose sensors with good accuracy, high sensitivity and low cost.1,2 Recently more and more analytical scientists have focussed their attention on enzyme-free glucose sensors. These nonenzymatic sensors have excellent capacities to achieve continuous target monitoring, good stability and stable repeatability.3,4 To date, the mainly effective electrocatalysts applied in non-enzymatic glucose sensors are metals and their oxides (Au,5 Pt,6 Ni,7 NiO,8 CuO,9 Co3O4,10 Fe3O4,11 etc.), or metal alloys (Pt–Au,12 Pt–Pb,13 Pt–Ni,14 Pt–Pd,15 etc.). However, compared with transition metal oxides, metals and metal alloys are too expensive to meet the practical demand in the detection of glucose and always suffer from anion poisoning.7 The widely used approach in the preparation of transition metal oxides can be described as the preparation of hydrogen and oxygen hydrocarbons by hydrothermal or solvothermal method, followed by annealing at different high temperatures.10,16By contrast, transition metal sulfides can be directly synthesized through hydrothermal or solvothermal process. Moreover, their morphologies can be easily controlled by changing the molar ratio of reactants or the proportion of the distilled water and the organic solvent.17 In the past few years, based on the transition metal sulfides standing out with the much easier synthetic process, some of them (such as CuS, Ni3S2, MoS2, etc.) have been widely used in lithium ion battery,18 supercapacitor19 and photocatalysis.20 Some metal sulfides have already been used in biosensors for detecting nonenzymatically glucose or immune sensor. Li21 et al. used CuS nanoflowers for enzyme-free sensing of hydrogen peroxide and glucose and showed a sensitivity of 5.86 μA mM−1 for glucose, Kim22 et al. performed NiS with reduced graphene oxide for nonenzymatic glucose sensor with a lower detection of 1 μM, Xu19 et al. synthesized 3D Ni3S2 nanosheet on Ni foam for nonenzymatic glucose sensor with a high sensitivity of 6148 μA mM−1 cm−2, and Liu23 et al. combined cobalt sulfide with gold nanoparticles for 17β-estradiol detection and with good sensitivity. However, to the best of our knowledge, cobalt sulfide has not been reported to be applied in nonenzymatic glucose sensor yet.
CoS, an important transition metal sulfide, has yielded the greatest returns on investment in the field of dye-sensitized solar cells,24 supercapacitor,25 photocatalysis26 and so on. Nanoflower-like CoS compounds, as their chemical bonds Co–S have several strengths and binding mechanisms, are especially significant to form ionic bonds which are easily bound in a molecule.27 At the same time it can enhance the effective area thus will provide more catalytic active sites. However, as a semiconductor material, CoS has not been widely applied in biosensor technology due to its low conductivity. Therefore, combining the highly electrocatalytic metal sulfide with conductive substrate to enhance the charge transmission will give us a fresh trend to develop a novel sensor. Conductive substrate (such as graphene,28 carbon nanotubes,29 carbon fibers,10 metal foil,8 multi-walled carbon nanotubes,30 conducting polymers31 etc.), either cost too much or have the shortcoming of being poisonous. Therefore searching for a cheap and non-poisonous material is the most important step. For now, more and more biomass materials with enough channels for the transmission of the electrons have been potential candidates to solve this problem in these fields.32–35 All of these biomass materials are easy to be obtained and synthesised simply, are not toxic and environmentally friendly. Meanwhile, these biomass materials with interconnected microporous channels are in favour of electrolyte penetration and ion diffusion by avoiding the boundedness of the onefold porous. In this study, a nanoflower-like CoS-decorated 3D porous carbon skeleton from rose has been simply synthesised by one step of solvothermal synthesis process and applied in the detection of glucose with excellent analytical performance. Thus, it is fit for the international trend of co-friendly environmental production.
