Nitro-based selective inhibitors against matrix metalloproteinase-7 over matrix metalloproteinase-1†
Mei-Hua Lia,
Yan-Feng Zhanga,
Hong-Rui Tianb,
Ming-Hua Zheng*a,
Ming-Yang Yanga,
Hu-Lin Fangb,
Yu-Zhong Xieac and
Jing-Yi Jin*b
aDepartment of Chemistry, College of Science, Yanbian University, Yanji City, Jilin Province 133002, China. E-mail: mhzheng@ybu.edu.cn; Fax: +86-4332732242; Tel: +86-433-2732449
bKey Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Ministry of Education, Yanbian University, Yanji City, Jilin Province 133002, China. E-mail: jyjin-chem@ybu.edu.cm; Fax: +86-4332732456; Tel: +86-4332733405
cCollege of Chemistry, Northeast Normal University, Changchun, Jilin Province 130024, China
First published on 27th November 2015
Abstract
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases, which catalyze the cleavage of the extracellular matrix and thus are tightly associated with various physiological and pathological processes. The MMP family contains at least 28 members, with only MMP-1, -2 and -7 experimentally validated as targets for cancer therapy. As reported in previous studies, the inhibition of MMP-1 could result in various side effects, including musculoskeletal syndrome, which should be avoided. However, “broad spectrum” inhibitors, which typically utilize hydroxamic acid as a strong zinc-binding group (ZBG), have been unapproved due to their lack of selectivity. Further, because both MMP-1 and -7 have similar S′1 hydrophobic pockets, it remains a challenge to explore the selective inhibition of MMP-7 over -1 up to now. In the presented paper, we firstly introduced a nitro group as a ZBG in the inhibition of MMP. A series of nitro-based dipeptidic compounds were thus synthesized and evaluated as MMP inhibitors. Combined with the kinetic assay results and computational analysis of the binding mode of the inhibitors with the active sites of the enzymes, it was disclosed that a reasonable adjustment of the P′3 side chains of the nitro-based MMP inhibitors could improve selectivity for the inhibition of MMP-7 over MMP-1.
Introduction
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases that play key roles in tissue remodeling associated with various physiological and pathological processes such as inflammation, multiple sclerosis, cancer, and so on.1 Inhibitors against MMPs have been thus extensively pursued as promising drug candidates for various pharmaceutical applications including cancer therapy.2 Among the 28 members of the MMP family, MMP-1, MMP-2 and MMP-7 have been validated as anticancer drug targets as suggested by Overall et al.3 This means that the MMP inhibitors directed to cancer therapy should selectively inhibit the three MMPs. However, most MMP inhibitors of the first generation utilized hydroxamic acid (HA) as the zinc binding group (ZBG), which generally resulted in “broad spectrum” inhibition, due to the strong chelation ability of HA.4 Typically, a broad-based inhibitor (Marimastat, Chart 1) could inhibit various MMPs and result in various side effects including severe musculoskeletal pain.5 MMP-1 inhibition was then suggested as the origin of the side effects and should be thus avoided.6 The failure of the reported MMP inhibitors in clinical trials might be ascribed to the deficiency of selectivity. The application of non-HA ZBGs was then suggested to improve the selectivity of the MMP inhibitors.2d In summary, it is necessary to develop selective MMP-2 or MMP-7 inhibitors, sparing MMP-1, and utilizing a non-HA ZBG such as that exemplified in the selective inhibition of MMP-2.7In the presented paper, we choose MMP-7 as the target enzyme because MMP-7 has been identified as the specific enzyme secreted by tumor cells, and could induce the evolution of tumor cells to cancer cells.8 On the other hand, a nitro group is utilized as the ZBG, which has been successfully introduced in the inhibition of other zinc-proteases.9 Here we hope to present our efforts to develop nitro-based inhibitors against MMP-7 over MMP-1.
For the design rationale for MMP inhibitors, many reported MMP inhibitors possess a ZBG attached to a peptide framework that binds to the “primed” binding regions,10 where the depth of the S′1 pocket is regarded as the key determinant for discrimination of various MMPs.11 Thus, a general approach to selective MMP inhibition concentrated on the size of the P′1 side chain of MMP inhibitors to satisfy the “so-called” S′1 specificity for different MMPs. However, because both MMP-1 and MMP-7 have similar S′1 pockets,2a,12 it seems infeasible that optimization of the structures of the P′1 side chains of the inhibitors could obtain the selective inhibition of MMP-7 over MMP-1, which may be the reason that selective inhibition of MMP-7 has been rarely reported until now.
According to the suggested ranking of the MMP sub-pockets based on the possibility of achieving selective interactions: S′1 > S′3 > S′2,13 we then designed a series of nitro-based dipeptide compounds 1a–d (Chart 1), which possessed different P′3 side chains, to investigate their possible selective inhibition of MMP-7 over MMP-1.
Experimental
Materials and methods
Unless otherwise noted, all chemicals were commercially available and used as received without further purification.Flash chromatography was performed with 100–200 mesh silica gel (Qingdao, China) and thin-layer chromatography (TLC) was carried out on silica coated glass sheets (Qingdao silica gel 60 F-254). Melting points were measured using a Thomas-Hoover capillary melting point apparatus and were uncorrected. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded with a Bruker AV 300 (300 MHz) instrument using tetramethylsilane as the internal standard. IR spectra were recorded on a Perkin-Elmer 1300 FT-IR spectrometer. High-resolution mass spectra were taken on a Shimadzu GC-MS-QP 2010. Elemental analyses were performed at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, China.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VTHF = 3
VTHF = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) followed by the addition of 1 mL of aqueous LiOH (20 mg, 0.84 mmol). The reaction mixture was kept stirring at room temperature for 6 hours. The reaction was quenched by the addition of 5 mL of water and extracted with ether (5 mL × 2). The remaining water layer was acidified to pH = 1 with 10% citric acid solution at 0 °C. After extracting with ether (5 mL × 5), the combined organic layer was dried over anhydrous Na2SO4. Evaporation under reduced pressure provided an oil as compound 3 (88.1 mg), which was then used in the subsequent esterification without further purification and characterization.
