Selective oxidation of 1,2-propanediol to lactic acid catalyzed by hydroxyapatite-supported Pd and Pd–Ag nanoparticles†
Yonghai Feng*a,
Wuping Xueb,
Hengbo Yin*b,
Minjia Mengb,
Aili Wangb and
Shuxin Liuc
aSchool of Material Science and Engineering, Jiangsu University, Zhenjiang, China 212013. E-mail: fengyonghai@ujs.edu.cn; Tel: +86-(0)511-88787591
bFaculty of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, China 212013
cSchool of Chemistry and Chemical Engineering, Mianyang Normal University, Mianyang, China 621000
First published on 3rd December 2015
Abstract
Selective catalytic oxidation of 1,2-propanediol is an alternative, effective, and environmentally benign method for producing lactic acid. Hydroxyapatite-supported Pd (Pd/HAP) and Pd–Ag (Pd–Ag/HAP) catalysts were prepared by the sol-immobilization method. The as-prepared catalysts were characterized by XRD, TEM, HRTEM, XPS, BET, and CO2-TPD techniques. The average particle sizes of Pd nanocubes in Pd/HAP catalysts were tuned from 6 to 18 nm by changing the amount of KBr and PVP, which significantly affected the 1,2-propanediol conversion and the lactic acid selectivity. Pd/HAP catalyst with cubic Pd nanoparticles was more selective to lactic acid than Pd/HAP catalyst with spherical Pd nanoparticles in the oxidation of 1,2-propanediol. The coalesced Pd and Ag nanoparticles in Pd–Ag/HAP bimetallic catalysts synergistically catalyzed the oxidation of 1,2-propanediol to lactic acid. The support HAP with high basicity promoted the oxidation of 1,2-propanediol to lactic acid. When the catalytic oxidation of 1,2-propanediol with O2 was carried out at 100 °C for 2 h in an alkaline solution, the lactic acid selectivity was 86.2% at the 1,2-propanediol conversion of 96.2% over Pd2/HAP-6 catalyst while the lactic acid selectivity was 88.8% at the 1,2-propanediol conversion of 86.3% over Pd1Ag1/HAP catalyst. The activation energies for the catalytic oxidation of 1,2-propanediol were in the order of Ea (Pd1Ag1/HAP) < Ea (Pd2/HAP-6) < Ea Ag2/HAP.
1. Introduction
In recent years, biomass derivatives and biodegradable products have attracted great attention due to the growing concern for fossil fuel depletion and broad promotion of environmental protection. Lactic acid, as a biomass derivative, is conventionally used in food and beverage fields as a preservative and pH adjusting agent. In chemical industry, lactic acid has been used as the monomer for the production of biodegradable polylactic acid (PLA).1 Growth in the PLA biodegradable plastics market has been the driving demand for lactic acid production with an annual growth rate of 22% from 2005 to 2015.2,3There are two lactic acid production processes, namely microbial fermentation and chemical synthesis. Microbial fermentation is the main production method for lactic acid by using a suitable strain of microorganism.4 However, the production of lactic acid by microbial fermentation is limited by the substrate and operating cost.3 The chemical synthesis method includes two steps, the reaction of acetaldehyde with HCN and the hydrolysis with sulfuric acid.5 Although the chemical route gives high reaction rate, it is not environmentally friendly due to the use of toxic HCN.6
Recently, selective catalytic oxidation of 1,2-propanediol has been considered as a green chemical route for lactic acid synthesis,5,7–16 which is due to the high lactic acid selectivity obtained in the catalytic oxidation of renewable 1,2-propanediol under mild reaction conditions.17 The raw material, 1,2-propanediol, can be facilely synthesized by catalytic hydrogenolysis of polyols, such as glucose, glycerol, sorbitol, and xylitol.18–26 Furthermore, with the scaling-up co-production of dimethyl carbonate and 1,2-propanediol by transesterification method,27 1,2-propanediol is facing the oversupply problem in market, especially in China, due to its limited demand in the production of organic solvent and unsaturated polyester resin.28,29
Monometallic Au nanoparticles have been found as effective catalysts for the oxidation of 1,2-propanediol to lactic acid. Their catalytic activities are strongly dependent on the particle size and shape of Au nanoparticle, type of support, and reaction condition.5,7,9 Prati et al.5 found that over 1% Au/C catalyst with Au particle size of 7.0 nm, the 1,2-propanediol conversion of 78% with lactic acid selectivity of ca. 100% was obtained under 0.3 MPa O2 at 90 °C for 1 h in an alkaline solution. Xu et al.9 found that Au/MgO with the Au particle sizes of 14–18 nm gave the lactic acid selectivity of 89.3% at the 1,2-propanediol conversion of 94.4% under 0.3 MPa of O2 at 60 °C for 6 h. Vos et al.7 reported that Au nanosols with the particle size of 2–4 nm gave the lactic acid selectivity of 98% with the 1,2-propanediol conversion of 69% under 0.5 MPa at 70 °C for 6 h. Au nanoparticle size, reaction temperature, and support type affected the catalytic oxidation of 1,2-propanediol to lactic acid.
As compared to the monometallic Au catalysts, bimetallic Au–Pd catalysts have high catalytic activities for the catalytic oxidation of 1,2-propanediol to lactic acid under mild reaction conditions.8,10–15 Hutchings et al.8 found that in the catalytic oxidation of 1,2-propanediol under 1 MPa O2 at 60 °C for 1 h, the catalytic activity and lactic acid selectivity over 1% AuPd/C catalyst were obviously higher than those over 1% Au/C and 1% Pd/C catalysts. It was explained as that in the bimetallic Au–Pd catalyst, Au reduced the barrier for C–H scission while Pd reduced the coverage of strongly bound adsorbates, giving high catalytic activity and lactic acid selectivity in the 1,2-propanediol oxidation.12,15 Our previous work reported that when the catalytic oxidation of 1,2-propanediol was carried out over Au0.75Pd0.25/HAP (hydroxyapatite) catalyst at 80 °C for 5 h in an alkaline solution, the lactic acid selectivity was 97.1% at the 1,2-propanediol conversion of 96.6%.15 It was suggested that Au and Pd nanoparticles synergistically catalyzed the oxidation of 1,2-propanediol to lactic acid, probably due to the electron transfer between Au and Pd atoms.
Monometallic Pd catalysts also showed high catalytic activity for the catalytic oxidation of 1,2-propanediol to lactic acid. Tsujino et al.30 firstly reported that the oxidation of 1,2-propanediol was observed on Pd/C catalyst at both the primary and secondary hydroxyl groups to yield lactic acid, hydroxyacetone, and pyruvic acid at 90 °C and a pH value of 8. Prati et al.5 reported that when 5% Pd/C was used as the catalyst for the catalytic oxidation of 1,2-propanediol under 0.3 MPa at 70 °C for 1 h, the lactic acid selectivity of 90% was obtained at the 1,2-propanediol conversion of 80%.