2. Experimental
2.1 Reagents
Cobalt nitrate (Co(NO3)2·6H2O), thiourea (CS(NH2)2), NaOH, glucose were purchased from Shanghai chemical reagent Co. Human serum samples were obtained from the school infirmary of Central China Normal University. The roses were bought from the flower market. All aqueous solutions were freshly prepared with distilled water.2.2 Preparation of CoS@C
The 3D continuous porous carbon skeleton of rose (called C) has been prepared as follows: one side of the rose petals was peeled up with tweezers while the other was easily stripped after frozen drying. After being annealed at 800 °C in nitrogen atmosphere, the 3D continuous porous carbon skeleton was obtained. Nanoflower-like CoS-decorated C was prepared through a simple one-pot solvothermal synthesis, 0.655 g (2.25 mmol) Co(NO3)2·6H2O and 0.171 g (2.25 mmol) thiourea were dissolved into 30 mL mixed solution containing 10 mL distilled water and 20 mL ethanol. By continuous stirring for 20 min, a homogeneous mixed solution was obtained and transferred into a 50 mL autoclave immediately. Then, appropriate 15 mg of prepared C (a solid) was immersed into the precursor solution and maintained at 160 °C for 24 h. After the autoclave was cooled down to room temperature naturally, the materials were washed with deionized water and ethanol for several times, and subsequently collected and dried in a vacuum oven at 60 °C for 5 h.2.3 Preparation of Nafion/CoS@C/GCE modified electrode
Prior to the modification, the surface of the glassy carbon electrode (GCE, with a diameter of 3 mm) was polished smoothly by using abrasive paper for metallograph of 2000 molybdenum and 0.05 μM alumina slurry, subsequently sonicated in ethanol and distilled water. After being dried with nitrogen gas, 7 μL of 3 mg mL−1 (the solution is deionized water) CoS@C was coated on the surface of GCE and dried naturally in air. Eventually, 5 μL of Nafion solution was homogeneously dropped on the surface of the electrode and was dried in air. As-prepared electrode was called Nafion/CoS@C/GCE. Other modified electrodes were prepared with similar procedures and used in the experiment for comparison.2.4 Apparatus and measurements
The morphology and the structure of the samples were characterized by field-emission scanning electron microscopy (SEM; JEOL, JSM-6700F), X-ray diffraction (XRD; X'Pert PRO MRD, PANalytical, Netherlands) and Raman spectroscopy (Lab RAMHR evolution, 532 nm). The electrochemical measurements were performed on a Model CHI440A Electrochemical Workstation (Shanghai CH Instruments, China) at room temperature with a three-electrode system using the as-prepared GCE as the working electrode, the saturated calomel electrode (SCE) as the reference electrode and a platinum wire as the counter electrode. All the electrochemical characteristics were measured in 0.1 M NaOH solution except for the electrochemical impedance spectroscope (EIS) testing which was carried out on a PARSTAT 4000 electrochemical workstation in 1.0 mM [Fe(CN)6]3−/4− containing 0.1 M KCl with the frequency range from 0.1 Hz to 100 kHz.3. Results and discussion
3.1 Characterization of bare carbon skeleton and CoS@C samples
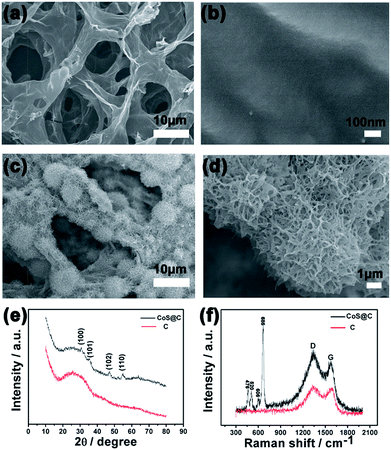
The morphologies of different samples were characterized by SEM at different magnifications. As shown in Fig. 1a, the carbon skeleton derived from rose has the 3D continuous micro-porous structure and pore dimensions are in the range of 15–30 μm. This result indicates that the carbon skeleton can offer enough channels for the electrolyte solution and improve the electron transmission rate. Fig. 1b shows the surface of the bare carbon skeleton is smooth. In contrast, Fig. 1c shows nanoflower-like CoS evenly grew on the surface of carbon skeleton after a solvothermal synthetic process, which endowed the material with highly effective surface area and more active sites. It is noteworthy that the structure of the carbon substrate with massive microporous skeleton was well preserved after the solvent thermal process, which is important for charge transmission and exchange of ions quickly. The high magnification image in Fig. 1d reveals that the CoS subunit nanoflower is made up of great deal of nanogrids through Ostwald ripening mechanism.17Fig. 1e reveals the XRD patterns of bare carbon skeleton and as-prepared CoS@C composite. Comparing with the bare carbon skeleton, the peaks located at 30°, 35.4°, 46.9° and 54.5° in CoS@C curve correspond to (100), (101), (102) and (110) planes of CoS (JCPDS 42-1467), respectively. The result clearly indicates that CoS has been coated successfully on the surface of the carbon skeleton during the solvothermal treatment and reflects purity phase and high crystallinity of the resultant material.