1) followed by the addition of 1 mL of aqueous LiOH (20 mg, 0.84 mmol). The reaction mixture was kept stirring at room temperature for 6 hours. The reaction was quenched by the addition of 5 mL of water and extracted with ether (5 mL × 2). The remaining water layer was acidified to pH = 1 with 10% citric acid solution at 0 °C. After extracting with ether (5 mL × 5), the combined organic layer was dried over anhydrous Na2SO4. Evaporation under reduced pressure provided an oil as compound 3 (88.1 mg), which was then used in the subsequent esterification without further purification and characterization.To the solution of CH2Cl2 containing compound 3, DCC (1.1 eq.) was added at 0 °C. After stirring for 40 minutes, a solution of CH2Cl2 containing p-nitro phenol (1.0 eq.) was added. The reaction mixture was then kept stirring at room temperature overnight. The reaction mixture was poured into a 5% NaHCO3 solution and extracted with CH2Cl2. The combined organic layer was dried over anhydrous Na2SO4. Evaporation under reduced pressure gave an oil, which was then purified by flash column chromatography (silica gel, n-hexane/EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide an oil as the product. Yield: 72.4%. [α]18D = −24.6° (c 2.00, CHCl3). IR (film): 866, 1012, 1113, 1161, 1192, 1346, 1489, 1524, 1591, 1614, 1765 cm−1. 1H NMR (300 MHz, CDCl3) δ: 1.01 (d, J = 6.4 Hz, 3H), 1.03 (d, J = 6.4 Hz, 3H), 1.59–1.63 (m, 1H), 1.73–1.92 (m, 2H), 2.48–2.57 (m, 1H), 2.68 (dd, J = 2.6, 10.4 Hz, 1H), 2.94 (dd, J = 4.0, 10.4 Hz, 1H), 3.24–3.29 (m, 1H), 7.33 (d, J = 9.0 Hz, 2H), 8.31 (d, J = 9.0 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ: 22.01, 22.48, 25.91, 37.80, 46.49, 46.88, 52.50, 122.46, 125.00, 145.41, 154.80, 171.04. HRMS calcd for C14H17O5: 279.1107. Found: 279.1112.
1) to provide an oil as the product. Yield: 72.4%. [α]18D = −24.6° (c 2.00, CHCl3). IR (film): 866, 1012, 1113, 1161, 1192, 1346, 1489, 1524, 1591, 1614, 1765 cm−1. 1H NMR (300 MHz, CDCl3) δ: 1.01 (d, J = 6.4 Hz, 3H), 1.03 (d, J = 6.4 Hz, 3H), 1.59–1.63 (m, 1H), 1.73–1.92 (m, 2H), 2.48–2.57 (m, 1H), 2.68 (dd, J = 2.6, 10.4 Hz, 1H), 2.94 (dd, J = 4.0, 10.4 Hz, 1H), 3.24–3.29 (m, 1H), 7.33 (d, J = 9.0 Hz, 2H), 8.31 (d, J = 9.0 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ: 22.01, 22.48, 25.91, 37.80, 46.49, 46.88, 52.50, 122.46, 125.00, 145.41, 154.80, 171.04. HRMS calcd for C14H17O5: 279.1107. Found: 279.1112.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide an oil as the product. Yield: 78.1%. [α]18D = −35.7° (c 1.52, CHCl3). IR (film): 1113, 1207, 1348, 1526, 1593, 1616, 1757 cm−1. 1H NMR (300 MHz, CDCl3) δ: 1.02 (d, J = 6.4 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 1.35–1.44 (m, 1H), 1.68–1.88 (m, 2H), 2.69 (d, J = 6.4 Hz, 1H), 3.01–3.03 (m, 1H), 3.62 (dd, J = 5.8, 10.8 Hz, 1H), 3.72 (dd, J = 5.6, 10.8 Hz, 1H), 4.04–4.07 (m, 1H), 7.32 (d, J = 9.0 Hz, 2H), 8.31 (d, J = 9.0 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ: 21.65, 23.32, 26.43, 37.49, 38.14, 48.47, 72.12, 122.28, 125.28, 145.53, 155.21, 172.08. HRMS calcd for C14H18BrNO5: 359.0368. Found: 359.0377.
1) to provide an oil as the product. Yield: 78.1%. [α]18D = −35.7° (c 1.52, CHCl3). IR (film): 1113, 1207, 1348, 1526, 1593, 1616, 1757 cm−1. 1H NMR (300 MHz, CDCl3) δ: 1.02 (d, J = 6.4 Hz, 3H), 1.05 (d, J = 6.4 Hz, 3H), 1.35–1.44 (m, 1H), 1.68–1.88 (m, 2H), 2.69 (d, J = 6.4 Hz, 1H), 3.01–3.03 (m, 1H), 3.62 (dd, J = 5.8, 10.8 Hz, 1H), 3.72 (dd, J = 5.6, 10.8 Hz, 1H), 4.04–4.07 (m, 1H), 7.32 (d, J = 9.0 Hz, 2H), 8.31 (d, J = 9.0 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ: 21.65, 23.32, 26.43, 37.49, 38.14, 48.47, 72.12, 122.28, 125.28, 145.53, 155.21, 172.08. HRMS calcd for C14H18BrNO5: 359.0368. Found: 359.0377.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 6.
1) to give compound 6.