In the previous researches, active carbon-, hydroxyapatite-, Mg(OH)2-, and MgO-supported monometallic and bimetallic Au and Pd (Pt) catalysts for the catalytic oxidation of 1,2-propanediol to lactic acid were widely investigated.5,7–15 However, the catalytic activities of hydroxyapatite-supported monometallic Pd nanoparticles with different particle sizes, shapes and bimetallic Ag–Pd nanoparticles on the catalytic oxidation of 1,2-propanediol to lactic acid have been rarely investigated.
In our present work, hydroxyapatite (HAP) nanorods with high basicity, good physicochemical stability, and eco-friendly property were used as supports for the preparation of supported Pd/HAP and Pd–Ag/HAP catalysts. The catalytic activities of the as-prepared Pd/HAP and Pd–Ag/HAP catalysts for the catalytic oxidation of 1,2-propanediol to lactic acid with O2 in a NaOH aqueous solution was investigated. A power function-type reaction kinetic model was used to evaluate the oxidation kinetics of 1,2-propanediol over the catalysts.
2. Experimental section
2.1. Materials
The chemicals, calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), phosphoric acid (H3PO4, 85%), sodium dihydrogen phosphate (NaH2PO4), ammonia solution (NH3·H2O, 25%), ascorbic acid (AA), silver nitrate (AgNO3), palladium chloride (PdCl2), polyvinylpyrrolidone (PVP, K30), potassium bromide (KBr), 1,2-propanediol, lactic acid (85%), formic acid, and acetic acid were of analytical reagent grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. Acetonitrile was of chromatographic grade and was purchased from Sinopharm Chemical Reagent Co., Ltd. Active carbon powder with the specific surface area of 430 m2 g−1 was also purchased from Sinopharm Chemical Reagent Co., Ltd. All the chemicals were used as received without further purification.2.2. Preparation of catalyst
The support, HAP nanorod, was prepared according to the previously reported method.31,32 Typically, aqueous solutions of Ca(NO3)2 (1 mol L−1) and H3PO4 (0.6 mol L−1) were added into a three-necked flask, which was kept in a water bath at 40 °C. Meanwhile, an ammonia aqueous solution (25%) was added dropwise into it to adjust the pH value of the reaction solution to 10. The resultant solution reacted at 40 °C for 4 h. Then, the reaction solution was transferred into a Teflon-lined autoclave and autoclaved at 100 °C for 10 h. The resultant powders were washed with distilled water until the filtrate conductivity was less than 2 mS m−1 and then dried at 120 °C overnight. The as-prepared sample was nanosized hydroxyapatite (HAP) with rod-like shape, which was used as the catalyst support.HAP-supported monometallic Pd catalysts with different Pd particle sizes were prepared by the sol-immobilization method. A typical catalyst preparation process was described as follows: 12 mL of an aqueous solution containing 0.16 g of AA and given amounts of PVP and KBr was added to a flask and preheated to 80 °C in a water bath under vigorous stirring for 10 min. Subsequently, 8 mL of PdCl2 aqueous solution (PdCl2, 0.07 g) was added. The reaction lasted for 3 h. The particle size of Pd nanoparticles was controlled by varying the amount of KBr and PVP with 0.3, 0.21; 0.6, 0.14; 1.2, 0.07 g, resulting in the formation of Pd nanocubes with the average particle sizes of ca. 6, 10, and 18 nm, respectively. After cooling to room temperature, the reduced Pd colloids were immobilized on HAP by adding 2.1 g of HAP nanorods under stirring for 2 h. The as-prepared Pd/HAP catalysts were filtered, washed with distilled water, and dried at 120 °C overnight. The as-prepared catalysts are denoted as Pd2/HAP-6, Pd2/HAP-10, and Pd2/HAP-18, respectively. The compositions of the Pd/HAP catalysts are listed in Table 1.
| Catalysts | Specific surface areas (m2 g−1) | Amounts of Ag and Pda (μmol g−1) | Particle sizes of Ag and Pdb (nm) | Binding energies (eV) | Total basicitiesc (μmol CO2 gcat−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Pd | Ag | Pd | Ag3d5/2 | Ag3d3/2 | Pd3d5/2 | Pd3d3/2 | |||
| a The amount of Ag and Pd in the catalysts were detected by ICP.b Particle sizes of Ag and Pd nanoparticles of the catalysts were calculated according to TEM images.c The base strengths of the catalysts were calculated from CO2-TPD curves. | ||||||||||
| Pd2/HAP-6 | 73.4 | 189.2 | 6.3 | 335.35 | 340.55 | 20.3 | ||||
| Pd2/HAP-10 | 74.3 | 186.8 | 10.4 | |||||||
| Pd2/HAP-18 | 75.2 | 185.7 | 17.6 | |||||||
| Pd0.2Ag1.8/HAP | 73.8 | 19.4 | 167.3 | 14.3 | 8.8 | 367.45 | 373.30 | 336.25 | — | 21.3 |
| Pd0.5Ag1.5/HAP | 73.3 | 47.8 | 139.6 | 11.5 | 11.5 | 367.75 | 373.45 | 336.10 | — | 20.8 |
| Pd1Ag1/HAP | 74.8 | 93.3 | 93.2 | 10.0 | 12.5 | 367.80 | 373.45 | 335.70 | 341.0 | 24.3 |
| Ag2/HAP | 71.6 | 186.2 | 0 | 18.8 | 367.40 | 373.25 | 20.4 | |||
| HAP | 77.3 | — | — | — | — | — | — | — | — | 42.8 |
To compare the effect of support on the 1,2-propanediol oxidation reaction, Pd2/C-6 catalyst was also prepared according to the method described for the preparation of Pd2/HAP-6. Active carbon was used as support instead of HAP.
HAP-supported bimetallic Pd–Ag catalysts with different Pd and Ag ratios (PdxAgy/HAP, x and y were the weight ratios of Pd and Ag to HAP) were prepared by the sol-immobilization method. Typically, 4 mL of aqueous solution of given amount of AgNO3 added dropwise into 12 mL aqueous solution of 0.3 g AA and 0.16 g PVP at 60 °C under vigorous stirring. The reaction lasted for 0.5 h. After cooling the as-prepared Ag colloids to room temperature, 0.1 g KBr, 0.08 g AA and 0.04 g PVP were added into the solution and preheated to 60 °C under vigorous stirring for 10 min. Subsequently, 4 mL of PdCl2 aqueous solution was added with a pipette. The reaction lasted for 1 h. After cooling to room temperature, the reduced Pd–Ag colloids were immobilized by adding 2.1 g of HAP nanorods under stirring for 2 h. The as-prepared Pd/HAP catalysts were filtrated, washed with distilled water, and dried at 120 °C overnight. The compositions of Pd–Ag/HAP catalysts are listed in Table 1.
The inductively coupled plasma (ICP) analysis showed that the contents of Pd and Ag in Pd/HAP and Pd–Ag/HAP catalysts were similar to those calculated according to their precursors, indicating that Pd and Ag were effectively supported on the surfaces of HAP nanorods by the sol-immobilization method.