Fig. 1f illustrates typical Raman scattering spectra of the bare carbon skeleton and the as-prepared CoS@C composite. Only two peaks at 1345 and 1598 cm−1 corresponding to the D and G peaks of graphite could be seen in the spectra after the roses were annealed at 800 °C. After solvothermal treatment (CoS@C sample), four obvious peaks at 476, 520, 606 and 685 cm−1 respectively matching the typical phonon modes Eg, F2g1, F2g2 and A1g of CoS materials have been detected.23 All of these peaks recorded that the sample has a pure phase, which is in perfect agreement with the result of XRD.
3.2 Electrochemical characterization
EIS was used to characterize the electrochemical behaviours and the electronic transmission. As shown in Fig. 2, the electron transfer resistance (Ret) value of the bare GCE is about 281 Ω. It is much smaller than the Ret value of Nafion/CoS/GCE, which is about 1384 Ω. This is due to the low-conductivity of CoS. However, when CoS was deposited on the carbon skeleton, the Ret value of Nafion/CoS@C/GCE decreased to 618 Ω, relatively smaller than that of the Nafion/CoS/GCE electrode. It can be attributed to the good electro-conductibility and good electronic transmission of the bare carbon skeleton. Meanwhile, it is indicated that the bare carbon skeleton plays an important role in both transmission of electrons and flow of the electrolyte solution. | ||
| Fig. 2 Nyquist plot of the GCE, Nafion/CoS/GCE and Nafion/CoS@C/GCE composite electrodes in 10 mL 1.0 mM [Fe(CN)6]3−/4− containing 0.1 M KCl with the frequency range from 0.1 Hz to 100 kHz. | ||
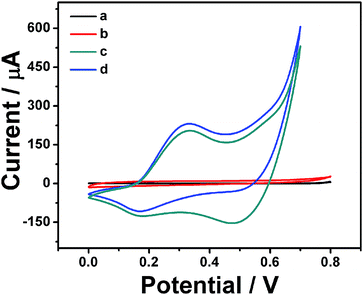
To demonstrate the good effect of CoS@C composite on the detection of glucose, the electrochemistry performance of different modified electrodes has been investigated in 10 mL 0.1 M NaOH solution by cyclic voltammetry (CV). As shown on Fig. 3, there were no peaks on the bare GCE (curve a) and bare carbon skeleton modified GCE (curve b) through cyclic voltammetry, indicating no redox reaction in the 0.1 M NaOH solution. However, after modifying the GCE with CoS@C, two pairs of apparently redox peaks emerged on the CV curve (curve c), illustrating obvious electrocatalytic activity of CoS. Meanwhile, the current of the reduced peak at the potential of +0.45 V on CoS@C modified GCE (curve d) increased sharply when adding 2 mM glucose. It indicates that glucose could be oxidized to glucolactone sharply. These results are strong evidence to prove the capability of the CoS@C to get a desired response for the nonenzymatic glucose sensor.