[(2′R,3′S)-4′-Bromo-2′-iso-butyl-3′-hydroxy]butyryl-(S)-tert-leucyl-phenylamide (6a). Oil. [α]19D = −22.2° (c 1.52, CHCl3). IR (film): 755, 877, 1023, 1248, 1442, 1469, 1488, 1546, 1647, 1688, 2980, 3298 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.88 (d, J = 6.4 Hz, 3H), 0.93 (d, J = 6.4 Hz, 3H), 1.12 (s, 9H), 1.59–1.68 (m, 3H), 2.78–2.83 (m, 1H), 3.33 (dd, J = 8.0, 10.8 Hz, 1H), 3.58 (dd, J = 6.4, 10.8 Hz, 1H), 4.02–4.06 (m, 1H), 4.46 (d, J = 9.0 Hz, 1H), 6.83 (d, J = 6.8 Hz, 1H), 7.09–7.34 (m, 5H), 7.50 (d, J = 9.0 Hz, 1H), 7.97 (s, 1H). 13C NMR (75 MHz, CDCl3) δ: 18.67, 22.38, 25.07, 26.72, 34.87, 35.59, 39.04, 46.76, 64.77, 120.21, 124.72, 129.00, 137.23, 168.81, 174.80. HRMS calcd for C20H31BrN2O3: 426.1518. Found: 426.1516. Anal. C20H31BrN2O3 requires C, 56.21; H, 7.31; N, 6.55. Found: C, 56.57; H, 7.26; N, 7.26.
[(2′R,3′S)-4′-Bromo-2′-iso-butyl-3′-hydroxy]butyryl-(S)-tert-leucyl-[(1′′S)-phenyl]-ethylamide (6b). M.p.: 156–157.5 °C. [α]19D = −28.8° (c 1.34, CHCl3). IR (film): 688, 700, 768, 1114, 1215, 1530, 1643, 1696, 2897, 2963, 3302 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.93 (d, J = 6.4 Hz, 3H), 0.95 (d, J = 6.4 Hz, 3H), 0.97 (s, 9H), 1.50 (d, J = 6.4 Hz, 3H), 1.55–1.65 (m, 2H), 1.70–1.76 (m, 1H), 2.75–2.81 (m, 1H), 3.27 (dd, J = 7.6, 9.8 Hz, 1H), 3.54 (dd, J = 4.6, 9.8 Hz, 1H), 3.85–3.89 (m, 1H), 4.12 (d, J = 6.2 Hz, 1H), 4.14–4.20 (m, 1H), 5.12 (d, J = 7.4 Hz, 1H), 5.86 (d, J = 6.2 Hz, 1H), 6.54 (d, J = 9.6 Hz, 1H), 7.32–7.37 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 21.36, 22.48, 22.71, 25.79, 26.67, 34.57, 35.32, 39.11, 46.48, 49.08, 60.81, 72.26, 126.28, 127.55, 128.71, 142.57, 169.18, 174.55. HRMS calcd for C22H35BrN2O3: 454.1831. Found: 454.1836. Anal. C22H35BrN2O3 requires C, 58.02; H, 7.75; N, 6.15. Found: C, 58.19; H, 7.69; N, 5.97.
[(2′R,3′S)-4′-Bromo-2′-iso-butyl-3′-hydroxy]butyryl-(S)-tert-leucyl-[(1′′R)-phenyl]-ethylamide (6c). M.p.: 162–163 °C. [α]23D = +31.9° (c 1.20, CHCl3). IR (film): 1110, 1215, 1496, 1517, 1641, 1647, 2960, 3018, 3305 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.84 (d, J = 6.4 Hz, 3H), 0.88 (d, J = 6.4 Hz, 3H), 1.07 (s, 9H), 1.43–1.49 (m, 2H), 1.50 (d, J = 6.4 Hz, 3H), 1.67–1.71 (m, 1H), 2.70–2.75 (m, 1H), 3.22 (dd, J = 7.4, 10.0 Hz, 1H), 3.46 (dd, J = 4.4, 10.0 Hz, 1H), 3.82–3.85 (m, 1H), 4.14 (d, J = 6.8 Hz, 1H), 4.16–4.20 (m, 1H), 5.12 (d, J = 7.8 Hz, 1H), 5.97 (d, J = 7.8 Hz, 1H), 6.53 (d, J = 9.2 Hz, 1H), 7.29–7.34 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 21.62, 22.30, 22.73, 25.68, 25.71, 34.53, 35.29, 39.02, 46.43, 48.98, 72.23, 126.02, 127.42, 128.69, 142.53, 169.17, 174.46. HRMS calcd for C22H35BrN2O3: 454.1831. Found: 454.1842. Anal. C22H35BrN2O3 requires C, 58.02; H, 7.75; N, 6.15. Found: C, 58.16; H, 7.73; N, 6.58.
[(2′R,3′S)-4′-Bromo-2′-iso-butyl-3′-hydroxy]butyryl-(S)-tert-leucyl-[(1′′,1′′)-diphenyl]-methylamide (6d). M.p.: 167–168 °C. [α]18D = −18.3° (c 1.48, CHCl3). IR (film): 689, 700, 796, 1215, 1495, 1518, 1643, 2961, 3294 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.90 (d, J = 6.4 Hz, 3H), 0.92 (d, J = 6.4 Hz, 3H), 1.04 (s, 9H), 1.43–1.55 (m, 2H), 1.67–1.75 (m, 1H), 2.70–2.74 (m, 1H), 3.23 (dd, J = 8.0, 10.2 Hz, 1H), 3.47 (dd, J = 5.8, 10.2 Hz, 1H), 3.82–3.87 (m, 1H), 4.02 (d, J = 8.0 Hz, 1H), 4.27 (d, J = 9.4 Hz, 1H), 6.23 (d, J = 8.0 Hz, 1H), 6.33 (d, J = 8.0 Hz, 1H), 6.52 (d, J = 9.4 Hz, 1H), 7.19–7.25 (m, 5H), 7.30–7.36 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 22.31, 22.82, 25.71, 26.67, 34.61, 35.42, 39.03, 46.57, 57.19, 60.85, 72.21, 127.13, 127.49, 127.63, 127.72, 128.63, 128.79, 140.79, 141.03, 168.45, 174.49. HRMS calcd for C27H37BrN2O3: 516.1988. Found: 516.1992. Anal. C27H37BrN2O3 requires C, 62.67; H, 7.21; N, 5.41. Found: C, 62.71; H, 7.27; N, 5.66.