2.3. Characterization
The X-ray powder diffraction (XRD) data of Pd/HAP and Pd–Ag/HAP catalysts were recorded on a diffractometer (D8 super speed Bruke AEX Company, Germany) using Cu Kα radiation (λ = 1.54056 Å) with Ni filter, scanning from 10° to 90° (2θ).High-resolution transmission electron microscopy (HRTEM) images were obtained on a microscope (JEM-2100) operated at an acceleration voltage of 200 kV to characterize the morphologies and the crystal structures of the Pd and Ag nanoparticles supported on HAP nanorods. The TEM specimens were prepared by placing a drop of Pd/HAP or Pd–Ag/HAP ethanol suspension onto a copper grid coated with a layer of amorphous carbon. The average particle sizes of the Ag and Pd nanoparticles were measured from the TEM images by counting at least 100 individual particles. The average particle sizes of the Ag and Pd nanoparticles were calculated by a weighted-average method according to the individual particle sizes of the all counted particles.
X-ray photoelectron spectra (XPS) of the catalysts were recorded on an ESCALAB 250 spectrometer (PHI5000VersaProbe, UlVAC-PHI Company, Japan) using Al Ka radiation (1486.6 eV). The binding energies were calculated with respect to C1s peak of contaminated carbon at 284.6 eV.
Temperature-programmed desorption of CO2 (CO2-TPD) was carried out in a fixed-bed continuous flow microreactor at atmospheric pressure. The catalysts (0.05 g) were dried at 400 °C for 2 h and then were CO2-saturated in a CO2 stream at 35 °C for 1 h. After purging with helium (30 mL min−1) at 100 °C for 1 h to remove the physically adsorbed CO2, the catalysts were heated at a linear heating rate of 10 °C min−1 up to 750 °C. In order to determine the amount of desorbed CO2 from CO2 desorption profiles, the areas under the curves were integrated by Gaussian deconvolution of the peaks and the amount of desorbed CO2 was expressed as micromoles of CO2 per gram of catalyst.
The Pd and Ag contents of the catalysts were analyzed by using inductively coupled plasma (ICP) technique (VISTA-MPX).
The specific surface areas of the catalysts were measured on a NOVA 2000e physical adsorption apparatus by the N2 adsorption/desorption method at −196 °C and calculated by the BET method.
2.4. Catalytic test
Catalytic oxidation of 1,2-propanediol was carried out in a 1000 mL capacity stainless steel autoclave equipped with a magnetically driven impeller. The autoclave was charged with appointed amount of 1,2-propanediol, water, sodium hydroxide, and catalyst. When the autoclave was heated up to the given temperature, O2 was fast introduced into the autoclave. After raising the pressure to the desired value, the reaction started and lasted for a certain time at given temperature under stirring at 700 rpm. After reaction, the autoclave was cooled to ambient temperature and depressurized.Before product analysis, the reaction mixture was acidified with hydrochloric acid (12 M) to the pH value of ca. 3. The concentration of remained 1,2-propanediol was analyzed on a gas phase chromatograph equipped with a PEG-20 M packed capillary column (0.25 mm × 30 m) and FID by the internal standard method with n-butanol as the internal standard. The products, such as lactic acid, acetic acid, and formic acid, were analyzed on a Varian HPLC system equipped with a reverse-phase column (Chromspher 5 C18, 4.6 mm × 250 mm) and a UV detector (λ = 210 nm) at 35 °C. The mobile phase was composed of H3PO4/NaH2PO4 (0.1 M NaH2PO4 acidified by H3PO4 to pH = 2) buffer aqueous solution and acetonitrile (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a flow rate of 0.6 mL min−1. The concentrations of the products were analyzed by the external standard method.
1) with a flow rate of 0.6 mL min−1. The concentrations of the products were analyzed by the external standard method.
3. Results and discussion
3.1. XRD, ICP, and BET analyses
The XRD patterns of support HAP, Pd2/HAP-6, Ag2/HAP, and Pd–Ag/HAP catalysts with different ratios of Ag and Pd to HAP are shown in Fig. 1. The XRD peaks of support HAP appearing at 2θ = 25.88, 28.97, 31.77, 32.2, 32.9, 34.05, 35.4, 39.2, 39.8, 42.03, 43.08, 46.7, 48.1, 49.47, 64.08, 77.18, and 78.23° were in good agreement with those of the standard HAP crystallite (Ca10(PO4)6(OH)2, JCPDS 09-0432), respectively, indicating that the structure of HAP support was not changed after supporting the metallic components. Five characteristic peaks of metallic silver appearing at 38.12, 44.28, 64.43, 77.47, and 81.54° (Ag, JCPDS 04-0783) could be found in the XRD patterns of the Ag2/HAP and Pd0.2Ag1.8/HAP catalysts while there were no characteristic peaks of metallic silver found in those of the Pd0.5Ag1.5/HAP and Pd1Ag1/HAP. For Pd2/HAP-6 catalyst, five characteristic peaks of metallic palladium appearing at 2θ = 40.11, 46.66, 68.12, 82.1, and 86.62° (Pd, JCPDS 46-1043) were observed. However, for the Pd–Ag/HAP catalysts, there were no characteristic peaks of metallic palladium found in the XRD patterns.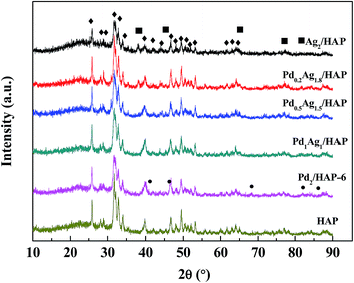 | ||
| Fig. 1 XRD patterns of support HAP, Pd2/HAP-6, Ag2/HAP, and Pd–Ag/HAP catalysts. ♦, HAP; ●, Pd; ■, Ag. | ||
ICP analysis showed that the contents of Ag and Pd in the catalysts were similar to those calculated according to their precursors (Table 1). The specific surface areas of the catalysts were in a range of 71.6–75.2 m2 g−1, which was close to that of support HAP.
The XRD, ICP, and BET analyses indicated that metallic Pd and Ag were formed and well dispersed on the surfaces of support HAP by the sol-immobilization method.