Fig. 4a shows the CV curves of the Nafion/CoS@C/GCE electrode at different scan rates (20, 40, 60, 80, 100 mV s−1) in 10 mL 0.1 M NaOH solution. With the scan rate increasing, the current enhances regularly with a trend that the anodic response shifts to a less positive value and the cathodic peak moves to a less negative one. Meanwhile the current of redox reaction has a good linear relationship with the square root of the scan rates (Fig. 4b), and the linear relationship between Ipa or Ipc and v1/2 are Ipc = 45.9546 + 47.183v1/2, Ipa = −75.852 − 40.8211v1/2, clearly indicating that the redox reaction is a surface-controlled electrochemical process.36 The 3D continuous carbon skeleton of rose provides an excellent morphology for fastening bioactive molecules and rapid channels for mass transmission.37 To further study the electrochemical catalytic mechanism of the composite, the electrochemical behaviours of different concentrations of glucose have been measured in the 0.1 M NaOH solution. As shown in Fig. 4c, two pairs of redox peaks (0.15 V/0.30 V, 0.45 V/0.55 V) have been found for all the curves, resulting from the transformation between CoS and CoSOH (0.15 V/0.30 V) and the next conversion between CoSOH and CoSO (0.45 V/0.55 V). The electrochemical redox reaction38–40 can be explained as follows:
| CoS + OH− ⇄ CoSOH + e− | (1) |
| CoSOH + OH− ⇄ CoSO + H2O + e− | (2) |
In these reactions, we found that the OH− plays an important role in the redox reaction. Furthermore, the current increased obviously when the different concentrations of glucose were added into the solution, demonstrating that the nanoflower-like CoS coated on 3D carbon skeleton of rose has an ideal electrocatalytic performance that can transform the glucose into glucolactone by conversion of Co4+ to Co3+ (0.45 V/0.55 V) following the equation:
| Co4+ + glucose →Co3+ + glucolactone | (3) |
Meanwhile it is also indicated that the bare carbon skeleton provides a large amount of high conductive pathways suitable for the transmission of electrons and that the cobalt sulfide offers a great deal of surface active sites to enhance the catalytic rate for the nonenzymatic glucose sensor.
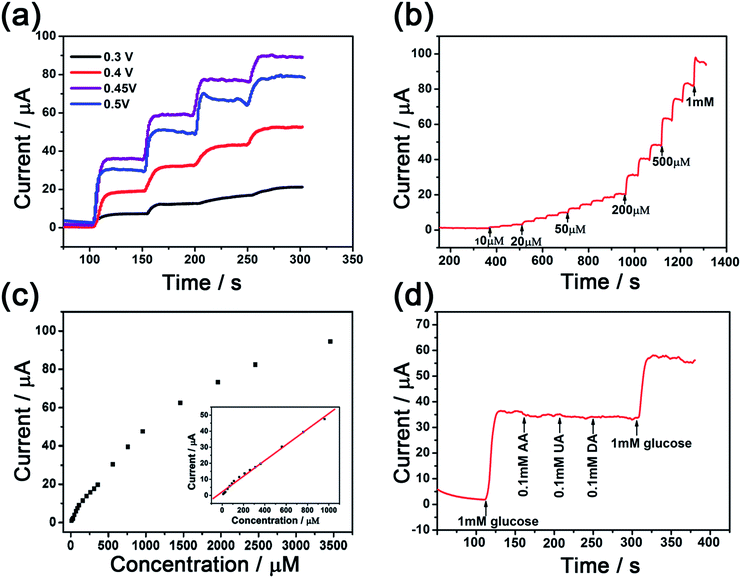
In order to find out the best potential for glucose detection, different potentials (from 0.3 V to 0.5 V) with a continuous addition of 1 mM glucose into 10 mL 0.1 M NaOH at 50 s intervals have been investigated and the results are shown in Fig. 5a. From 0.3 V to 0.45 V, the current responses increased regularly, while the current responses decreased and became unstable when the potential increased from 0.45 V to 0.5 V, which was due to the effect of a higher background current. From 0.3 V to 0.5 V, the change of the current step confessed that 0.45 V where the highest current step has been obtained is the best potential for the further determinations of the glucose. Comparing with other cobalt oxide composites applied in nonenzymatic glucose sensing,41 the potential has been reduced obviously in the anodic overpotential, suggesting a high electrocatalytic activity of the CoS@C nanocomposite in the direct oxidation of glucose. It is sure about that the overpotential shifted negatively42 can be attributed to a kinetic effect43 which we can attribute to the good platform of bare carbon skeleton that can improve the rate of the transmission of the electrons. At the same time, the step change of the current reduced slowly every time owing to the active sites of the composite having been conquered by the added glucose, which also means that it is a surface controlled process. Fig. 5b shows the amperometric response of Nafion/CoS@C/GCE with different concentrations of glucose added into 10 mL 0.1 M NaOH with successively stirring at a working potential of +0.45 V. The glucose has been detected at a short time within 3 s. The corresponding calibration versus glucose concentration has been shown in Fig. 5c. Inset is the calibration curve of the linear relationship from 10 μM to 960 μM, it is calculated that the sensitivity is about 697 μA mM−1 cm−2 with the linear equation: I (μA) = 2.2133 + 0.0488C (μM) (R = 0.997) and the limit of detection (LOD) is 2 μM at the signal-to-noise ratio (S/N = 3). Comparing with other Co-based nonenzymatic glucose sensors, such as 471.5 μA mM−1 cm−2 for 3D