[(2′R,3′S)-4′-Bromo-2′-iso-butyl-3′-hydroxy]butyryl-(S)-tert-leucyl-methylamide (6e). Oil. [α]21D = −10.6° (c 0.58, CHCl3). IR (film): 753, 806, 1310, 1497, 1587, 1614, 2954, 3491 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.84 (d, J = 6.4 Hz, 3H), 0.88 (d, J = 6.4 Hz, 3H), 0.95 (s, 9H), 1.45–1.62 (m, 3H), 2.31–2.52 (m, 1H), 2.71 (d, J = 9.2 Hz, 3H), 3.24 (dd, J = 7.6, 10.0 Hz, 1H), 3.43 (dd, J = 6.8, 10.0 Hz, 1H), 3.91–4.05 (m, 1H), 4.14 (d, J = 7.0 Hz, 1H), 5.27 (br, 1H), 6.33 (d, J = 7.0 Hz, 1H), 6.58 (d, J = 7.2 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ: 22.42, 23.60, 25.79, 27.02, 34.66, 35.38, 38.98, 46.72, 58.92, 65.72, 169.50, 173.13. HRMS calcd for C15H29BrN2O3: 364.1362. Found: 364.1368. Anal. C15H29BrN2O3 requires C, 49.32; H, 8.00; N, 7.67. Found: C, 49.36; H, 8.05; N, 8.13.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 1.
1) to give compound 1.
[(2′R,3′S)-2′-Iso-butyl-3′-hydroxy-4′-nitro]butyryl-(S)-tert-leucyl-phenylamide (1a). M.p.: 155–156 °C. [α]23D = −26.4° (c 0.88, CHCl3). IR (film): 669, 798, 1080, 1147, 1211, 1310, 1378, 1454, 1539, 1578, 1643, 1661, 2872, 2962, 3315 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.87 (d, J = 6.4 Hz, 3H), 0.89 (d, J = 6.4 Hz, 3H), 1.12 (s, 9H), 1.53–1.63 (m, 3H), 2.49–2.54 (m, 1H), 3.80–3.84 (m, 1H), 4.32–4.67 (m, 4H), 6.99 (d, J = 8.8 Hz, 1H), 7.11–7.15 (m, 1H), 7.31–7.35 (m, 2H), 7.45–7.49 (m, 2H), 7.96 (s, 1H). 13C NMR (75 MHz, CDCl3) δ: 22.16, 22.64, 25.63, 26.75, 34.71, 38.69, 47.25, 61.68, 69.53, 79.15, 120.27, 124.88, 129.03, 137.05, 169.03, 174.22. HRMS calcd for C20H31N3O5: 393.2264. Found: 393.2270. Anal. C20H31N3O5 requires C, 61.05; H, 7.94; N, 10.68. Found: C, 61.09; H, 8.01; N, 11.15.
[(2′R,3′S)-2′-Iso-butyl-3′-hydroxy-4′-nitro]butyryl-(S)-tert-leucyl-[(1′′S)-phenyl]-ethylamide (1b). M.p.: 163–164 °C. [α]19D = −26.5° (c 0.56, CHCl3). IR (film): 669, 798, 1080, 1147, 1211, 1310, 1378, 1454, 1539, 1578, 1643, 1661, 2872, 2962, 3315 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.91 (d, J = 6.4 Hz, 3H), 0.95 (d, J = 6.4 Hz, 3H), 0.96 (s, 9H), 1.41–1.47 (m, 1H), 1.51 (d, J = 6.8 Hz, 3H), 1.65–1.72 (m, 1H), 1.85–1.94 (m, 1H), 2.37–2.40 (m, 1H), 4.14 (d, J = 9.0 Hz, 1H), 4.31–4.42 (m, 3H), 4.48–4.53 (m, 1H), 5.01 (dd, J = 7.0, 12.8 Hz, 1H), 5.07–5.14 (m, 1H), 5.96 (d, J = 9.0 Hz, 1H), 6.62 (d, J = 9.4 Hz, 1H), 7.31–7.37 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 21.29, 22.37, 22.68, 25.72, 26.69, 34.50, 38.74, 46.93, 61.16, 69.63, 79.18, 125.28, 127.65, 128.76, 142.42, 169.18, 173.93. HRMS calcd for C22H35N3O5: 421.2577. Found: 421.2572. Anal. C22H35N3O5 requires C, 62.69; H, 8.37; N, 9.97. Found: C, 62.77; H, 8.34; N, 10.05.
[(2′R,3′S)-2′-Iso-butyl-3′-hydroxy-4′-nitro]butyryl-(S)-tert-leucyl-[(1′′R)-phenyl]-ethylamide (1c). M.p.: 145–146 °C. [α]23D = +42.6° (c 0.62, CHCl3). IR (film): 700, 759, 1022, 1093, 1211, 1238, 1259, 1371, 1384, 1537, 1633, 1643, 2872, 2928, 3309 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.85 (d, J = 6.4 Hz, 3H), 0.87 (d, J = 6.4 Hz, 3H), 1.05 (s, 9H), 1.41–1.47 (m, 1H), 1.52 (d, J = 7.0 Hz, 3H), 1.60–1.71 (m, 1H), 1.85–1.91 (m, 1H), 2.37–2.40 (m, 1H), 4.19 (d, J = 9.0 Hz, 1H), 4.31–4.42 (m, 3H), 4.48–4.53 (m, 1H), 5.10 (dd, J = 7.0, 12.8 Hz, 1H), 6.31 (d, J = 9.0 Hz, 1H), 6.75 (d, J = 9.0 Hz, 1H), 7.22–7.34 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 21.62, 22.19, 22.74, 25.72, 34.42, 38.64, 47.23, 49.11, 61.03, 79.16, 126.05, 127.49, 128.68, 142.51, 169.34, 173.80. HRMS calcd for C22H35N3O5: 421.2577. Found: 421.2581. Anal. C22H35N3O5 requires C, 62.69; H, 8.37; N, 9.97. Found: C, 62.62; H, 8.34; N, 9.38.
