Label-assisted laser desorption/ionization mass spectrometry (LA-LDI-MS): use of pyrene aldehyde for detection of biogenic amines, amino acids and peptides†
Abstract
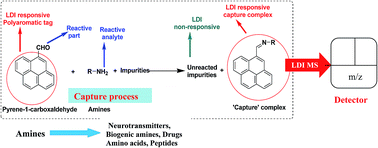
Label-assisted laser desorption/ionization (LA-LDI) technique has recently been applied to the detection of small molecules through a time of flight (TOF) mass spectrometric measurement. By excluding the external matrix, the mass spectrum becomes much cleaner being free of matrix related peaks and noises. Peaks corresponding to compounds/complexes formed between the analytes and the label are mostly seen in the spectrum. In this paper we report a LA-LDI mass spectrometry based method for detection of amines, including the ones ubiquitous to biological samples like biogenic amines, amino acids, di- and tripeptides using 1-pyrene carboxaldehyde as the LDI label.

 Please wait while we load your content...
Please wait while we load your content...