hierarchical porous cobalt oxide,34 13.52 μA mM−1 cm−2 for Co3O4 nanoparticles,44 669.78 μA mM−1 cm−2 for cobalt-oxide nanostructures42 and 162.8 μA mM−1 cm−2 for cobalt oxide/hydroxide nanostructures,45 the sensitivity of CoS@C is higher than these Co-based sensors. This result further illustrated that connecting the bare carbon skeleton with cobalt sulfide not only can improve the sensitivity which is attributed to the large area and amount of active sites of the cobalt sulfide, but also offer more convenient channels for the transmission of electrolyte and ion diffusion owing to the interconnected microporous structure of the bare carbon skeleton.
3.3 Reproducibility, stability, and anti-interference property of the Nafion/CoS@C/GCE electrode
The current of responses after adding 1 mM glucose into a 10 mL 0.1 M NaOH solution was measured to examine the reproducibility of the Nafion/CoS@C/GCE electrode. A relative standard deviation (RSD) of 2.3% has been obtained on the response current with eight successive measurements. To further demonstrate the reproducibility of the composite, four same electrodes were prepared and a RSD of 3.6% has been gotten. After being stored at 4 °C for two weeks, the response current of this glucose biosensor retained 92.6% of its initial response current indicating good stability. At the same time, to further satisfy the detection of glucose in human blood or food exactly, ascorbic acid (AA), uric acid (UA) and dopamine (DA) as the interferences have been tested (Fig. 5d). At first, 1 mM glucose had been added in 10 mL 0.1 M NaOH with successively stirring at the working potential of +0.45 V, subsequently the current enhanced quickly to form an obvious current step. Then AA, UA, DA were added at 50 s intervals, the current did not change very clearly, indicating that the material does have an effect on detecting glucose without other interferences and that the sensor exhibits good selectivity resulting from AA, UA, DA have the repelling effect on both Nafion and CoS@C composite34 in 0.1 M NaOH solution.3.4 Real sample analysis
In order to evaluate the accuracy of this fabricated sensor, the glucose in human serum samples has been detected by this method and a blood sugar analyzer (SBA-40C). Three serum samples obtained from the hospital of the University have been added in 10 mL 0.1 M NaOH solution and detected respectively. As shown in Table 1, the recovery rates are from 96.3% to 98.7% and the RSD are satisfactory, which demonstrated the feasibility of measurements in real serum samples.4. Conclusion
In summary, CoS was synthesized through a facial one-pot solvothermal synthesis process, using 3D continuous porous carbon skeleton from rose as the substrate. It is the first time that CoS was applied in the detection of nonenzymatic glucose sensor. On the other hand, the 3D continuous porous carbon skeleton from rose not only offered enough channels for mass transfer, but also improved the conductivity of the material. All of these features can evidently enhance the current of the detection of glucose. Meanwhile, nanoflower-like cobalt sulfide with large effective surface areas can offer more active sites to further increase the catalysis of the oxidation of glucose. Therefore the CoS@C offers the potential to achieve continuous glucose monitoring, good stability and stable reproducibility. As the nanoflower-like structure of CoS provided abundant highly electrocatalytic active sites and the carbon skeleton of rose featured plenty of interconnected channels, the CoS@C material applied for glucose detection is promising for the international trend of co-friendly environmental production especially in the blood or the food industry.Acknowledgements
This work was financially supported by self-determined research funds of CCNU from the colleges' basic research and operation of MOE (No. CCNU 15A02035 and CCNU 15DZ007), the Key Scientific Project of Wuhan City (No. 2013011801010598), the Scientific Project of AQSIQ (No. 2013IK093) and the National Natural Science Foundation of China (No. 50802032 and 11275082), as well as self-determined research funds of WHU from the colleges' basic research and operation of MOE (2042014gf048).Notes and references
- N. J. Ronkainen, H. B. Halsall and W. R. Heineman, Chem. Soc. Rev., 2010, 39, 1747 RSC.