[(2′R,3′S)-2′-Iso-butyl-3′-hydroxy-4′-nitro]butyryl-(S)-tert-leucyl-[(1′′,1′′)-diphenyl]-methylamide (1d). M.p.: 159–161 °C. [α]21D = −23.6° (c 0.68, CHCl3). IR (film): 785, 1217, 1389, 1500, 1631, 2962, 3314 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.89 (d, J = 6.4 Hz, 3H), 0.91 (d, J = 6.4 Hz, 3H), 1.03 (s, 9H), 1.41–1.52 (m, 1H), 1.55–1.69 (m, 2H), 2.36–2.42 (m, 1H), 4.13–4.25 (m 2H), 4.34–4.45 (m, 3H), 6.23 (d, J = 9.0 Hz, 1H), 6.52 (d, J = 7.8 Hz, 1H), 6.64 (d, J = 9.0 Hz, 1H), 7.20–7.25 (m, 5H), 7.30–7.36 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 22.22, 22.78, 25.67, 26.70, 34.48, 38.70, 47.12, 57.35, 61.21, 69.61, 79.08, 127.14, 127.57, 127.63, 127.78, 128.65, 128.82, 140.72, 140.96, 169.55, 173.84. HRMS calcd for C27H37N3O5: 483.2733. Found: 483.2736. Anal. C27H37BrN2O3 requires C, 67.06; H, 7.71; N, 8.69. Found: C, 67.31; H, 7.78; N, 9.12.
[(2′R,3′S)-2′-Iso-butyl-3′-hydroxy-4′-nitro]butyryl-(S)-tert-leucyl-methylamide (1e). M.p.: 138–139 °C. [α]21D = −11.9° (c 0.21, CHCl3). IR (film): 989, 1211, 1312, 1490, 1521, 1578, 1630, 2950, 3422 cm−1. 1H NMR (300 MHz, CDCl3) δ: 0.84 (d, J = 6.4 Hz, 3H), 0.88 (d, J = 6.4 Hz, 3H), 1.09 (s, 9H), 1.47–1.68 (m, 3H), 2.21–2.34 (m, 1H), 2.76 (d, J = 9.2 Hz, 3H), 3.91–4.09 (m, 2H), 4.16 (d, J = 7.0 Hz, 1H), 4.37 (dd, J = 8.2, 13.0 Hz, 1H), 5.10 (br, 1H), 6.15 (d, J = 7.0 Hz, 1H), 6.81 (d, J = 7.4 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ: 22.46, 23.14, 24.51, 25.88, 32.41, 35.66, 37.98, 47.44, 60.18, 82.31, 168.25, 174.33. HRMS calcd for C15H29N3O5: 331.2107. Found: 331.2111. Anal. C15H29N3O5 requires C, 54.36; H, 8.82; N, 12.68. Found: C, 54.39; H, 8.81; N, 13.08.
Kinetics
Human recombinant MMP-1, MMP-2 and MMP-7 were purchased from Enzo Life Science and used without further purification for kinetic assays. The fluorescent substrate {Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 [Mca = (7-methoxycourmarin-4-yl)-acetyl; Dpa = N-3-(2,4-dinitrophenyl)-L-α,β-diaminopropionyl)]} and 50 mM HEPES buffer solution (pH = 7.5, containing 10 mM CaCl2 and 0.05% Brij-35) used for the kinetic assays were obtained from the MMP inhibitor Profiling Kit (BML-AK-016). A BioTek FLx 800 fluorescence plate reader with 96-well plates was used for fluorescence profiling.Enzyme stock solutions were diluted with the assay buffer solution to the desired concentrations as mentioned in Instruction Manual BML-AK016 from Enzo Life Science. All nitro-based inhibitors were dissolved in DMSO and further diluted into the assay buffer. The content of DMSO in the final measurement was kept at 1% (v/v). Before the kinetic assay, the following controls were firstly set up (positive control, inhibitor control, vehicle control, test compound control, and substrate control). On the other hand, we also built a standard curve relating Mca-Pro-Leu-OH (a calibration standard packed in the kit) to arbitrary fluorescence units (RFUs).
Before addition of the fluorescent substrate, the enzyme was incubated with varying concentrations of inhibitors (0.2–2.0 Ki) for 1 hour at 37 °C. The enzymatic hydrolysis mixtures were agitated by shaking for 1 second after each fluorescence measurement. The final concentrations of MMP-1, -2 and -7 were 0.765 U μL−1, 0.047 U μL−1, and 0.064 U μL−1, respectively (1 U = 100 pmol min−1@37 °C, 100 μM thiopeptide, as described in the kit). Upon cleavage of the fluorescent substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (final concentration = 4 μM) at the Gly–Leu bond, Mca fluorescence (λex = 340 nm, λex = 400 nm) was measured at 60 second intervals for 60 minutes. According to the plots of RFUs versus time for each sample, the range of initial time points was determined during which the reaction was linear. The slope of each line was fitted as the initial velocity (v) of the enzymatic hydrolysis using OriginPro 7.5. We measured the velocity for each concentration of the tested inhibitors in triplicate. The averaged slope was used as v in the subsequent determination of Ki values. Here, v and v0 are the initial velocity in the presence and absence of the inhibitor, respectively.
Molecular docking
The X-ray structures of the enzymes were obtained from the Protein Data Bank (PDB ID: 966C15 and 1MMQ16 for MMP-1 and -7, respectively). After extracting the bound inhibitor from the original structure, water molecules and the possibly missing residues were added using Swiss-pdb Viewer 3.7.17 Before docking, the inhibitors 1 were optimized at the B3LYP/6-31G* level18 using Gaussian 09.19The ligands were docked onto the active site of the enzyme using AutoDock 4.0.20 The partial atomic charges for the ligands were obtained at the B3LYP/6-31(G)* level. The nonbonded zinc parameters used were as follows: radius = 0.87 Å, well depth = 0.35 kcal mol−1, charge = 0.95 e.21 The grid size was set to 80 × 80 × 80 Å points with a grid spacing of 0.375 Å centered on the zinc ion at the active site of the enzyme. The docking simulations were performed using the Lamarckian genetic algorithm with 200 solutions for each ligand obtained. The best poses were based on two criteria, the distance of the nitro group from the catalytic zinc ion and the binding free energies.