3.2. TEM analysis
For the monometallic Pd/HAP catalysts, the TEM images of Pd2/HAP-6 Pd2/HAP-10, and Pd2/HAP-18 show that the resultant Pd nanocubes with the average particle sizes of 6.3, 10.4, and 17.6 nm were well dispersed on the surfaces HAP nanorods, respectively (Fig. 2a–c). The HRTEM images (insets) show the lattice fringes of the Pd nanocubes were examined to 0.224, 0.194 nm, close to {1 1 1} and {2 0 0} lattice spacing of fcc palladium and the Pd nanocubes had single crystalline structure. The TEM images also show that the HAP nanorods had the average length of ca. 80 nm and the average diameter of ca. 18 nm, respectively.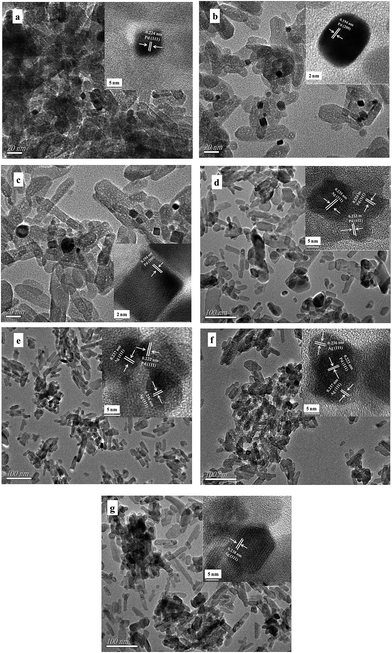 | ||
| Fig. 2 TEM images of (a) Pd2/HAP-6, (b) Pd2/HAP-10, (c) Pd2/HAP-18, (d) Pd0.2Ag1.8/HAP, (e) Pd0.5Ag1.5/HAP, (f) Pd1Ag1/HAP, and (g) Ag2/HAP catalysts. The insets are the corresponding HRTEM images. | ||
Fig. 2d–f show the TEM images of the bimetallic Pd0.2Ag1.8/HAP, Pd0.5Ag1.5/HAP, and Pd1Ag1/HAP catalysts. The TEM images show the average particle sizes of metallic Pd and Ag nanoparticles were 8.8–12.5 nm and 10–14.3 nm, respectively (Table 1). The HRTEM images (insets) show that the metallic Ag nanoparticles coalesced with metallic Pd nanoparticles.
For the monometallic Ag2/HAP catalyst, the TEM image (Fig. 2g) shows that Ag nanoparticles with the average particle size of 18.8 nm were well dispersed on the surfaces of HAP nanorods. The HRTEM image (inset) shows the lattice fringes of the Ag nanoparticles was examined to 0.238 nm, close to {1 1 1} lattice spacing of fcc silver, revealing that the Ag nanoparticles had single crystalline structure.
3.3. XPS analysis
The XPS measurement was employed to determine the chemical states of metallic nanoparticles in Pd2/HAP-6, Ag2/HAP, and Pd–Ag/HAP catalysts. The XPS of Pd3d and Ag3d are shown in Fig. 3 and the binding energies are listed in Table 1. | ||
| Fig. 3 XPS spectra of (a) Pd3d and (b) Ag3d for Pd2/HAP-6, Ag2/HAP, and Pd–Ag/HAP catalysts with different Pd and Ag loadings. | ||
For Pd2/HAP-6 catalyst, the binding energies of Pd3d5/2 and Pd3d3/2 were 335.35 and 340.55 eV, respectively, higher than those (335.05 for Pd3d5/2 and 340.30 eV for Pd3d3/2) of pure metallic Pd nanoparticles, which were prepared in the absence of HAP. The positive binding energy shifts could be due to the possible electron transfer from Pd nanoparticles to HAP. Similar phenomenon was found when Au was supported on HAP.33 It is reasonable to conclude that Pd nanoparticles had the characteristics of electron donation to HAP. For Ag2/HAP catalyst, the binding energies of Ag3d5/2 and Ag3d3/2 were 367.40 and 373.25 eV, respectively. Splitting of the Ag3d doublet is 5.9 eV, implying the presence of metal silver,34 which was consistent with the XRD analysis. However, the binding energy of Ag3d5/2 and Ag3d3/2 of Ag2/HAP were lower than those (368.10 for Ag3d5/2 and 374.05 eV for Ag3d3/2) of pure Ag nanoparticles. It was reported that when silver in Ag/HAP sample had three chemical states of Ag+, Ag2+, and Ag0, respectively. The binding energy of silver increased with the decrease in the oxidation state.35,36 This negative binding energy shifts implied that silvers in Ag+, Ag2+, and Ag0 chemical states were present in Ag2/HAP probably due to the interaction between Ag nanoparticles and HAP.
For Pd–Ag/HAP bimetallic catalysts, the binding energies of Pd3d5/2 were in an order of Pd0.2Ag1.8/HAP > Pd0.5Ag1.5/HAP > Pd1Ag1/HAP > Pd2/HAP. The binding energies of Pd3d increased with decreasing the Pd content, indicating that electrons were transferred from Pd to Ag.37 Generally, the binding energies of Ag3d decreased with decreasing the Ag content over Pd–Ag bimetallic nanoparticles.37 However, for Pd–Ag/HAP bimetallic catalysts, the binding energies of Ag3d5/2 and Ag3d3/2 were in an order of Ag2/HAP < Pd0.2Ag1.8/HAP < Pd0.5Ag1.5/HAP ≈ Pd1Ag1/HAP. With increasing the Pd content in Pd–Ag/HAP catalysts, more silvers in Ag0 chemical state are present due to the electron transfer from Pd to Ag.
For the Pd–Ag/HAP bimetallic catalysts, the binding energies of Pd3d and Ag3d increased with decreasing their particle sizes, indicating that the small-sized metallic nanoparticles had stronger interaction than the large-sized ones, causing more electron transfer.
The XPS analysis revealed that there existed a strong interaction between Ag and Pd nanoparticles over Pd–Ag/HAP catalysts, probably due to their coalescence (HRTEM analysis).
3.4. CO2-TPD analysis
CO2-TPD measurement was carried out to investigate the surface basicities of support HAP, Pd2/HAP-6, Ag2/HAP, and Pd–Ag/HAP catalysts. The CO2-TPD profiles of the samples are shown in Fig. 4 and the basic strengths are listed in Table 1. For HAP support, two CO2 desorption peaks appearing at 190 °C and 502 °C were observed in the CO2-TPD profile, meaning that both weak- and strong-strength basic sites were present on the surface of HAP.38 With loading Pd and Ag on HAP support, the profiles showed single CO2 desorption peaks appearing at 193–248 °C, meaning that weak-strength basic sites were present on the surfaces of HAP-supported Pd and Ag catalysts. Moreover, the total basicities of these catalysts decreased to ca. 50% that of pure HAP, indicating that covering HAP surface with metallic Pd and Ag significantly caused the decrease in its basicity. | ||
| Fig. 4 CO2-TPD patterns of support HAP, Pd2/HAP-6, Ag2/HAP, and Pd–Ag/HAP catalysts with different Pd and Ag loadings. | ||
3.5. Catalytic oxidation of 1,2-propanediol
When HAP and active carbon were used as supports, the conversion of 1,2-propanediol and the selectivity of lactic acid over Pd2/HAP-6 catalyst were more than those over Pd2/C-6 catalyst (Table 2). It is reported that a basic site on support is beneficial for abstracting proton from a OH group, enhancing the selective oxidation of diols.40,41 The basic sites present in support HAP could interact with OH group of 1,2-propanediol, favoring the formation of lactic acid.