- R. J. Chen, H. C. Choi, S. Bangsaruntip, E. Yenilmez, X. Tang, Q. Wang, Y.-L. Chang and H. Dai, J. Am. Chem. Soc., 2004, 126, 1563 CrossRef CAS PubMed.
- K. Tian, M. Prestgard and A. Tiwari, Mater. Sci. Eng., C, 2014, 41, 100 CrossRef CAS PubMed.
- A. Tarlani, M. Fallah, B. Lotfi, A. Khazraei, S. Golsanamlou, J. Muzart and M. Mirza-Aghayan, Biosens. Bioelectron., 2015, 67, 601 CrossRef CAS PubMed.
- B. K. Jena and C. R. Raj, Chem.–Eur. J., 2006, 12, 2702 CrossRef CAS PubMed.
- H. Qiu and X. Huang, J. Electroanal. Chem., 2010, 643, 39 CrossRef CAS.
- L. M. Lu, L. Zhang, F. L. Qu, H. X. Lu, X. B. Zhang, Z. S. Wu, S. Y. Huan, Q. A. Wang, G. L. Shen and R. Q. Yu, Biosens. Bioelectron., 2009, 25, 218 CrossRef CAS PubMed.
- G. Li, X. Wang, L. Liu, R. Liu, F. Shen, Z. Cui, W. Chen and T. Zhang, Small, 2015, 11, 731 CrossRef CAS PubMed.
- L. C. Jiang and W. D. Zhang, Biosens. Bioelectron., 2010, 25, 1402 CrossRef CAS.
- T. Chen, X. Li, C. Qiu, W. Zhu, H. Ma, S. Chen and O. Meng, Biosens. Bioelectron., 2014, 53, 200 CrossRef CAS PubMed.
- Y. Ke, Y. Zeng, X. Pu, X. Wu, L. Li, Z. Zhu and Y. Yu, RSC Adv., 2012, 2, 5676 RSC.
- Y. Yamauchi, A. Tonegawa, M. Komatsu, H. Wang, L. Wang, Y. Nemoto, N. Suzuki and K. Kuroda, J. Am. Chem. Soc., 2012, 134, 5100 CrossRef CAS PubMed.
- Y. Bai, Y. Sun and C. Sun, Biosens. Bioelectron., 2008, 24, 579 CrossRef CAS PubMed.
- H. Gao, F. Xiao, C. B. Ching and H. Duan, ACS Appl. Mater. Interfaces, 2011, 3, 3049 CAS.
- X. Bo, J. Bai, L. Yang and L. Guo, Sens. Actuators, B, 2011, 157, 662 CrossRef CAS.
- Y. Mu, D. Jia, Y. He, Y. Miao and H.-L. Wu, Biosens. Bioelectron., 2011, 26, 2948 CrossRef CAS PubMed.
- W. Dong, X. Wang, B. Li, L. Wang, B. Chen, C. Li, X. Li, T. Zhang and Z. Shi, Dalton Trans., 2011, 40, 243 RSC.
- J. Cheng, Y. Pan, J. Zhu, Z. Li, J. Pan and Z. Ma, J. Power Sources, 2014, 257, 192 CrossRef CAS.
- H. Huo, Y. Zhao and C. Xu, J. Mater. Chem. A, 2014, 2, 15111 CAS.