Results and discussion
Chemistry
Synthesis of the designed nitro-modified dipeptide compounds is shown in Scheme 1. Our initial attempt to obtain 2-iso-butyl-3-hydroxy-4-nitrobutanoic acid failed (ESI†). We then selected the corresponding activated carboxylate, i.e. 2-iso-butyl-3-hydroxy-4-nitrobutanoic acid p-nitrophenyl ester, as the substrate of the condensation with the L-t-Leu amides. Compound 5 was thus prepared for the subsequent nitro substitution. However, we found that a great deal of p-nitro phenol appeared in the reaction of sodium nitrate with compound 5, which indicated that the p-nitrophenoxy anion should be a better leaving group than the bromo group at the 4-position. Therefore, we revised our synthetic route as shown. It should be noted that the condensation with the L-t-Leu amides could only proceed in the presence of NaOAc. We believed that a possible anhydride intermediate might be crucial to the smooth attacking of the amine group of the L-t-Leu amides to the carbonyl of compound 5. Final nitro-substitution of compounds 6a–e gave the desired compounds 1a–e, respectively.MMP inhibition
Inhibitory activities of compounds 1a–e were thus examined in vitro against recombinant human MMP-1, -2 and -7 using a fluorogenic substrate assay. The final concentration of the substrate was controlled at 4 μM, which was far less than the Km values for each tested MMP.22 Competitive inhibition could be thus described by the rate equation (eqn (1)),| d[P]/dt = kcat[E][S]/Km(1 + [I]/Ki) | (1) |
| v0/v = k0/k = 1 + [I]/Ki | (2) |
All Ki values are collected in Table 1. Firstly, compared with Marimastat (IC50 values were 20 and 5 nM on MMP-7 and -1, respectively),2f the inhibitory activities of compound 1e against both enzymes significantly decreased, which could be ascribed to the weaker coordination of the nitro group to the catalytic zinc than HA. Secondly, compounds 1 exhibited the similar inhibitory potency with submicromolar level against MMP-1 and -2, whereas the Ki values of compounds 1a–d on MMP-7 ranged from 3.7 to 14.8 nM. It means that compounds 1a–d were more potent inhibitors against MMP-7 than MMP-1. If we defined the selectivity as the ratio of Ki(MMP-1)/Ki(MMP-7), it could be observed that the selectivity of compound 1b was the highest as 38.1. Selective inhibition of MMP-7 over MMP-1 is thus proved to be feasible by adjustment of the sizes of P′3 side chains. Especially, compound 1b exhibited the highest inhibitory activities against MMP-7, which was about twofold more potent than its diastereomer 1c. It is a very interesting finding that the stereochemistry of the P′3 chains has such subtle effects on the inhibition of MMP-7, which may be further utilized in the design of MMP inhibitors. Finally, it should be noted that the change of P′3 from a methyl to aromatic group could significantly improve the inhibitory potency on MMP-7 as well as the selectivity.
| Compound | Ki/(nM) | Selectivity | ||
|---|---|---|---|---|
| MMP-7 | MMP-1 | MMP-2 | ||
| 1a | 9.4 ± 0.2 | 249 ± 8.2 | 155 ± 5.7 | 26.5 |
| 1b | 3.7 ± 0.2 | 141 ± 5.1 | 116 ± 4.2 | 38.1 |
| 1c | 8.9 ± 0.1 | 198 ± 4.8 | 106 ± 3.3 | 22.2 |
| 1d | 14.8 ± 0.3 | 358 ± 8.2 | 130 ± 4.8 | 24.2 |
| 1e | 158 ± 7.4 | 192 ± 5.7 | 190 ± 5.1 | 1.22 |
Computational analysis
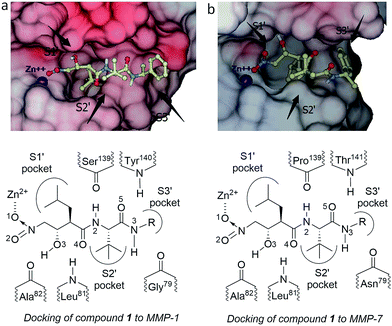
Since Browner’s group firstly reported the crystal structures of the complexes of MMP-7 and inhibitors in 1995,24 other related X-ray crystallography studies have been rarely reported, which may be ascribed to the difficulties in obtaining the single crystal of MMP-7. Although we have incubated the complexes of other zinc-proteases and nitro-based inhibitors,9 our group also failed to achieve the single crystal of MMP-7. To investigate the effects of the P′3 chains of compounds 1 on the inhibitory activities on MMPs, we then conducted computational docking of the presented inhibitors into the active sites of MMP-1 and -7, respectively. The predicted interactions between the typical inhibitor 1b and MMPs are depicted in Fig. 2. Docking results could be divided into two classes according to the binding modes of the inhibitors at the active site of MMP, where one was described by the bidentate chelation of the nitro group to the catalytic zinc ion and the other was described by the monodentate mode. However, using the experimentally exhibited inhibitory potency orders as the standard, it was surprisingly found that the series with monodentate coordination were in good agreement. Such a coordination mode of the nitro group has been rarely reported in the previous structural analysis of the complexes of the other zinc-proteases with inhibitors.9 We thus assumed that a longer peptide chain should provide more hydrogen bonding and/or more hydrophobic effects to remedy the loss of the chelated coordination to the catalytic zinc in binding energies. Such a strategy has been successfully applied in the selective inhibitors against MMP-13 as reported.25All possible interactions between the inhibitors and the residues (the catalytic zinc and the other amino acid residues) at the active site of the two MMPs are collected in Table 2. Although the exhibited docking results could not precisely show the details of the enzyme–inhibitor complexes compared with the X-ray diffraction, we then focused on the possible main interactions between the inhibitor and the residues at the active site of the enzyme. According to the docked conformations of compound 1 at the active sites of MMP-1 and -7, it was found that both the number and the strength of the hydrogen bonds contributed significantly to the inhibitory potencies. Compared with the active site of MMP-1, the relatively large S′3 hydrophobic pocket of MMP-7 allows it to accommodate an aromatic ring with less loss of the vital hydrogen bonds to the amino acid residues at the active site of the enzymes.
| Compound | Atom | MMP-1 | MMP-7 | ||
|---|---|---|---|---|---|
| Residue | Distance (Å) | Residue | Distance (Å) | ||
| 1a | O1 | Zinc | 3.06 | Zinc | 3.08 |
| O2 | Zinc | Zinc | |||
| O3 | Ala82 | 3.08 | Ala82 | 1.84 | |
| O4 | Leu81 | 2.81 | Leu81 | 3.08 | |
| N2 | Ser139 | 4.02 | Pro139 | 2.18 | |
| O5 | Tyr140 | Thr141 | 3.05 | ||
| N3 | Gly79 | Asn79 | 2.67 | ||
| 1b | O1 | Zinc | 3.05 | Zinc | 3.05 |
| O2 | Zinc | Zinc | |||
| O3 | Ala82 | 2.97 | Ala82 | 1.67 | |
| O4 | Leu81 | 3.86 | Leu81 | 3.01 | |
| N2 | Ser139 | 2.62 | Pro139 | 2.21 | |
| O5 | Tyr140 | Thr141 | 3.05 | ||
| N3 | Gly79 | 3.72 | Asn79 | 1.91 | |
| 1c | O1 | Zinc | 3.08 | Zinc | 3.13 |
| O2 | Zinc | Zinc | |||
| O3 | Ala82 | 2.15 | Ala82 | 2.03 | |
| O4 | Leu81 | Leu81 | 3.05 | ||
| N2 | Ser139 | 3.11 | Pro139 | 3.36 | |
| O5 | Tyr140 | 2.19 | Thr141 | 3.00 | |
| N3 | Gly79 | Asn79 | |||
| 1d | O1 | Zinc | 3.14 | Zinc | 3.06 |
| O2 | Zinc | Zinc | |||
| O3 | Ala82 | 4.02 | Ala82 | 3.08 | |
| O4 | Leu81 | 3.44 | Leu81 | 2.75 | |
| N2 | Ser139 | Pro139 | 3.70 | ||
| O5 | Tyr140 | Thr141 | 3.38 | ||
| N3 | Gly79 | 3.46 | Asn79 | 2.17 | |
| 1e | O1 | Zinc | 3.11 | Zinc | 3.15 |
| O2 | Zinc | Zinc | |||
| O3 | Ala82 | 2.51 | Ala82 | 2.66 | |
| O4 | Leu81 | 2.60 | Leu81 | ||
| N2 | Ser139 | Pro139 | 4.20 | ||
| O5 | Tyr140 | 3.99 | Thr141 | 4.05 | |
| N3 | Gly79 | 3.80 | Asn79 | 3.95 | |
Conclusions
We firstly introduced a nitro group as a ZBG in the inhibition of MMP. According to the ranking of the sub-pockets based on achieving selectivity in MMP inhibition, a series of nitro-based dipeptide compounds were designed as MMP inhibitors by adjustment of the P′3 chains. Synthesis of the designed compounds was finally achieved, utilizing the activated carboxylate as the key intermediate. Kinetic assays disclosed that the nitro-based compounds exhibited weaker inhibitory activities compared with the HA-based inhibitor of MMP. Further we found that aromatic side chains as the P′3 could enhance both the potency for MMP-7 and the selectivity of MMP-7 over MMP-1. Further docking studies showed that both the number and the strength of the hydrogen bonds should be crucial to the selective inhibition of MMP-7 without regard for the slight variations in the zinc binding mode. We noted that the presented nitro-based compounds 1a–d inhibited MMP-7 with moderate selectivity. However, the results were clearly enough to indicate that the delicate modification of the side chains of the inhibitors could improve the selective inhibition of MMP-7 over MMP-1. Further advances are highly anticipated based on both experimental and theoretical exploration.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC 21062023). JY also thanks Prof. X. X. Fang (Jilin University) for the help with the enzyme kinetics.Notes and references
- For reviews: (a) W. Zitka, J. Kukacka, S. Krizkova, D. Huska, V. Adam, M. Masarik, R. Prusa and R. Kizek, Curr. Med. Chem., 2010, 17, 3751 CrossRef; (b) J. D. Raffett and R. A. Khalil, Biochem. Pharmacol., 2008, 75, 346 CrossRef PubMed; (c) H.-J. Raffett and W. C. Parks, Matrix Biol., 2007, 26, 587 CrossRef PubMed.
- For reviews: (a) N.-G. Li, Z.-H. Shi, Y.-P. Tang and J.-A. Duan, Curr. Med. Chem., 2009, 16, 3805 CrossRef CAS; (b) D. Georgiadis and A. Yiotakis, Bioorg. Med. Chem., 2008, 16, 8781 CrossRef CAS PubMed; (c) G. Tu, W. Xu, H. Huang and S. Li, Curr. Med. Chem., 2008, 15, 1388 CrossRef CAS; (d) E. Nuti, T. Tuccinardi and A. Rossello, Curr. Pharm. Des., 2007, 13, 2087 CrossRef CAS; (e) J. F. Fisher and S. Mobashery, Cancer Metastasis Rev., 2006, 25, 115 CrossRef CAS PubMed; (f) M. Whittaker, C. D. Floyd, P. Brown and A. J. Gearing, Chem. Rev., 1999, 99, 2735 CrossRef CAS PubMed; (g) W. N. Lipscomb and N. Sträter, Chem. Rev., 1996, 96, 2375 CrossRef CAS PubMed.
- C. M. Overall and O. Kleifeld, Nat. Rev. Cancer, 2006, 6, 227 CrossRef CAS PubMed.
- (a) G. Murphy and H. Nagase, Mol. Aspects Med., 2008, 29, 209 CrossRef PubMed; (b) F. E. Jacobsen, J. A. Lewis and S. M. Cohen, ChemMedChem, 2007, 2, 152 CrossRef CAS PubMed; (c) B. G. Rao, Curr. Pharm. Des., 2005, 11, 295 CrossRef CAS PubMed; (d) C. M. Overall and C. López-Otin, Nat. Rev. Cancer, 2002, 2, 657 CrossRef CAS PubMed.
- (a) J. Hu, P. E. van den Steen, Q.-X. A. Sang and G. Opdenakker, Nat. Rev. Drug Discovery, 2007, 6, 480 CrossRef CAS PubMed; (b) C. M. Overall and O. Kleifeld, Br. J. Cancer, 2006, 94, 941 CrossRef CAS; (c) G. Giaccone, F. Shepherd, C. Debruyne, V. Hirsh, M. Smylie, S. Rubin, H. Martins, A. Lamont, M. Krzakowski and B. Zee, Eur. J. Cancer, 2001, 37, s152 CrossRef; (d) W. P. Steward, Cancer Chemother. Pharmacol., 1999, 43, s56 CrossRef CAS PubMed; (e) K. Holmbeck, P. Bianco, J. Caterina, S. Yamada, M. Kromer, S. A. Kuznetsov, M. Mankani, P. G. Robey, A. R. Poole, I. Pidoux, J. M. Ward and H. Birkedal-Hanse, Cell, 1999, 99, 81 CrossRef CAS; (f) J. W. Hutchinson, G. M. Tierney, S. L. Parsons and T. R. C. Davis, J. Bone Jt. Surg., Br. Vol., 1998, 80-B, 907 CrossRef.
- (a) L. Dahlber, R. C. Billinghurst, P. Manner, F. Nelson, G. Webb, M. Ionescu, A. Reiner, M. Tanzer, D. Zukor, J. Chen, H. E. van Wart and A. R. Poole, Arthritis Rheum., 2000, 43, 673 CrossRef; (b) R. Scatena, Expert Opin. Invest. Drugs, 2000, 9, 2159 CrossRef CAS.
- Z.-H. Shi, N.-G. Li, Q.-P. Shi, H. Tang, Y.-P. Tang, W. Li, L. Yin, J.-P. Yang and J.-A. Duan, Bioorg. Med. Chem. Lett., 2013, 23, 1206 CrossRef CAS PubMed.
- (a) M. Li, H. Yamamoto, Y. Adachi, Y. Maruyama and Y. Shinomura, Exp. Biol. Med., 2006, 231, 20 Search PubMed; (b) H. C. Crawford, C. R. Scoggins, U. K. Washington, L. M. Matrisian and S. D. Leach, J. Clin. Invest., 2002, 109, 1437 CrossRef CAS.
- (a) G. R. Tian, S.-H. Wang, S.-F. Wang, L.-Q. Meng, H. Li, Z.-H. Zeng and J.-Y. Jin, MedChemComm, 2011, 2, 698 RSC; (b) S.-F. Wang, G. R. Tian, W.-Z. Zhang and J.-Y. Jin, Bioorg. Med. Chem. Lett., 2009, 19, 5009 CrossRef CAS PubMed; (c) S.-H. Wang, S.-F. Wang, W. Xuan, Z.-H. Zeng, J.-Y. Jin, J. Ma and G. R. Tian, Bioorg. Med. Chem., 2008, 16, 3596 CrossRef CAS PubMed.
- R. E. Babine and S. L. Bender, Chem. Rev., 1997, 97, 1359 CrossRef CAS PubMed.
- L. Devel, B. Czarny, F. Beau, D. Georgiadis, E. Stura and V. Dive, Biochimie, 2010, 921, 1501 CrossRef PubMed.
- B. Pirard and H. Matter, J. Med. Chem., 2006, 49, 51 CrossRef CAS PubMed.
- (a) B. Pirard, Drug Discovery Today, 2007, 12, 640 CrossRef CAS PubMed; (b) R. Subramaniam, M. K. Haldar, S. Tobwala, B. Ganguly, D. K. Srivatava and S. Mallik, Bioorg. Med. Chem. Lett., 2008, 18, 3333 CrossRef CAS.
- S. S. Lee, Z.-H. Li, D. H. Lee and D. H. Kim, J. Chem. Soc., Perkin Trans. 1, 1995, 2877 RSC.
- B. Lovejoy, A. R. Welch, S. Carr, C. Luong, C. Broka, R. R. Hendricks, J. A. Campbell, K. A. Walker, R. Martin, H. van Wart and M. F. Browner, Nat. Struct. Biol., 1999, 6, 217 CrossRef CAS PubMed.
- M. F. Browner, W. W. Smith and A. L. Castelhano, Biochemistry, 1995, 34, 6602 CrossRef.
- N. Guex and M. C. Peitsch, Electrophoresis, 1997, 18, 2714 CrossRef CAS PubMed.
- C. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 37, 785 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, J. Comput. Chem., 2009, 30, 2785 CrossRef CAS PubMed.
- X. Hu and H. S. William, J. Mol. Graphics Modell., 2003, 22, 115 CrossRef CAS PubMed.
- C. G. Knight, F. Willenbrock and G. Murphy, FEBS Lett., 1992, 296, 263 CrossRef CAS PubMed.
- J. Feder, L. R. Brougham and B. S. Wildi, Biochemistry, 1974, 13, 1186 CrossRef CAS PubMed.
- M. F. Browner, W. W. Smith and A. L. Castelhano, Biochemistry, 1995, 34, 6602 CrossRef CAS PubMed.
- D. A. Gao, Z. Xiong, A. Heim-Riether, L. Amodeo, E. M. August, X. Cao, L. Ciccarelli, B. K. Collins, K. Harrington, K. Harverty, M. Hill-Drzewi, X. Li, S. Liang, S. M. Margarit, N. Moss, N. Nagaraju, J. Proudfoot, R. Roman, S. Schlyer, L. S. Keenan, S. Taylor, B. Wellenzohn, D. Wiedenmayer, J. Li and N. A. Farrow, Bioorg. Med. Chem. Lett., 2010, 20, 5039 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Enzyme inhibition kinetics and molecular docking results. See DOI: 10.1039/c5ra22271k |
| This journal is © The Royal Society of Chemistry 2015 |