| Catalysts | Conversions (%) | Selectivities (%) | ||
|---|---|---|---|---|
| Lactic acid | Formic acid | Acetic acid | ||
| a Reaction conditions: 1,2-propanediol aqueous solution, 200 mL, 0.28 mol L−1; NaOH concentration, 0.56 mol L−1; reaction temperature, 100 °C; O2 pressure, 1.0 MPa; catalyst, 0.5 g, reaction time, 2 h.b The Pd2/HAP-6-S catalyst was composed by immobilization of Pd nanospheres with the particle size of ca. 6 nm on HAP.c The catalyst was composed by mixing 0.25 g Pd2/HAP and 0.25 g Ag2/HAP. | ||||
| Pd2/HAP-6-Sb | 99.3 | 55.4 | 14.1 | 30.5 |
| Pd2/C-6 | 91.6 | 69.4 | 10.3 | 20.3 |
| Pd2/HAP-6 | 96.2 | 86.2 | 4.5 | 9.3 |
| Pd2/HAP-10 | 79.4 | 89.1 | 2.9 | 7.9 |
| Pd2/HAP-18 | 21.1 | 91.5 | 2.3 | 6.3 |
| Pd0.2Ag1.8/HAP | 22.6 | 69.4 | 9.8 | 20.8 |
| Pd0.5Ag1.5/HAP | 64.3 | 85.0 | 4.5 | 10.5 |
| Pd1Ag1/HAP | 86.3 | 88.8 | 3.1 | 8.1 |
| Ag2/HAP | 15.7 | 57.2 | 14.4 | 28.4 |
| Pd2/HAP + Ag2/HAPc | 53.9 | 83.5 | 5.3 | 11.2 |
| HAP | 0 | — | — | — |
When using Pd2/HAP-6, Pd2/HAP-10, and Pd2/HAP-18 catalysts to catalyze the oxidation of 1,2-propanediol, it was found that the 1,2-propanediol conversions over Pd2/HAP catalysts were in an order of Pd2/HAP-6 (96.2%) > Pd2/HAP-10 (79.4%) > Pd2/HAP-18 (21.1%), whereas the lactic acid selectivities were in an order of Pd2/HAP-6 (86.2%) < Pd2/HAP-10 (89.1%) < Pd2/HAP-18 (91.5%) (Table 2), indicating that small-sized Pd nanocubes on HAP support not only favored the oxidation of 1,2-propanediol to lactic acid, but also slightly decreased the lactic acid selectivity to some extent due to oxidative cleavage of intermediates to formic and acetic acids.
When the bimetallic Pd–Ag/HAP catalysts were used for the catalytic oxidation of 1,2-propanediol, the conversions of 1,2-propanediol were in an order of Pd1Ag1/HAP (86.3%) > Pd0.5Ag1.5/HAP (64.3%) > Pd0.2Ag1.8/HAP (22.6%) > Ag2/HAP (15.7%) (Table 2). The selectivities of lactic acid were in an order of Pd1Ag1/HAP (88.8%) > Pd0.5Ag1.5/HAP (85.0%) > Pd0.2Ag1.8/HAP (69.4%) > Ag2/HAP (57.2%). The results indicated that high Pd content in Pd–Ag/HAP catalysts favored the catalytic oxidation of 1,2-propanediol to lactic acid and Pd played the main role in the reaction process. For Pd1Ag1/HAP catalyst, it had lower Pd content and larger Pd particle size than Pd2/HAP-6 and Pd2/HAP-10 catalysts. Generally, the Pd1Ag1/HAP catalyst would exhibit much lower catalytic activity as compared to the Pd2/HAP-6 and Pd2/HAP-10 catalysts, considering that the catalytic activity of Ag nanoparticles was negligible. However, the catalytic activity for the oxidation of 1,2-propanediol to lactic acid over Pd1Ag1/HAP was comparable to those over Pd2/HAP-6 and Pd2/HAP-10. It was reasonable to suggest that the Ag and Pd bimetallic nanoparticles in Pd–Ag/HAP synergistically catalyzed the oxidation reaction.
In order to certify the synergistic effect of Pd and Ag nanoparticles, the catalytic activities over Pd1Ag1/HAP and Pd2/HAP + Ag2/HAP (both catalysts with the same amount of Pd and Ag as those in Pd1Ag1/HAP catalyst were physically mixed.) were investigated. As shown in Table 2, the 1,2-propanediol conversion over Pd1Ag1/HAP catalyst (86.3%) was obviously higher than that over the Pd2/HAP + Ag2/HAP catalysts (53.9%). The results proved that the coalesced Pd and Ag bimetallic nanoparticles in the Pd–Ag/HAP catalyst synergistically catalyzed oxidation of 1,2-propanediol to lactic acid. The strong interaction (electrons transfer) between the coalesced Pd and Ag bimetallic nanoparticles in the Pd–Ag/HAP catalyst probably played an important role in the catalytic oxidation reaction.
| Catalysts | Temperatures (°C) | Conversions (%) | Selectivities (%) | ||
|---|---|---|---|---|---|
| Formic acid | Lactic acid | Acetic acid | |||
| a Reaction conditions: 1,2-propanediol aqueous solution, 200 mL, 0.28 mol L−1; NaOH concentration, 0.56 mol L−1; O2 pressure, 1.0 MPa; catalyst, 0.5 g, reaction time, 2 h. | |||||
| Pd1Ag1/HAP | 120 | 95.3 | 5.7 | 79.8 | 14.5 |
| 100 | 86.4 | 3.1 | 88.8 | 8.1 | |
| 80 | 62.3 | 3.1 | 91.0 | 5.9 | |
| Pd2/HAP-6 | 120 | 99.4 | 5.9 | 80.5 | 13.6 |
| 100 | 96.2 | 4.5 | 86.2 | 9.3 | |
| 80 | 53.3 | 2.8 | 91.1 | 6.1 | |
The 1,2-propanediol conversions and product selectivities in the catalytic oxidation of 1,2-propanediol under different 1,2-propanediol concentrations at 100 °C over Pd1Ag1/HAP and Pd2/HAP-6 catalysts are listed in Table 4. The conversions of 1,2-propanediol decreased from 96.4% to 67.7% and from 100% to 76.1% while the lactic acid selectivities increased from 81.5% to 91.7% and from 79.6% to 92.5%, respectively, with increasing the 1,2-propanediol concentrations from 0.14 to 0.42 mol L−1. Both Pd1Ag1/HAP and Pd2/HAP-6 catalysts had comparable catalytic activities for the catalytic oxidation of 1,2-propanediol to lactic acid under different 1,2-propanediol concentrations. At low 1,2-propanediol concentration, more catalytic active sites available on the surfaces of catalysts led to the cleavage of intermediates (probably hydroxyacetone or pyruvaldehyde), giving high selectivities of acetic and formic acids.
| Catalysts | 1,2-Propanediol concentrations (mol L−1) | Conversions (%) | Selectivities (%) | ||
|---|---|---|---|---|---|
| Formic acid | Lactic acid | Acetic acid | |||
| a Reaction conditions: 1,2-propanediol aqueous solution, 200 mL; NaOH concentration, 0.56 mol L−1; reaction temperature, 100 °C; O2 pressure, 1.0 MPa; catalyst, 0.5 g, reaction time, 2 h. | |||||
| Pd1Ag1/HAP | 0.42 | 67.7 | 2.9 | 91.7 | 5.4 |
| 0.28 | 86.4 | 3.1 | 88.8 | 8.1 | |
| 0.14 | 96.4 | 6.4 | 81.5 | 12.1 | |
| Pd2/HAP-6 | 0.42 | 76.1 | 2.6 | 92.5 | 4.9 |
| 0.28 | 96.2 | 4.5 | 86.2 | 9.3 | |
| 0.14 | 100 | 6.5 | 79.6 | 13.9 | |
When 1,2-propanediol was catalytically oxidized under O2 pressures of 0.5–1.5 MPa, the 1,2-propanediol conversions over Pd1Ag1/HAP and Pd2/HAP-6 catalysts were around 86% and 96%, respectively, after reacting for 2 h (Table 5). The lactic acid selectivities were around 88% and 86%. Increasing the O2 pressures from 0.5 to 1.5 MPa had a minor effect on the oxidation of 1,2-propanediol to lactic acid, which could be explained as that the adsorption of O2 was probably saturated on the catalysts surfaces when the O2 pressure was higher than 0.5 MPa.
| Catalysts | O2 pressures (MPa) | Conversions (%) | Selectivities (%) | ||
|---|---|---|---|---|---|
| Formic acid | Lactic acid | Acetic acid | |||
| a Reaction conditions: 1,2-propanediol aqueous solution, 200 mL, 0.28 mol L−1; NaOH concentration, 0.56 mol L−1; reaction temperature, 100 °C; catalyst, 0.5 g, reaction time, 2 h. | |||||
| Pd1Ag1/HAP | 1.5 | 86.7 | 4.6 | 86.6 | 8.8 |
| 1 | 86.4 | 3.1 | 88.8 | 8.1 | |
| 0.5 | 85.3 | 3.2 | 89.3 | 7.5 | |
| Pd2/HAP-6 | 1.5 | 97.5 | 5.0 | 83.4 | 11.6 |
| 1 | 96.2 | 4.5 | 86.2 | 9.3 | |
| 0.5 | 94.6 | 4.6 | 85.9 | 9.5 | |
Fig. 5 shows the 1,2-propanediol conversions and the product selectivities in the catalytic oxidation of 1,2-propanediol over Pd1Ag1/HAP catalyst under different NaOH concentrations. It was found that increasing NaOH concentration favored the catalytic oxidation of 1,2-propanediol to lactic acid. When the NaOH concentrations were 0.28, 0.56, 0.84, and 1.12 mol L−1, 1,2-propanediol was completely converted after reacting at 100 °C for 3 h. With increasing the reaction time from 0.5 to 3 h, the lactic acid selectivities slightly decreased from 86.8% to 81.5%, 90.1% to 88.2%, 91.7% to 91%, and 92% to 91%, respectively. The selectivities of formic and acetic acids were less than 3.6% and 8.2% under high NaOH concentrations of 0.56–1.12 mol L−1, respectively. When the NaOH concentration was more than 0.28 mol L−1, further increasing NaOH concentration had a minor effect on 1,2-propanediol conversion and product selectivity.
The 1,2-propanediol conversions and product selectivities over Pd1Ag1/HAP catalyst at 100 °C with different catalyst loadings are shown in Fig. 6. When the catalyst loadings were 0.5, 1.0, and 1.5 g, the reaction times for complete conversion of 1,2-propanediol were 3, 2, and 1.5 h, respectively. The selectivities of lactic acid were around 89%, 88%, and 86%. The total selectivities of formic and acetic acids were less than 15%. The results showed that increasing catalyst loading increased the oxidation rate of 1,2-propanediol to lactic acid. With high catalyst loading, varying the catalyst loading had a minor effect on the lactic acid selectivity.
3.6. Reaction kinetics
A power-function type kinetic equation was used to evaluate the effect of 1,2-propanediol concentration, O2 pressure, and reaction temperature on the oxidation rate of 1,2-propanediol. The effect of NaOH concentration on the reaction rate was ignored herein because when the NaOH concentration was more than 0.28 mol L−1, further increasing NaOH concentration had no obvious effect on the oxidation of 1,2-propanediol under our present experimental conditions. To eliminate the effect of diffusion, Pd1Ag1/HAP with different loadings in the range of 0.25–1.5 g was used for the oxidation of 1,2-propanediol with the concentration of 0.28 mol L−1. A linear correlation between the catalyst loading and the 1,2-propanediol conversion was observed at first 0.5 h (Fig. S2†). This result indicated that the initial oxidation rate was controlled only by chemical reaction rather than mass diffusion. On the other hand, side reactions for the formation of formic and acetic acids were ignored because the initial reaction rate was used to fit the reaction kinetics and the amount of formic and acetic acids were very small at initial reaction step.The power function-type reaction kinetic equation can be expressed as follows.
 | (1) |
Linear eqn (2) is obtained by taking the natural logarithm of both sides of the eqn (1).
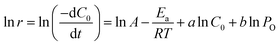 | (2) |
To calculate the reaction orders (a, b) and activation energy (Ea) according to eqn (2), the initial rates were calculated according to the data shown in Fig. S3.† The initial reaction rates of 1,2-propanediol under different reaction conditions were calculated at the first 0.5 h. The values of pre-exponential factors (A), activation energies (Ea), and reaction orders (a, b) over Pd1Ag1/HAP, Pd2/HAP-6, and Ag2/HAP catalysts are calculated by the multiple linear regression method and listed in Table 6.
| Catalysts | ln(A) | Standard errors | A | −Ea/R (10−3 K) | Standard errors | Ea (kJ mol−1) | a | Standard errors | b | Standard errors | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd1Ag1/HAP | 5.0 | 0.8 | 148.3 | −2.5 | 0.3 | 20.8 | 0.3 | 0.1 | 0.05 | 0.1 | 0.9352 |
| Pd2/HAP-6 | 6.6 | 1.2 | 734.6 | −2.9 | 0.4 | 24.1 | 0.6 | 0.1 | 0.04 | 0.1 | 0.9614 |
| Ag2/HAP | 6.8 | 1.7 | 897.2 | −3.7 | 0.6 | 30.8 | 0.4 | 0.2 | 0.07 | 0.2 | 0.9324 |
All experimental data gave good correlation coefficients for the power-function type kinetics in the range of 0.9324–0.9614, indicating that the power-function type kinetic model was appropriate for evaluating the effect of 1,2-propanediol concentration, O2 pressure, and reaction temperature on the oxidation of 1,2-propanediol to lactic acid. Over Pd1Ag1/HAP, Pd2/HAP-6, and Ag2/HAP catalysts, the reaction kinetics were listed as follows.
 | (3) |
 | (4) |
 | (5) |
The activation energies over Pd1Ag1/HAP, Pd2/HAP-6, and Ag2/HAP were 20.8, 24.1 and 30.8 kJ mol−1, respectively. The activation energy over Pd1Ag1/HAP catalyst was lower than those over Pd2/HAP-6 and Ag2/HAP catalysts. The frequency factor over the Pd1Ag1/HAP catalyst was also lower than those over Pd2/HAP-6 and Ag2/HAP catalysts. The results indicated that there could be different kinds of catalytic active sites on the surfaces of the catalysts.
The reaction orders with respect to 1,2-propanediol concentration over Pd1Ag1/HAP, Pd2/HAP-6, and Ag2/HAP catalysts were estimated to 0.3, 0.6 and 0.4, respectively. The low reaction orders indicated that Pd1Ag1/HAP catalyst more strongly adsorbed 1,2-propanediol than Pd2/HAP-6 and Ag2/HAP catalysts.42 The reaction orders with respect to O2 pressure over Pd1Ag1/HAP, Pd2/HAP-6, Ag2/HAP catalysts were close to zero, being consistent with the catalytic result that the 1,2-propanediol conversions kept constant in the O2 pressure range of 0.5–2 MPa over Pd1Ag1/HAP, Pd2/HAP-6, Ag2/HAP catalysts, respectively.
3.7. Reaction route
HAP-supported monometallic Pd catalyst showed high catalytic activity in selective oxidation of 1,2-propanediol to lactic acid, which could be due to that Pd was beneficial for decreasing the coverage of strongly bound adsorbates in the catalytic oxidation of 1,2-propanediol, resulting in high lactic acid selectivity.12 On the other hand, the basic sites of the support HAP also played important roles in the enhancement of selective oxidation of 1,2-propanediol to lactic acid.40,41 Furthermore, Pd–Ag/HAP bimetallic catalyst decreased the activation energy for the oxidation of 1,2-propanediol, giving high catalytic activity in the selective oxidation of 1,2-propanediol to lactic acid.The mechanism for the oxidation of 1,2-propanediol is complex, which may involve the oxidation of the primary and secondary hydroxyl groups, as well as the base-induced tautomeric equilibrium and Cannizzaro reaction, as shown in Scheme 1.8,9,14,15 When the oxidation of 1,2-propanediol over Pd/HAP or Pd1Ag1/HAP catalyst was carried out under an optimal reaction condition, lactic acid was formed as the main product, revealing that the primary hydroxyl oxidation is dominant. If the secondary hydroxyl oxidation occurs, 1,2-propanediol converted to hydroxyacetone, and subsequently to pyruvaldehyde, as well as to low carbon acids such as acetic acid or formic acid.8,9 By comparison of the catalytic results (Table 2), the oxidative cleavage products (formic acid and acetic acid) were evidently generated when Ag2/HAP and Pd2/C-6 were used as catalysts. This indicates that the hydroxyl oxidation can be controlled by changing the active component and support. However, neither hydroxyacetone or pyruvaldehyde was detected under our experimental conditions, indicating that hydroxyacetone immediately converted to lactaldehyde and pyruvaldehyde, which rapidly converted to lactic acid in an alkaline solution.8
 | ||
| Scheme 1 Reaction routes for catalytic oxidation of 1,2-propanediol over Pd–Ag/HAP or Pd/HAP catalysts in an alkaline solution.8,9,14,15 | ||
3.8. Recycling performance
The recycling performances of Pd1Ag1/HAP and Pd2/HAP-6 catalysts in the catalytic oxidation of 1,2-propanediol were also investigated. After reacting at 100 °C for 2 h, the used catalyst was filtered and dried at 120 °C for 12 h before next recycling. As shown in Table S1,† for the fresh Pd1Ag1/HAP catalyst, the 1,2-propanediol conversion and lactic acid selectivity were 86.3% and 88.8%, respectively. After recycling for 4 times, the 1,2-propanediol conversion and lactic acid selectivity over the spent Pd1Ag1/HAP catalyst were 79.6% and 87%, respectively. The TONs showed that the spent Pd1Ag1/HAP still had high catalytic activity for 1,2-propanediol oxidation. After recycling for 4 times, the weight percentages of Pd and Ag in the spent Pd1Ag1/HAP catalyst were 0.93% and 0.95%, respectively, indicating that only a small amount of Pd and Ag was leached during the reaction as compared to those in the fresh catalyst. The 1,2-propanediol conversions over Pd2/HAP-6 catalyst decreased from 96.2% to 70.6% with increasing the recycling times from 1 to 4, probably due to that about 20% Pd of the fresh Pd2/HAP catalyst was leached after recycling for 4 times. The results showed that the Pd–Ag/HAP bimetallic catalyst had better recycling performance than the Pd/HAP monometallic catalyst.4. Conclusions
The Pd/HAP and Pd–Ag/HAP catalysts were prepared by the sol-immobilization method. The particle sizes of Pd nanocubes were tuned from 6 to 18 nm by changing the amount of KBr and PVP. Pd and Ag nanoparticles coalesced in Pd–Ag/HAP catalysts.When the catalytic oxidation of 1,2-propanediol with O2 was carried out at 100 °C for 2 h in NaOH aqueous solution, over Pd2/HAP-6 catalyst, the lactic acid selectivity was 86.2% at the 1,2-propanediol conversion of 96.2%, and over Pd1Ag1/HAP catalyst, the lactic acid selectivity was 88.8% at the 1,2-propanediol conversion of 86.3%.
Small-sized Pd nanocubes in Pd/HAP catalysts favored the 1,2-propanediol oxidation to lactic acid as compared to large-sized ones. Pd/HAP catalysts with cubic Pd nanoparticles exhibited higher lactic acid selectivity than those with spherical Pd nanoparticles in oxidation of 1,2-propanediol. The coalesced Pd and Ag nanoparticles in the Pd–Ag/HAP catalysts synergistically catalyzed the selective oxidation of 1,2-propanediol to lactic acid.
The activation energies in the catalytic oxidation of 1,2-propanediol over the catalysts were in an order of Ea (Pd1Ag1/HAP) < Ea (Pd2/HAP-6) < Ea (Ag2/HAP).
Acknowledgements
The authors sincerely thank Professor K. Chen (Jiangsu University) for supporting the TEM and HRTEM measurements. This work was financially supported by research funds from Jiangsu Province Education Bureau (No. 11KJB530002, 1102120C), Jiangsu University for Young Researchers (No. 11JDG028, 2010-4849), China Postdoctoral Foundation Committee (No. 2011M500866) and Programs of Senior Talent Foundation of Jiangsu University (No. 15JDG024).References
- W. Chumeka, P. Pasetto, J. F. Pilard and V. Tanrattanakul, Polymer, 2014, 55, 4478–4487 CrossRef CAS.
- A. Södergard and M. Stolt, Prog. Polym. Sci., 2002, 27, 1123–1163 CrossRef.
- G. Juodeikiene, D. Vidmantiene, L. Basinskiene, D. Cernauskas, E. Bartkiene and D. Cizeikiene, Catal. Today, 2015, 239, 11–16 CrossRef CAS.
- Y. J. Wee, J. N. Kim and H. W. Ryu, Food Technol. Biotechnol., 2006, 44, 163–172 CAS.
- L. Pratil and M. Rossi, J. Catal., 1998, 176, 552–560 CrossRef.
- P. Pal, J. Sikder, S. Roy and L. Giorno, Chem. Eng. Process., 2009, 48, 1549–1559 CrossRef CAS.
- P. G. N. Mertens, M. Bulut, L. E. M. Gevers, I. F. J. Vankelecom, P. A. Jacobs and D. E. De Vos, Catal. Lett., 2005, 102, 1–2 CrossRef.
- N. Dimitratos, J. A. Lopez-Sanchez, S. Meenakshisundaram, J. M. Anthonykutty, G. Brett, A. F. Carley, S. H. Taylor, D. W. Knighta and G. J. Hutchings, Green Chem., 2009, 11, 1209–1216 RSC.
- H. Ma, X. Nie, J. Cai, C. Chen, J. Gao, H. Miao and J. Xu, Sci. China: Chem., 2010, 53, 1497–1501 CrossRef CAS.
- Y. Ryabenkova, P. J. Miedziak, N. F. Dummer, S. H. Taylor, N. Dimitratos, D. J. Willock, D. Bethell, D. W. Knight and G. J. Hutchings, Top. Catal., 2012, 55, 1283–1288 CrossRef CAS.
- Y. Ryabenkova, Q. He, P. J. Miedziak, N. F. Dummer, S. H. Taylor, A. F. Carley, D. J. Morgan, N. Dimitratos, D. J. Willock, D. Bethell, D. W. Knight, D. Chadwick, C. J. Kiely and G. J. Hutchings, Catal. Today, 2013, 203, 139–145 CrossRef CAS.
- M. B. Griffin, A. A. Rodriguez, M. M. Montemore, J. R. Monnier, C. T. Williams and J. W. Medlin, J. Catal., 2013, 307, 111–120 CrossRef CAS.
- C. D'Agostino, Y. Ryabenkova, P. J. S. H. T. Miedziak, G. J. Hutchings, L. F. Gladdena and M. D. Mantle, Catal. Sci. Technol., 2014, 4, 1313–11322 Search PubMed.
- Y. Feng, H. Yin, A. Wang, D. Gao, X. Zhu, L. Shen and M. Meng, Appl. Catal., A, 2014, 482, 49–60 CrossRef CAS.
- Y. Feng, H. Yin, D. Gao, A. Wang, L. Shen and M. Meng, J. Catal., 2014, 316, 67–77 CrossRef CAS.
- D. Tongsakul, S. Nishimura and K. Ebitani, J. Phys. Chem. C, 2014, 118, 11723–11730 CAS.
- R. M. Williams and J. W. Medlin, Surf. Sci., 2014, 619, 30–38 CrossRef CAS.
- D. Liang, C. Liu, S. Deng, Y. Zhu and C. Lv, Catal. Commun., 2014, 54, 108–113 CrossRef CAS.
- Y. Feng, H. Yin, A. Wang, L. Shen, L. Yu and T. Jiang, Chem. Eng. J., 2011, 168, 403–412 CrossRef CAS.
- A. Bienholz, H. Hofmann and P. Claus, Appl. Catal., A, 2011, 391, 153–157 CrossRef CAS.
- L. Zhao, J. H. Zhou, Z. J. Sui and X. G. Zhou, Eng. Sci., 2010, 65, 30–35 CrossRef CAS.
- C. Montassier, J. C. Ménézo, L. C. Hoang, C. Renaud and J. Barbier, J. Mol. Catal., 1991, 70, 99–110 CrossRef CAS.
- T. J. A. Foskey, D. M. Heinekey and K. I. Goldberg, ACS Catal., 2012, 2, 1285–1289 CrossRef.
- S. Wang, K. Yin, Y. Zhang and H. Liu, ACS Catal., 2013, 3, 2112–2121 CrossRef CAS.
- B. Li, J. Wang, Y. Yuan, H. Ariga, S. Takakusagi and K. Asakura, ACS Catal., 2011, 1, 1521–1528 CrossRef CAS.
- J. Hu, Y. Fan, Y. Pei, M. Qiao, K. Fan, X. Zhang and B. Zong, ACS Catal., 2013, 3, 2280–2287 CrossRef CAS.
- B. M. Bhanage, S. Fujita, Y. Ikushima and M. Arai, Green Chem., 2003, 5, 429–432 RSC.
- http://www.docin.com/p-445508830.html.
- http://mcgroup.co.uk/news/20140418/propylene-glycol-market-reach-supplydemand-balance-2015.html.
- T. Tsujino, T. Ohigashi, S. Sugiyama, K. Kawashiro and H. Hayashi, J. Mol. Catal., 1992, 71, 25–35 CrossRef CAS.
- A. Wang, H. Yin, D. Liu, H. Wu, M. Ren, T. Jiang, X. Cheng and Y. Xu, Mater. Lett., 2007, 61, 2084–2088 CrossRef CAS.
- A. Wang, D. Liu, H. Yin, H. Wu, Y. Wada, M. Ren, T. Jiang, X. Cheng and Y. Xu, Mater. Sci. Eng., C, 2007, 27, 865–869 CrossRef CAS.
- J. Fang, J. Li, B. Zhang, X. Yuan, H. Asakura, T. Tanaka, K. Teramura, J. Xie and N. Yan, Nanoscale, 2015, 7, 6325–6333 RSC.
- C. Wagner, A. Naumkin, A. Kraut-Vass, J. Allison, C. Powell and J. Rumble Jr, NIST Standard Reference Database 20, ver. 3.4, 2006, http://www.srdata.nist.gov/xps/index.htm Search PubMed.
- Y. Yan, X. Zhang, C. Li, Y. Huang, Q. Ding and X. Pang, Appl. Surf. Sci., 2015, 332, 62–69 CrossRef CAS.
- P. A. Kumar, M. P. Reddy, L. K. Ju and H. H. Phil, Catal. Lett., 2008, 126, 78–83 CrossRef CAS.
- B. Zhang, Y. Yuan, K. Philippot and N. Yan, Catal. Sci. Technol., 2015, 5, 1683 CAS.
- D. Gao, Y. Feng, H. Yin, A. Wang and T. Jiang, Chem. Eng. J., 2013, 233, 349–359 CrossRef CAS.
- W. Niu, Y. Gao, W. Zhang, N. Yan and X. Lu, Angew. Chem., Int. Ed., 2015, 54, 8271–8274 CrossRef CAS PubMed.
- J. Zhang, M. Gandelman, L. J. W. Shimon, H. Rozenberg and D. Milstein, Organometallics, 2004, 3, 4026–4033 CrossRef.
- K. Shimizua, K. Kon, K. Shimura and S. S. M. A. Hakim, J. Catal., 2013, 300, 242–250 CrossRef.
- S. Hirasawa, H. Watanabe, T. Kizuka, Y. Nakagawa and K. Tomishige, J. Catal., 2013, 300, 205–216 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM image of Pd2/HAP-6-S (Fig. S1), the relationship between catalyst loading and 1,2-propanediol conversion (Fig. S2), conversions of 1,2-propanediol over Pd1Ag1/HAP, Pd2/HAP-6, and Ag2/HAP catalysts under different reaction conditions (Fig. S3), and the recycling performances of Pd1Ag1/HAP and Pd2/HAP-6 catalysts (Table S1). See DOI: 10.1039/c5ra21410f |
| This journal is © The Royal Society of Chemistry 2015 |