- H. Li, K. Yu, X. Lei, B. Guo, C. Li, H. Fu and Z. Zhu, Dalton Trans., 2015, 44, 10438 RSC.
- Y. J. Yang, J. Zi and W. Li, Electrochim. Acta, 2014, 115, 126 CrossRef CAS.
- S. Radhakrishnan and S. J. Kim, RSC Adv., 2015, 5, 44346 RSC.
- K. J. Huang, Y. J. Liu, J. Z. Zhang, J. T. Cao and Y. M. Liu, Biosens. Bioelectron., 2015, 67, 184 CrossRef CAS.
- J. Y. Lin, J. H. Liao and T. Y. Hung, Electrochem. Commun., 2011, 13, 977 CrossRef CAS.
- Q. Zhang, C. Xu and B. Lu, Electrochim. Acta, 2014, 132, 180 CrossRef CAS.
- S. Kong, Z. Jin, H. Liu and Y. Wang, J. Phys. Chem. C, 2014, 118, 25355 CAS.
- K. J. Huang, J. Z. Zhang, G. W. Shi and Y. M. Liu, Mater. Lett., 2014, 131, 45 CrossRef CAS.
- S. Wu, Q. He, C. Tan, Y. Wang and H. Zhang, Small, 2013, 9, 1160 CrossRef CAS PubMed.
- M. Choudhary, S. K. Shukla, A. Taher, S. Siwal and K. Mallick, ACS Sustainable Chem. Eng., 2014, 2, 2852 CrossRef CAS.
- H. Nie, Z. Yao, X. Zhou, Z. Yang and S. Huang, Biosens. Bioelectron., 2011, 30, 28 CrossRef CAS PubMed.
- J. Wu and L. Yin, ACS Appl. Mater. Interfaces, 2011, 3, 4354 CAS.
- Z. Li, L. Zhang, B. S. Amirkhiz, X. Tan, Z. Xu, H. Wang, B. C. Olsen, C. M. B. Holt and D. Mitlin, Adv. Energy Mater., 2012, 2, 431 CrossRef CAS.
- W. Xiong, Y. Gao, X. Wu, X. Hu, D. Lan, Y. Chen, X. Pu, Y. Zeng, J. Su and Z. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 19416 CAS.
- L. Han, D. P. Yang and A. Liu, Biosens. Bioelectron., 2015, 63, 145 CrossRef CAS.
- Z. H. Zhu, W. Xiong, X. Hu, X. Wu, Y. Zeng, B. Wang and G. He, J. Mater. Chem. A, 2015, 3, 17209 Search PubMed.
- X. Dong, X. Wang, L. Wang, H. Song, H. Zhang, W. Huang and P. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 3129 CAS.
- Y. Ma, M. Zhao, B. Cai, W. Wang, Z. Ye and J. Huang, Biosens. Bioelectron., 2014, 59, 384 CrossRef CAS PubMed.
- J. Xu, Q. F. Wang, X. W. Wang, Q. Y. Xiang, B. Liang and G. Z. Shen, ACS Nano, 2013, 6, 5453 CrossRef PubMed.
- J. Y. Lin and S. W. Chou, RSC Adv., 2013, 3, 2043 RSC.
- C. Y. Chen, Z. Y. Shih, Z. Yang and H. T. Chang, J. Power Sources, 2012, 215, 43 CrossRef CAS.
- S. J. Li, J. M. Du, J. Chen, N. N. Mao, M. J. Zhang and H. Pang, J. Solid State Electrochem., 2013, 18, 1049 CrossRef.
- S. Ci, S. Mao, T. Huang, Z. Wen, D. A. Steeber and J. Chen, Electroanalysis, 2014, 26, 1326 CrossRef CAS.
- M. F. Wang, Q. A. Huang, X. Z. Li and Y. Wei, Anal. Methods, 2012, 4, 3174 RSC.
- C. Karuppiah, S. Palanisamy, S.-M. Chen, V. Veeramani and P. Periakaruppan, Sens. Actuators, B, 2014, 196, 450 CrossRef CAS.
- A. Salimi, R. Hallaj and S. Soltanian, Electroanalysis, 2009, 21, 2693 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |