Organometallic polymers for electrode decoration in sensing applications
Xueling Feng
,
Kaihuan Zhang
,
Mark A. Hempenius
and
G. Julius Vancso
*
Materials Science and Technology of Polymers, MESA+ Institute for Nanotechnology, University of Twente, 7500 AE Enschede, The Netherlands. E-mail: g.j.vancso@utwente.nl; Fax: +31 53 489 3823; Tel: +31 53 489 2967
First published on 1st December 2015
Abstract
Macromolecules containing metals combine the processing advantages of polymers with the functionality offered by the metal centers. This review outlines the progress and recent developments in the area of electrochemical chemo/biosensors that are based on organometallic polymers. We focus on materials in which the metal centers provide function, allowing these materials to be used in electrochemical sensing applications based on various transduction mechanisms. Examples of chemo/biosensors featuring organometallic polymers that possess Fe, Os, Co and Ru are discussed.
1. Introduction
Organometallic polymers or metallopolymers refer to a wide range of metal-containing polymers, which have attracted rapidly expanding interest due to their unique chemical and physical properties and potential applications.1–9 Different metallic centers in these substances can adopt various coordination numbers, oxidation states and different coordination geometries. Additionally, different chain geometries, degrees of polymerization, types of bonding (covalent or supramolecular) and variation of other parameters of the primary chemical structure provide access to new and versatile classes of functional materials.10 The presence of metals in their main chain or side groups enables organometallic polymers to potentially play an unprecedented role in advanced technology in areas including nanoscale manufacturing,11,12 ceramics precursors,13 ferromagnetic materials,14 separation, drug delivery,15 molecular motors16 and actuators,17 photovoltaic devices,18 catalysis,19 sensing, energy conversion and storage, etc.Interest in the development of new, easily processible materials that feature metal centers in synthetic polymer chains motivated scientists to tackle the synthesis of poly(vinylferrocene) by radical-polymerization.20 Since this discovery, numerous synthetic approaches have been developed and adapted including polycondensation,21,22 controlled radical polymerization, living ionic polymerization, ring-opening polymerization,23–25 and electropolymerization26,27 to form organometallic polymers that include main-chain or side-chain metal centers. Synthetic advances have also expanded from those that make use of traditional covalent bonds to incorporate metal centers in polymers, to approaches which use potentially reversible, “dynamic” binding by non-covalent coordination interactions that yield organometallic supramolecular polymers.1,28 Numerous reviews and monographs highlight and summarize the developments in the organometallic polymer field.25,28–36
Stimulus responsive, or smart macromolecular materials which exhibit abrupt conformational and chemical changes in response to small variations of external stimuli, are of intense current interest.37–39 The incorporation of metal centers in organometallic polymers offers many unique opportunities in the area of stimuli responsive materials. In most cases, organometallic polymers have intrinsic redox and luminescent properties inherited from the presence of metal centers and have been explored, e.g. as potential sensing materials, as they are capable of responding to external stimuli and by signals, which can be measured or recorded.40,41
Chemo/biosensors have been intensively researched due to their impact on numerous fields, such as in industrial processes, in vitro diagnostics (IVD), food quality control, chemical threat detection and environmental monitoring.42 A chemo/biosensor is a device that detects the presence of particular chemical substances, a class of chemicals, or the occurrence of certain chemical reactions and biological cues qualitatively or quantitatively.40 Chemo/biosensors have been developed for cations, anions, acids, vapors, volatile organics, biomolecules and for numerous other systems.42–44 Usually, the sensor contains a receptor which can selectively respond to a particular analyte, or register chemical or biological changes; a transducer which converts this response into electrical (or other) signals and a processor which collects, amplifies, and provides a read-out of the signal.41 Transducers include amperometric, potentiometric, gravimetric, piezoelectric, thermal or optical devices.
Many commercial sensors have been developed based on inorganic-semiconductor or organic-polymeric films that react with the analyte molecules.42,45 The changes in chemical or physical properties of these films are monitored. Typically the concentration and chemical or physical characteristics of the analytes would determine the magnitude of the response. Although a variety of chemo/biosensors have been successfully commercialized, there is still a strong need for improvement in sensor fabrication with new materials and transduction mechanisms to enhance the sensing sensitivity, selectivity, reliability and robustness of the sensing process.
Owing to the intrinsic luminescent properties of some metals, organometallic polymers featuring such metallic centers have been widely used as luminescence sensors by monitoring the fluorescence or phosphorescence change of the sensing system due to the presence of analytes. Several reviews and articles discuss the development of luminescent chemo/biosensors based on organometallic polymers.9,40,46–50 Organometallic polymers are also used as mechanical probes,51 to fabricate sensitive membranes in surface acoustic wave devices for humidity sensing,52–55 or in quartz crystal microbalance (QCM) devices to detect and quantify organic vapors.56 Owing to the intrinsic redox and affinity properties of the metal, organometallic polymers have been employed in a variety of electrochemical sensors by detecting the current, redox potential or resistance changes of the sensing system.
In this review, we survey the recent developments and highlight some milestones related to designing electrochemical chemo/biosensors with organometallic polymers (Fig. 1).
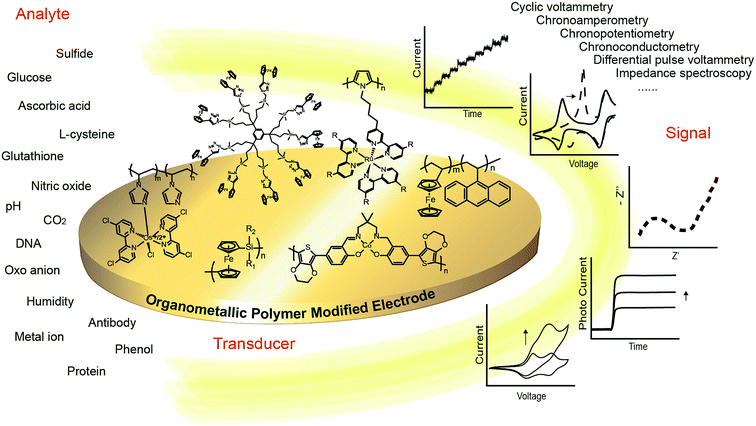 | ||
| Fig. 1 A schematic of electrochemical chemo/biosensors based on organometallic polymer modified electrodes. | ||
2. Scope of organometallic polymers and metalorganic structures
Organometallic polymers can contain a variety of metal centers including main group (p-block) metals such as Sn and Pb,57 transition metals such as Fe, Ir, Ru,47 Cr, Os,58 Pt,57 Ag, Co,59 or lanthanides and actinides such as Eu.2 The position of the metal centers in the polymers and the nature of the linkages between them define the various structural types.1 Based on the location of the metal centers, organometallic macromolecules can be divided into polymers with metal moieties embedded within the polymer backbone (Fig. 2, polymer 1, 2) and in the pendant side groups (Fig. 2, polymer 3).59 Considering the geometrical structure of macromolecules, the organometallic polymers may be linear (Fig. 2, polymer 2), star-shaped (Fig. 2, polymer 4) or dendritic (Fig. 3).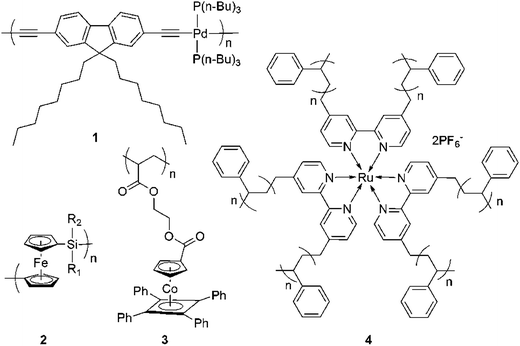 | ||
| Fig. 2 Examples of organometallic polymers. (1) Pd(II)-containing fluorene-based polymetallaynes,57 (2) poly(ferrocenylsilanes),25 (3) side chain Co(I) polymers featuring cyclopentadienyl-cobalt-cyclobutadiene (CpCoCb) units59 and (4) Ru(II)-containing star-shaped polymer.60 | ||
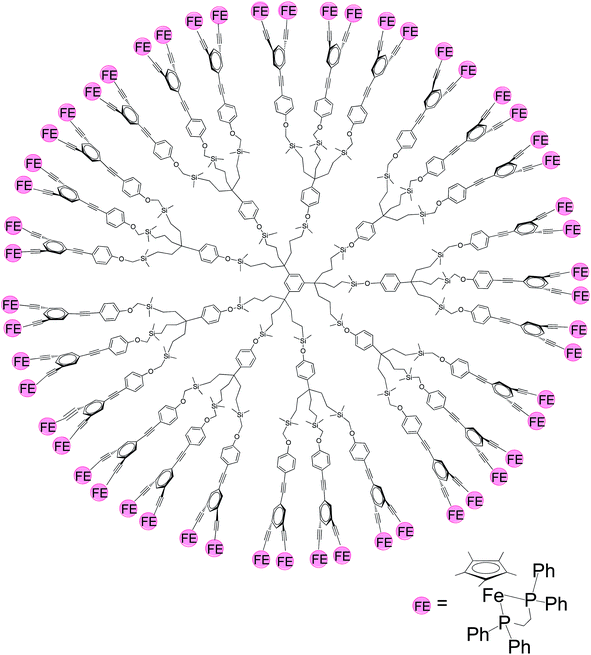 | ||
| Fig. 3 Dendrimer with redox-active Cp*FeII(dppe)-alkynyl centers (Cp* = η5-C5Me5, dppe = 1,2-bis(diphenylphosphino)ethane).62 | ||
The linkages binding the metals can be covalent, or non-covalent. Covalent linkages enable irreversible or “static” binding of the metal while non-covalent coordination can allow potentially reversible “dynamic” binding, forming organometallic supramolecular polymers (Fig. 4).1 Metal–organic frameworks (MOFs), consisting of metal ions coordinated to organic molecules, are special organometallic polymers which represent an interesting class of crystalline molecular materials synthesized by combining metal-connecting points and bridging ligands with one-, two-, or three-dimensional structures.61
 | ||
| Fig. 4 Organometallic polymer obtained by “dynamic binding” using M2+ complexation by the tritopic bis-terpyridine cyclam ligand.63 Reprinted with permission from ref. 63. Copyright (2013) Elsevier Inc. | ||
3. Electrochemical sensors
Electrochemical chemo/biosensors should possess high selectivity, excellent sensitivity, low cost, ease of use, portability and simplicity of construction.64 The analytes and reactions being monitored by electrochemical methods typically cause a measurable current (amperometry), a measurable charge accumulation or potential (potentiometry), or alter the conductive properties of the medium between electrodes (conductometry).41 Many signal transduction schemes require a physical interface which generally involves chemically modified electrodes65 to tune electrochemical responses to analytes and improve detection sensitivity, selectivity and device stability. When preparing chemically modified electrodes, a thin film with a particular chemical composition and certain architecture is usually coated onto, or chemically bound to, the electrode surface in a rationally designed way, providing the desired properties to the electrode.66Major basic designs of thin polymer films include end-tethered polymer chains, films from functional particles, electrostatic layer-by-layer assembled films,67 block-copolymer films, crosslinked thin polymer or hydrogel thin films, porous films, etc.68–70 The techniques involved to obtain these films include drop-casting and spin-coating, inkjet printing, doctor blading, layer-by-layer assembly, grafting to and from methods, electropolymerization, etc.71–73
Electrodes, modified with organometallic polymers, possess many interesting features that can be exploited for electroanalytical and sensor applications. The properties of the electrode and its sensing ability are easily controlled by carefully choosing the proper metal, the ligands and the decoration architectures. For example, by adapting the ligands to a certain metal (e.g. iron, cobalt), the redox properties can be tuned as the standard electrode potential is influenced by the ligands (Table 1). Additionally, organometallic polymer decorated electrodes often have a large surface with high redox-active center loadings.
| Half-reactions | E0/V |
|---|---|
| Fe3+ + e− → Fe2+ | +0.77 |
| Fe(phen)33+ + e− → Fe(phen)32+ | +1.13 |
| Fe(CN)63− + e− → Fe(CN)64− | +0.36 |
| [Ferrocenium]+ + e− → Ferrocene | +0.40 |
| Co3+ + e− → Co2+ | +1.92 |
| Co(NH3)63+ + e− → Co(NH3)62+ | +0.06 |
| Co(phen)33+ + e− → Co(phen)32+ | +0.33 |
| Co(C2O4)33− + e− → Co(C2O4)34− | +0.57 |
3.1 Sensors based on ferrocene-containing organometallic polymers
Metallocenes exhibit remarkable electronic and optical properties which make them versatile building blocks for incorporation into polymer systems. Ferrocene (Fc) and its derivatives represent the most common metallocenes applied in organometallic polymers. Discovered in 1951,75 ferrocene has a “sandwich” like structure with two cyclopentadienyl (Cp) rings coordinated to one Fe(II) cation as a neutral complex. The complex is small with dimensions of 4.1 Å × 3.3 Å, while the ferrocenium ion, the oxidized form of ferrocene, has dimensions of 4.1 Å × 3.5 Å.76 Considering the steric requirements, van der Waals radii have been recommended for indicating the molecular dimensions.77 Thus, the neural species is ca. 6.7 Å long along the cylinder axis and ca. 5.7 Å wide.78 The dimensions of the ferrocenium ion are only slightly larger (cylinder axis of ca. 6.8 Å, diameter ca. 5.9 Å). Because of the favorable electrochemical characteristics, such as low oxidation potential (pH-independent), fast electron-transfer rate, high levels of stability in its two redox states, low cost, and well-defined synthetic procedures for many derivatives, ferrocene has proved to be an effective building block in electrocatalysis and electrochemical sensing materials.79,80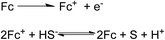 | ||
| Scheme 1 The electrocatalytic reaction of sulfide with ferrocene moieties in a pH/sulfide electrochemical sensor.81 | ||
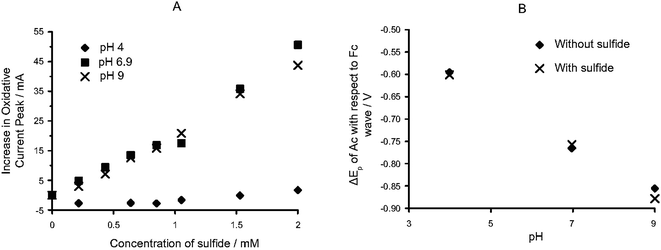 | ||
| Fig. 5 (A) Dual pH/sulfide electrochemical sensor. Calibration plot of the normalized peak current of the ferrocene vs. sulfide concentration, at various pH values. (B) Calibration plot of the variation in the peak potential of the anthracene units with respect to the ferrocene units, as a function of pH.81 Reprinted with permission from ref. 81. Copyright (2006) Wiley-VCH. | ||
Electrodes covered with this organometallic polymer are also pH sensitive. The two-electron oxidation potential of the anthracene moiety was linearly related to the pH value (Fig. 5B), and followed a Nernstian response with the protons, while the redox-active but pH-insensitive ferrocene moiety acts as the reference species (Fig. 6). Additionally, the pH response was found to be temperature independent, showing an insignificant variation (<10 mV) over a range of temperatures. The ferrocene units here had a dual role, as an internal calibrating agent for the system and as an electrocatalyst involved in the sensing mechanism. Owing to the support of the polymer chain, the signal of the ferrocene group in this organometallic polymer at elevated temperatures showed a superior stability compared to that of ferrocene in solutions.82
 | ||
| Fig. 6 The proposed electrochemical pathway for anthracene and ferrocene moieties in a pH/sulfide electrochemical sensor.81 | ||
Using similar organometallic polymers, the pH sensing ability was enhanced by associating the polymers with carbon nanotubes (CNTs).83 In such systems the plot of the anthracene moiety peak potential against pH was linear up to at least pH 11.6, showing a wider pH sensing range.
Zhang et al. reported the fabrication of cationic poly(allylamine)ferrocene grafts on the surface of a gold electrode modified with negatively charged alkanethiols by electrostatic interaction.84 The modified electrode was used as an ascorbic acid sensor. The cyclic voltammogram of the decorated electrode showed, upon addition of ascorbic acid, an increase of the catalytic current and a decrease of the overpotential of ascorbic acid, which provides evidence for excellent electrocatalytic performance of the ferrocene-containing polymer in ascorbic acid oxidation. The modified electrode has many advantages as it is simple to fabricate, has a fast response and good chemical and mechanical stability.
Organometallic polymers containing ferrocene moieties have also been designed and synthesized to construct amperometric biosensors with enzymes in which the Fc moieties act as mediators to enable the shuttling of electrons between the enzymes and the electrode.85 In most cases, the electrode surfaces were prepared by drop casting using mixtures of organometallic polymers and enzymes. Examples of redox ferrocene-containing organometallic polymers used in enzymatic sensing include poly(vinylferrocene-co-hydroxyethyl methacrylate),86 ferrocene-containing polyallylamine,87 poly(glycidyl methacrylate-co-vinylferrocene),88 ferrocene-branched chitosan derivatives89 etc.
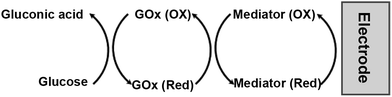
The first generation of oxidase-based amperometric biosensors was based on the immobilization of oxidase enzymes on the surface of various electrodes. For these systems, the efficiency of electron transfer from the enzymes to the electrode has been found to be poor in the absence of a mediator. Taking the glucose biosensors as a model, the electron transfer between glucose oxidase (GOx) active sites and the electrode surface is the limiting factor in the performance of amperometric glucose biosensors. Because of the thick protein layer surrounding its flavin adenine dinucleotide (FAD) redox center as an inherent barrier, glucose oxidase does not directly transfer electrons to conventional electrodes.85 In GOx biosensors employing organometallic redox mediators, the metal center shuttles electrons between the FAD center and the electrode surface, thus significantly improving the performance of the sensors. The mediation cycle is shown in Fig. 7, and the reactions involved are as follows (Scheme 2).
 | ||
| Fig. 7 Sequence of events occurring in mediator-based glucose biosensors.85 Reprinted with permission from ref. 85. Copyright (2008) American Chemical Society. | ||
 | ||
| Scheme 2 Redox reactions in mediator-based glucose biosensors.85 | ||
M(ox) and M(red) are the oxidized and reduced forms of the mediator, respectively. In this process two electrons are transferred from glucose to the redox centers of the GOx. These electrons then are transferred to the mediator, forming the reduced form of the mediator. The reduced form is re-oxidized at the electrode, giving a current signal proportional to the glucose concentration as the oxidized form of the mediator is regenerated.90
Hydrogel films were obtained by crosslinking the drop casted films of enzymes and organometallic polymers to improve the stability and performance of the related biosensors. New materials were developed, with the aim of tailoring the interaction between the redox polymer and the enzyme and optimizing the electron transfer between them. Polymer flexibility or segmental mobility, degree of functional density and hydration properties would all have impact on the performance of the sensor.91 For example, redox polymer hydrogel films with glucose oxidase were formed by photoinitiated free-radical polymerization of poly(ethylene glycol) and vinylferrocene with a film thickness of ∼100 μm.90 Electrodes decorated with a crosslinked thin film of ferrocene-bearing poly(ethyleneimine) (PEI) and glucose oxidase hydrogel have also been utilized as glucose sensor.91,92 Efforts to reduce the film thickness have been made, as this was believed to enhance the sensing ability. To this end, by using crosslinkable polymers, it was possible to generate polymer coatings with varying thickness. Rühe and co-workers described the synthesis of poly(dimethylacrylamide) polymers containing both electroactive ferrocene moieties and photoreactive benzophenone groups which reacted as crosslinker.93 The ferrocene containing polymer was mixed with GOx and was deposited as a thin film on the electrode surface. The polymer layer was cross-linked and became firmly adhered to the electrode as a hydrogel thin film upon brief irradiation with UV light. Glucose-oxidizing electrodes with very high catalytic current responses were obtained.
In another study, a thermoresponsive poly(N-isopropylacrylamide) (PNIPAM)-ferrocene polymer was synthesized and attached to a cysteamine-modified gold electrode by a simple grafting to method, forming a thin hydrophilic organometallic polymer film (Fig. 8).94 The organometallic polymer acted as a covalently bound mediator. The flexible, brush-like redox polymer thin layers allowed an efficient interaction with the enzyme [soluble glucose dehydrogenzase (sGDH)] and enabled electrical communication between the cofactor pyrrolinoquinoline quinone (PQQ) of sGDH in the presence of glucose. At elevated temperature, the polymer shrank and the brush-like structure disappeared. Thus, the electron transfer between the electrode and sGDH could be controlled.
 | ||
| Fig. 8 Fabrication of a covalently bound PNIPAM-ferrocene thin film on a gold electrode by a simple grafting to method.94 Adapted with permission from ref. 94. Copyright (2007) American Chemical Society. | ||
Polymer brushes containing ferrocene groups have been explored for decorating electrodes for electrochemical sensing. For example, Kang and co-workers developed an enzyme-mediated amperometric biosensor on ITO electrodes via surface-initiated atom-transfer radical polymerization (ATRP) of ferrocenylmethyl methacrylate (FMMA) and glycidyl methacrylate (GMA) (Fig. 9) under chemical control.95 By ATRP, a ferrocene-containing organometallic polymer brush film was introduced on the electrode surface. Glucose oxidase was subsequently immobilized via coupling reactions to the glycidyl group in GMA segments. With the introduction of a redox-P(FMMA) block as the electron-transfer mediator, the enzyme-mediated ITO electrode exhibited high sensitivity.
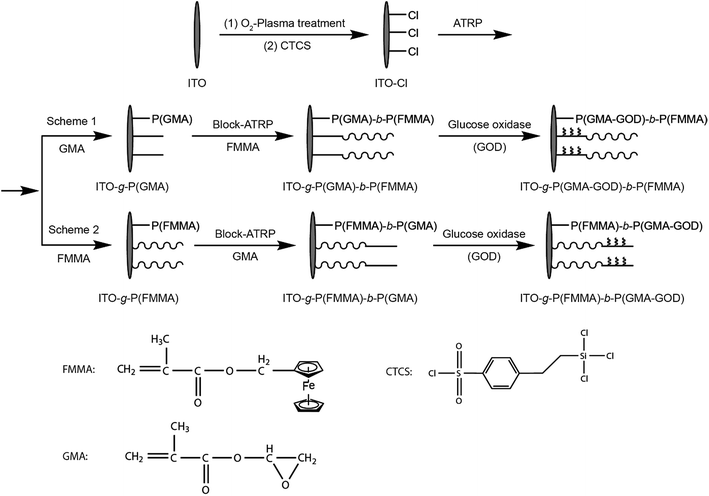 | ||
| Fig. 9 Ferrocene containing polymer brushes by SI-ATRP and the immobilization of Glucose oxidase on the thin film.95 Adapted with permission from ref. 95. Copyright (2009) Elsevier. | ||
In the above case, the ferrocene moieties of PFMMA segments in the polymer brush provide redox-active properties to the polymer while the GMA segments offer possible sites for coupling with functional groups, e.g. GOx. Liu et al. used the same organometallic polymer brushes obtained by consecutive SI-ATRP of FMMA and GMA as label-free electrochemical immunosensors for sensitive detection of tumor necrosis factor-alpha antigen (TNF-α).96 The redox-active ferrocene moieties in the PFMMA segment were introduced on the Au electrode surface to generate redox responsive signals, while the abundant epoxy groups in PGMA segments offered various possibilities for coupling TNF-α antibodies by an aqueous carbodiimide coupling reaction. The antibody-coated electrode was used to detect target antigens by capturing TNF-α onto the electrode surface through immunoreactions, which would cause a drop of the redox currents of the film (Fig. 10). The oxidation peak currents decreased linearly with TNF-α concentration in the range of 0.01 ng mL−1 to 1 μg mL−1 with a detection limit of 3.94 pg mL−1. By monitoring the oxidation peak current of the electrode, an electrochemical biosensor for certain antigens with good sensitivity was realized.
 | ||
| Fig. 10 Label-free electrochemical immunosensors based on ferrocene-containing polymer brushes.96 Adapted with permission from ref. 96. Copyright (2012) Elsevier. | ||
Garrido and co-workers prepared poly(methacrylic acid) brushes on a diamond electrode which was dual-functionalized with the redox enzyme glucose oxidase and aminomethyl ferrocene. The authors demonstrated the amperometric detection of glucose by these organometallic polymer brushes.97 The GOx and ferrocene moieties were well-distributed within the polymer brushes. This attempt offers an interesting strategy for the fabrication of smart electrodes for biosensors by electrical wiring of enzymes with a redox-responsive polymer.
A signal amplification strategy for electrochemical detection of DNA and proteins, based on ferrocene containing polymer brushes, was also reported.98 The DNA capture probe (thiolated ssDNA) with a complementary sequence to the target DNA was immobilized on the electrode Au surface. After the formation of sandwiched DNA duplexes with probe DNA, target DNA and the initiator-labeled detection probe DNA, poly(2-hydroxyethyl methacrylate) (PHEMA) brushes were grown from the duplexes in a controlled manner. The growth of long chain polymeric material provided abundant sites for subsequent coupling of electrochemically active ferrocene moieties. These ferrocene-containing polymer brushes in turn significantly enhanced the electrochemical signal output. The measured redox current of ferrocene was proportional to the logarithm of DNA concentration from 0.1 to 1000 nM.
The electrostatic layer-by-layer (LbL) assembly technique has been broadly employed as a simple and convenient approach in fabricating nanostructures with precise control of film structure and composition (in the film-surface normal direction).99–101 The LbL assembly is usually based on the alternating adsorption of oppositely charged polyelectrolytes via electrostatic interaction. Furthermore, in addition to electrostatics, various other approaches for film assembly have been utilized to construct covalently bonded layers. For example, by covalent LbL assembly of periodate-oxidized glucose oxidase and the redox polymer poly(allylamine)ferrocene on cystamine modified Au electrode surfaces, highly stable glucose oxidase multilayer films were prepared as biosensing interfaces (Fig. 11).102 The electrode modified with the multilayer displayed excellent catalytic activity for the oxidation of glucose. The sensitivity of the sensor depended on the number of bilayers. The catalytic current with a certain glucose concentration was linearly related to the number of assembled layers. By controlling the number of bilayers on the Au electrode, the sensor sensitivity could be tuned.
 | ||
| Fig. 11 Layer-by-layer construction of GOx/PAA-Fc multilayer films on a Au electrode surface.102 Reprinted with permission from ref. 102. Copyright (2004) Elsevier. | ||
Electropolymerization is another suitable deposition approach for the formation of ferrocene-containing polymeric systems to obtain directly coupled layers at the electrode surface.26 In this method, electropolymerizable monomers functionalized with ferrocene units were used, e.g. thiophene, pyrrole, aniline, or vinyl groups. Organometallic polymer films could be formed by this simple and reproducible process with controllable thickness and morphology. For example, Surinder et al. prepared a copolymer film of pyrrole and ferrocene carboxylate modified pyrrole (P(Py-FcPy)) on indium-tin-oxide (ITO) substrates by electrochemical polymerization. Glucose oxidase (GOx), the “model enzyme”, was entrapped during deposition in the fabrication of this electrochemical biosensor.103 The redox properties of the pyrrole copolymer, enhanced by the presence of ferrocene moieties, showed a favorable electron transfer with an improved electrochemical signal for electrochemical biosensors. This example demonstrates the feasibility of fabricating sensitive electrochemical biosensors using ferrocene modified polypyrrole films.
For example, our group fabricated various PFS grafts on electrodes through different approaches (simple “grafting to”, covalent layer-by-layer assembly and electrografting) and investigated the sensing abilities of these redox-active interfaces.
Poly(ferrocenyl(3-iodopropyl)methylsilane) was covalently immobilized onto amine-modified surfaces by amination of iodopropyl side groups of PFS with a simple “grafting to” method, forming thin, uniform and relatively dense PFS films (Fig. 12).108 CV studies showed that the tethered PFS grafts on the electrode could effectively catalyze the oxidation of ascorbic acid which formed the basis for the use of PFS-decorated electrodes as electrochemical sensor.
 | ||
| Fig. 12 Schematic representation of the covalent surface-attachment of PFS chains (a) on a silicon substrate and (b) on a gold substrate. Reproduced from ref. 108 with permission from The Royal Society of Chemistry. | ||
This simple “grafting-to” method was extended further to include a covalent LbL deposition process. The sequential buildup of PFS layers was realized by using the amine alkylation reaction between PFS bromopropyl side groups and poly(ethylene imine) (Fig. 13).109 The thickness and composition of the grafts on the electrode could be precisely tuned. Owing to the formation of covalent bonds between the layers, these covalently interconnected layers do not disassemble upon oxidation and reduction, in contrast to PFS layers featuring similar backbones held together by electrostatic forces.15,110,111 PFS/PEI multilayers were successfully employed in the electrochemical sensing of ascorbic acid and hydrogen peroxide. In Fig. 13b the sensor response of PFS multilayers with different number of bilayers is compared to consecutive additions of H2O2. Obviously, the amount of accessible ferrocene moieties per unit area at the electrode directly affects the performance of the sensor.
 | ||
| Fig. 13 (a) Schematic representation of PFS/PEI multilayer fabrication on amine-functionalized substrates. (b) Amperometric response of (PFS/PEI)4-PFS and (PFS/PEI)8-PFS to H2O2 at −0.1 V (vs. Ag/AgCl) constant potential, where each step represents 25 μM H2O2. Adapted with permission from ref. 109. Copyright (2013) American Chemical Society. | ||
In another study by us, ultrathin, robust, dense, redox active organometallic PFS films were introduced to Au substrates using the electrografting method. The PFS grafts were formed within 5 min by cathodic reduction of Au substrates in a solution of imidazolium-functionalized PFS in the ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate (Fig. 14).112 The electrografting of the organometallic polymer followed the equation shown in Fig. 14. The imidazolium side group of the PFS forms a complex with the auride ion (Au−) generated during the cathodic reduction of the Au substrate leading to the formation of new phases with the general formula [PFS-MID+Au−]. The Au electrodes were modified with PFS in this novel, simple and efficient method and employed as an ascorbic acid sensor. The amperometric response of the modified electrode to successive additions of ascorbic acid was evaluated at a fixed potential of 0.52 V (vs. Ag/AgCl), showing a rapid response and a high sensitivity.
 | ||
| Fig. 14 Electrografting of PFS on Au substrate in ionic liquid. Adapted with permission from ref. 112. Copyright (2014) American Chemical Society. | ||
A PFS based glucose sensor was fabricated by decorating porous carbon electrodes with a layer of glucose oxidase and by a film of polyisoprene-b-poly(ferrocenyldimethylsilane) (PI-b-PFDMS) by drop-coating followed by chemical crosslinking with OsO4.113 It was found that the morphology of the film could be controlled by varying the block ratio of the copolymer and the composition of the casting solvent. By treatment with OsO4, a cross-linked and stable film was obtained. Glucose oxidase was employed as model enzyme and PI-b-PFDMS was used as electron mediator (Fig. 15). The role of block copolymer morphology in the mediation of electron transport between the electrode and reaction center was investigated. The Fc moieties packed within the self-assembled structures were very useful to improve the electron transfer rate between the GOx and the electrode. The utilization of a biocontinuous microphase-separated block copolymer structure revealed a remarkable enhancement in catalytic currents and good glucose sensitivities at low glucose concentrations.
Astruc and co-workers synthesized a series of ferrocenyl dendrimers suitable for electrochemical sensing.116–121 1,2,3-Triazolylferrocenyl dendrimers prepared by click chemistry are selective electrochemical sensors for both transition-metal cations and oxo anions (H2PO4− and ATP2−) with dramatic dendritic effects.118 The ferrocenyl termini, which were directly attached to the triazole rings in the dendrimers, served as a redox monitor, showing a single, fully reversible CV wave (Fig. 16). When an oxo anion (H2PO4− or ATP2−, but not HSO4−) or a transition-metal cation (Cu+, Cu2+, Pd2+, or Pt2+) salt was added, the redox peak position of the ferrocenyl dendrimers shifted when they recognized certain oxo anions or transition metals. According to the Echegoyen–Kaifer model,122 the process is a sign of a relatively “strong redox recognition”, indicated by only a shift of the initial CV wave.
 | ||
| Fig. 16 Structure of ferrocene-containing dendrimers and the redox sensing of both oxo anions (A−) and metal cations (M+) by poly-1,2,3-triazolylferrocenyl dendrimers: cyclic voltammograms of dendrimers (a) without and (b) in the presence of (n-Bu4N)(H2PO4); (c) in the presence of [Pd (MeCN)4](BF4)2.118 Adapted with permission from ref. 118. Copyright (2007) Wiley-VCH. | ||
For oxo anions, the peak position shifted to a less positive potential (Fig. 16b), showing that the dendrimer-oxo anion assembly is easier to oxidize than the dendrimer itself. For metal cations, the oxidation peak appeared at a more positive potential (Fig. 16c) than the initial peak, indicating that the cation-dendrimer assembly is more difficult to oxidize than the dendrimer. Thus the metallodendrimers containing ferrocene termini served as redox sensors for selective recognition of anions and cations.
3.2 Functionalization and applications with Os-containing compounds
Osmium is a transition metal in the platinum group and it can form compounds with oxidation states ranging from −2 to +8. Os(II), Os(III) and Os(IV) complexes are the most widely used ones in electrochemical studies.76 Osmium-based redox organometallic polymers have attracted interest as efficient redox platforms for catalysis and biosensing because of their facile and reversible electron-transfer capability, and the possibility to tune the redox potential by changing the ligand and the backbone structure.123 Fig. 17 and Table 2 summarize the structures of several osmium-based polymers possessing redox centers distributed along the backbone,124 such as poly(vinylimidazole) (PVI), poly(4-vinylpyridine) (P4VP), or polypyrrole and others125 and their redox potential under certain conditions. Unlike the ferrocene moieties which are neutral groups within the polymer, Os complexes with ligands often introduce charges into the polymer. | ||
| Fig. 17 Molecular structures of osmium-based polymers Os1 to Os6.124 | ||
Osmium-based polymers are excellent candidates as effective mediators for shuttling electrons between electrode and analytes and have been applied in biosensors for measuring ascorbic acid,126 lactate,127 H2O2,128 dopamine,129 etc.
There are many examples where osmium-containing organometallic polymers are used to “wire” enzymes in order to create amperometric biosensors. In enzyme electrodes, the polymer structure on the electrode is one of the key factors that influences the electron transfer rate, surface coverage of redox active centers, charge transport and propagation. Diffusion and permeation of soluble species through the polymer thus affect the performance of polymer-decorated electrodes in sensing.130
Like ferrocene-containing organometallic polymers, Os-containing polymers can also form stable hydrogels in aqueous solution and provide excellent matrices for immobilizing enzymes on electrode surfaces.137–139 When enzymes and mediators are co-immobilized in the film, they are concentrated and closely connected which leads to strong bioelectrocatalytic activities. Much effort has been made to enhance the conductivity and performance of osmium-polymer-hydrogel-based biosensors.125,140–144 For example, new linkers have been introduced between the osmium complex and the polymer backbone,145 co-electrodeposition techniques were employed to form crosslinked thin films from enzymes and polymers,146 and carbon nanotubes or graphene were integrated with the polymers.147
Zafar et al. assembled FAD-dependent, glucose dehydrogenase (GcGDH) based hydrogel thin films with different Os polymers on graphite electrodes for glucose sensing.144 Six different Os-containing polymers with PVI or P4VP backbone, whose redox potentials were tuned by the ligands, were employed in the immobilization of the enzyme. The type of Os-containing polymer and enzyme/Os polymer ratio significantly affect the performance of the biosensors.
A thermo-, pH-, and electrochemical-sensitive hydroxypropylcellulose-g-poly(4-vinylpyridine)-Os (bipyridine) (HPC-g-P4VP-Os(bpy)) graft copolymer (Fig. 18) was synthesized by Huang et al.130 A biosensor for glucose detection was fabricated by immobilizing GOx on the graft copolymer-decorated electrode. The water-soluble HPC backbone with excellent swelling ability provided an excellent environment for enzyme activity while the Os complex served as the redox mediator. The sensor showed an enhanced sensitivity for glucose detection up to 0.2 mM.
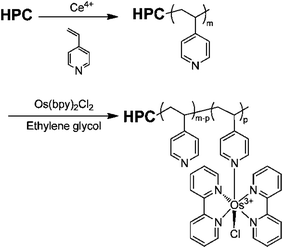 | ||
| Fig. 18 Preparation of HPC-g-P4VP-Os(bpy).130 Adapted with permission from ref. 130. Copyright (2012) American Chemical Society. | ||
Stable and porous films were formed by drop-coating electrodes with PVP-Os/chitosan and enzyme composites, showing an enhanced electrocatalytic activity for glucose sensing.58 Porous structures (Fig. 19B) resulted from random inter and intra polymeric cross-linking between two positively charged polymers, PVP-Os and chitosan by using glutaraldehyde, while the PVP-Os film had a homogeneous and smooth morphology (Fig. 19A). When testing the GOx/PVP-Os- and GOx/PVP-Os/chitosan-modified electrodes, the latter was found to exhibit a more than three times higher catalytic current. The enhanced catalytic conversion rate of the chitosan composite for glucose oxidation is a result of the stable incorporation of the enzyme into the porous and highly hydrophilic hydrogel. The porous structure enables the fast movement of chemicals involved in the glucose oxidation reaction.
 | ||
| Fig. 19 SEM images of the PVP-Os polymer (A) and PVP-Os/chitosan composite (B).58 Adapted with permission from ref. 58. Copyright (2013) Wiley-VCH. | ||
Zhang and Shen et al. also used the LbL assembly technique to modify the electrode with a polycation-bearing Os complex and glucose oxidase in the 1990s. The cyclic voltammetry curves they reported indicated that the osmium transferred the electrons successfully between the immobilized enzyme and the electrode surface.148
Minko, Katz et al. developed a smart sensing system based on an organometallic polymer containing Os centers in the side chains.149,150 A poly(4-vinylpyridine) (P4VP) brush functionalized with Os(dmo-bpy)22+ (dmo-bpy = 4,4′-dimethoxy-2,2′-bipyridine) redox groups was grafted to an ITO electrode. The electron exchange between the polymer-bound Os complex and the electrode was tuned by the swelling degree of the polymer chain. At pH < 4.5, due to the protonation of the pyridine groups, the film swelled, allowing electron exchange (Fig. 20). At pH > 6, the polymer was in a collapsed state and the electrochemical process was inhibited because of frozen polymer chain motion.149
 | ||
| Fig. 20 Reversible pH-controlled transformation of the Os-containing organometallic polymer on the electrode surface between electrochemically active and inactive states.150 Adapted with permission from ref. 150. Copyright (2008) American Chemical Society. | ||
The structural changes of the polymer enabled reversible transformations at the electrode surface between the active and the inactive states. The electrochemical activity of the Os-containing polymer modified electrode was combined with a biocatalytic reaction of glucose in the presence of soluble glucose oxidase (GOx), showing reversible activation of the bioelectrocatalytic process. The pH-controlled, switchable redox activity enabled the modified electrode to serve as a “smart” interface for a new generation of electrochemical biosensors with a signal controlled activity.150
Os-containing polymers also have potential uses in gene detection arrays.151–154 For example, an Os-containing polymer in combination with the enzyme soybean peroxidase (SBP) was used to detect a single base pair mismatch in an 18-base oligonucleotide.151 A single-stranded 18-base probe oligonucleotide was covalently attached to an Os-containing redox polymer film on a microelectrode, while the target single-stranded 18-base oligonucleotides were bound to the enzyme. Hybridization of the probe and target oligonucleotides (Fig. 21) brought the enzyme close to the modified electrode which switched on the electrocatalytic reduction of H2O2 to water. By monitoring the current enhancement, the single base mismatch in an oligonucleotide could be amperometrically sensed with the organometallic polymer-coated electrode.
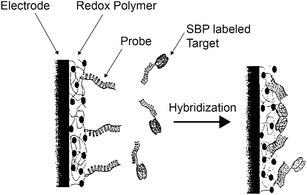 | ||
| Fig. 21 A DNA base-pair mismatch detection system based on an Os-containing polymer.151 Reprinted with permission from ref. 151. Copyright (1999) American Chemical Society. | ||
Employing a similar mechanism, an enzyme-amplified amperometric nucleic acid biosensor was proposed by Gao et al. based on sandwich-type assays.154 Capture probe, sample DNA and detection probe with GOx formed a sandwich structure on the electrode by hybridization. The Os-containing organometallic polymer was introduced on the electrode surface by electrostatic interaction, activating and mediating the enzymatic reactions of the enzyme labels (Fig. 22). With high electron mobility and good kinetics provided by the organometallic polymer, the nucleic acid molecules were amperometrically detected at femtomolar levels.
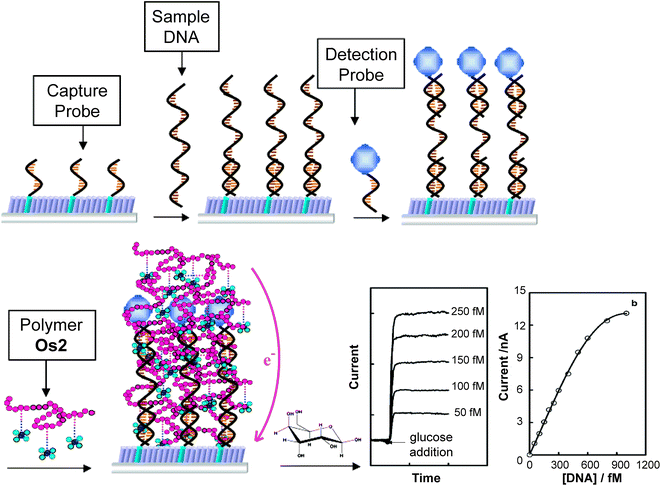 | ||
| Fig. 22 Illustration of the nucleic acid electrochemical activator bilayer detection platform.154 Adapted with permission from ref. 154. Copyright (2004) American Chemical Society. | ||
3.3 Immobilization and use of Co-containing molecules
Cobalt-based organometallic polymers are also well-suited for sensory applications as the coordination ability of the cobalt enables further bonding of specific analytes.155 Compared to other metal centers, cobalt is less sensitive to water and oxygen in ambient conditions.156 Swager et al. prepared a series of cobalt-containing conducting organometallic polymers and demonstrated that communication between the metal center and polymer backbone could be tuned by the reversible binding of small molecules. The energy levels of the metal-based orbitals could be altered which made these polymers highly suitable for small ligating molecules detection.34Fox instance, a selective and effective detection system for the physiologically important species nitric oxide has been developed based on chemoresistive changes in a cobalt-containing conducting organometallic polymer film device.156 The corresponding metal-containing monomer, featuring polymerizable 3,4-(ethylenedioxy)thiophene (EDOT) groups, was electropolymerized onto the working electrode surfaces, forming a conducting organometallic film (Fig. 23A). The polymer film was highly conductive and the metal was intimately involved in the conduction pathway. When NO was exposed to the microelectrodes decorated with this cobalt-containing conducting polymer, coordination of the ligand occurred, which changed the orbital energies of the complex, resulting in an increase in electrical resistance (Fig. 23B). The cobalt metal center adopted a square pyramidal coordination arrangement to accommodate the addition of a bent NO ligand to form polymer-(NO) complexes. The device was insensitive to gases such as CO2, O2 and CO while showing a large, irreversible resistance change when exposed to NO2.
 | ||
| Fig. 23 (A) Fabrication of conducting organometallic polymer electrode devices by electropolymerization across interdigitated microelectrodes (IME). (B) Chemoresistive response to NO gas exposure in dry N2. The unconditioned film is shown in black, the conditioned film at 0.262 V (vs. Fc/Fc+) for 2 min is shown in red, and the poly-EDOT film is shown in blue.156 Adapted with permission from ref. 156. Copyright (2006) American Chemical Society. | ||
3.4 Electrode decoration with Ru-containing polymers
Organometallic polymers containing ruthenium are often used in photoelectrochemical sensors.157–160 The ruthenium moieties within the polymer serve as photoelectrochemically active materials. Take [Ru(bpy)3]2+(bpy = 2,2′-bipyridine) as an example, where the excited state of Ru(II) is generated upon irradiation with light. The [Ru(bpy)3]2+ can react as electron donor or acceptor, producing an anodic or cathodic photocurrent (Fig. 24).161 | ||
| Fig. 24 Schematic illustrations of (A) anodic and (B) cathodic photocurrent generation mechanisms by a ruthenium complex.161 Adapted with permission from ref. 161. Copyright (2014) American Chemical Society. | ||
Based on this phenomenon, Cosnier et al. fabricated several photoelectrochemical immunosensors for the detection of biologically important species.157 For example, a biotinylated tri(bipyridyl) ruthenium(II) complex (Fig. 25) with pyrrole groups was electropolymerized on the electrode to form a biotinylated Ru-containing polypyrrole film. A cathodic photocurrent could be generated under illumination in the presence of an oxidative quencher. The immunosensor platform was built by subsequently attaching avidin and biotinylated cholera toxin (the probe) to the Ru-containing organometallic polymer decorated electrode via the avidin-biotin reaction. The photocurrent of the layered system decreased as the increase in steric hindrance thwarted the diffusion of quencher molecules to the underlying Ru-containing polymer film. When the analyte, consisting of cholera toxin antibodies (anti-CT), was introduced to the system, the photocurrent further decreased, due to the specific binding of the antibodies to the electrode. By monitoring the variation of the photocurrent, detection of the corresponding antibody was realized from 0 to 200 μg mL−1.157
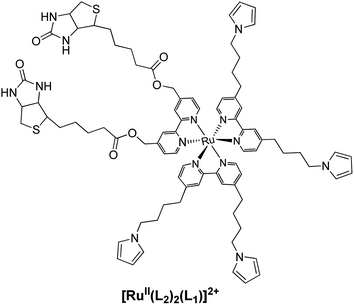 | ||
| Fig. 25 Structure of the biotinylated tri(bipyridyl) ruthenium(II) complex.157 | ||
Similarly, a label-free photoelectrochemical immunosensor and aptasensor were fabricated based on another Ru(II) containing organometallic copolymer. The bifunctional copolymer158 was electropolymerized on the electrode using pyrenebutyric acid, Nα′,Nα-bis(carboxymethyl)-L-lysine amide (NTA-pyrene) and [tris-(2,2′-bipyridine)(4,4′-(bis(4-pyrenyl-1-ylbutyloxy)-2,2′-bipyridine)] ruthenium(II) hexafluorophosphate (Ru(II)-pyrene complex). The pyrene groups, present in both compounds, underwent oxidative electropolymerization on platinum electrodes. The resulting copolymer contained NTA moieties, which functioned as an immobilization system for biotin- and histidine-tagged biomolecules, and Ru(II)-pyrene served as the photoelectrochemical transducing molecule.
Upon illumination, an excited state of Ru(II) can be formed and further quenched by sacrificial electron donors or acceptors, generating photocurrent. For the construction of an immunosensor for cholera antitoxin antibodies (anti-CT) detection, biotin-Cu(NTA) interactions were used to modify the electrode with biotin-conjugated cholera toxin molecules (CT) (Fig. 26A). The resulting copolymer-CT immunosensor was exposed to different anti-CT concentrations and the photocurrent responses were recorded. The normalized immunosensor response increased linearly with increasing antibody concentration (Fig. 26B). By immobilizing thrombin binding aptamer (TBA) to the Ru-containing copolymer film, a photoelectrochemical aptasensor for thrombin was also developed (Fig. 26C and D).
 | ||
| Fig. 26 Photoelectrochemical immunosensor and aptasensor. (A) Operating principle of the photoelectrochemical immunosensors. (B) Calibration curve for sensing anti-CT concentrations ranging from 0 to 8 μg mL−1. (C) Photocurrent measurement for the electrode (a) before and (b) after thrombin binding aptamer anchoring and (c) after incubation with thrombin (12 pM) and (D) calibration curve for photoelectrochemical aptasensing for thrombin concentrations ranging from 0 to 10 pM. All measurements were recorded in de-aerated 10 mM sodium ascorbate 0.1 M PBS solution.158 Reprinted with permission from ref. 158. Copyright (2013) Elsevier. | ||
3.5 Electrochemical sensors with metal–organic coordination polymers
Metal–organic coordination polymers (MOCP), also known as metal–organic frameworks (MOFs) or coordination networks utilize metal–ligand bonds to form polymer backbones. The wide range of choices for the organic linkers and metal ions for MOF construction have permitted the rational structural design of various MOFs with targeted properties.61,162–164 Ultrahigh porosity, large accessible surface areas, tunable structure, open metal sites, and high thermal and chemical stability of MOFs make them promising candidates for potential applications in many fields. Here we focus on the applications of MOFs in electrochemical sensing.Some MOFs or MOF complexes exhibited excellent electrocatalytic activity, which is suitable for electrochemical sensor fabrication. For example, a two-dimensional Co-based metal–organic coordination polymer (Co-MOCP) was prepared by a simple solvothermal synthesis.165 1,3,5-Tri(1-imidazolyl)benzene, a typical imidazole-containing tripodal ligand with N donors, was used for the construction of the 2-D coordination architectures with Co2+. The electrode decorated with Co-MOCP was used for the electrocatalytic oxidation of reduced glutathione (GSH).165 This electrochemical sensor showed a wide linear range (from 2.5 μM to 0.95 mM), low detection limit (2.5 μM), and high stability towards GSH, which renders it a good platform for GSH sensing.
Heterogeneous MOFs were also proposed for sensor fabrication. Hosseini et al. developed L-cysteine166 and hydrazine167 electrochemical sensors with Au–SH–SiO2 nanoparticles immobilized on Cu-MOFs. Guo et al. demonstrated the electrocatalytic oxidation of NADH and reduction of H2O2 with macroporous carbon (MPC) supported Cu-based MOF hybrids.168
Cu terephthalate MOFs were integrated with graphene oxide (GO) and deposited onto a glassy carbon electrode. The hybrid film was treated with electro-reduction to convert GO in the composite to graphene, the highly conductive reduced form.169 Because of the synergistic effect from graphene's high conductivity and the unique electron mediating action of Cu-MOF, the decorated electrode showed a high sensitivity and low interference towards acetaminophen (ACOP) and dopamine (DA). By monitoring the oxidation peak current of the two drugs with differential pulse voltammetric (DPV) measurements, the concentrations of ACOP and DA could be determined.
Owing to high porosity and impressive absorption ability, MOFs could be used as novel and efficient immobilization matrices for enzymes. Glucose oxidase-based glucose biosensors and tyrosinase-based phenolic biosensors were fabricated with Au or Pt based organometallic polymers.170 The coordinated organometallic polymer network can immobilize enzymes with high load/activity, showing improved mass-transfer efficiency, and the thus-prepared glucose and phenolic biosensors exhibited excellent performance with long-term stability. Fig. 27 displays the one-pot fabrication process of the functional electrode and the biosensing mechanism. 2,5-Dimercapto-1,3,4-thiadiazole (DMcT) which enables coordination of two or more metal ions was chosen to react with Au ions to form a porous structure in the presence of tyrosinase. Chronoamperometric measurements were used to monitor the current variation under different phenolic concentrations. The decorated electrode showed enhanced enzyme catalysis efficiency and excellent sensing performance towards phenol, resulting from the porous structure of the organometallic network which provided adequate space for enzyme entrapment and facilitated the mass transfer of the analytes and products.
 | ||
| Fig. 27 Illustration of the fabrication of tyrosinase-based phenolic biosensors and the biosensing mechanism.170 Reprinted with permission from ref. 170. Copyright (2011) American Chemical Society. | ||
Mao et al. studied a series of zeolitic imidazolate frameworks (ZIFs) as a matrix for integrated dehydrogenase-based electrochemical biosensors.171 ZIFs with various pore sizes, surface areas and functional groups were investigated as matrix for co-immobilizing electrocatalysts (i.e., methylene green, MG) and dehydrogenases (i.e., glucose dehydrogenase, GDH). ZIF-70 [Zn(Im)1.13(nIm)0.87, Im = imidazole, nIm = 2-nitroimidazole] showed outstanding adsorption capacities toward MG and GDH and was used to construct a biosensor by drop-casting MG/ZIF-70 on a glassy carbon electrode, followed by coating GDH onto the MG/ZIF-70 composite. In a continuous-flow system, the biosensor was linearly responsive to glucose in the range of 0.1–2 mM.
Electrochemical sensors for the differential pulse anodic stripping voltammetric determination of lead based on multi-wall carbon nanotubes@Cu3(BTC)2 (BTC = benzene-1,3,5-tricarboxylate)172 and amino-functionalized Cu3(BTC)2 (ref. 173) were also reported. The sensing systems showed excellent calibration responses towards lead at low concentrations, resulting from the absorbing effect of the MOFs.
MOFs showed superior sorption properties towards small molecules. The high porosity and reversible sorption behavior suggests that the MOFs are suitable candidates for fabricating gas sensors. The absorption by, or desorption of molecules from the MOFs often induces changes in the dielectric properties of these materials.174 By utilizing this characteristic, MOFs were applied as sensor materials for impedimetric gas sensors. For example, Achmann et al. constructed the first impedance sensor with Fe-1,3,5-benzenetricarboxylate-MOF (Fe-BTC) for humidity sensing, which responded linearly in the range of 0 to 2.5 vol% water.174
A rubidium ion containing metal–organic framework CD-MOF has been shown as a candidate for CO2 detection. The organometallic polymer CD-MOF showed an extended cubic structure comprising units of six γ-cyclodextrins (CD), linked by rubidium ions, which could react with gaseous CO2 to form CO2-bound CD-MOF. The absorption process is reversible (Fig. 28). The pristine CD-MOF exhibited a high ionic conductivity. When binding with CO2, a large drop in the conductivity (∼550-fold) was monitored by electrochemical impedance spectroscopy. The CO2 sensors that were fabricated based on this principle were capable of measuring CO2 concentrations quantitatively.175 Fig. 28 also shows the cyclic change of conductivity of the CD-MOF with sequential CO2 absorption and desorption. The plot of average conductivity value vs. CO2 concentration shows that the sensitivity of the conductivity change is relatively high at low CO2 concentration. This example demonstrates that MOFs have a promising future in the field of quantitative sensing applications.
 | ||
| Fig. 28 CO2 sensor based on a rubidium ion containing metal–organic framework CD-MOF.175 Adapted with permission from ref. 175. Copyright (2014) American Chemical Society. | ||
4. Conclusions
This review summarizes the role of organometallic polymers as active components in electrochemical sensors. As illustrated, the presence of metal centers in the polymeric materials can introduce a variety of useful properties and render them a versatile and promising class of functional, soft materials. The resulting analytical performance of the chemo/biosensors relies intimately on the properties of the materials utilized to build the devices. Strategies of immobilization of organometallic polymers on electrode surfaces and opportunities for the resulting decorated electrodes in sensing are discussed. As is presented here, rational design of composition or structure of the organometallic components and improvements in fabrication techniques continuously advance the development of electrode surfaces towards greater sensing selectivity and lower limits of detection. Importantly, breakthroughs in the design and synthesis of organometallic polymers would open new avenues to further enhance performance and broaden the applicability and scope of electrochemical sensors.The advent of nanotechnology techniques over the last decade has been promoting progress in the area of sensing applications, as well. In the future, efforts have to be made to integrate the advantages of nanotechnology and MEMS/microfluidic technology with the specific characteristics of organometallic polymers for the development of fully automatic, label-free, highly sensitive, real-time chemo/biosensing. The MEMS/microfluidics devices, in particular, hold great promise for the fabrication of miniaturized, portable chemo/biosensors and biochips which, for example, may enable routine health checks at home, real-time environmental detection, etc. Such miniaturized and simplified devices also have great economic potential in the diagnostic market. Alternatively, employing the organometallic polymer to construct an array-based device that acts as a chemical nose or is a constituent of other artificial organs, would also be attracting.
Taking the current knowledge to real-life applications is an important goal for the future. Organometallic polymers offer many exciting future opportunities and challenges in the electrochemical sensing and we hope that this review will assist to inspire future achievements and breakthroughs.
References
- G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439–3468 CrossRef CAS.
- J. C. Eloi, L. Chabanne, G. R. Whittell and I. Manners, Mater. Today, 2008, 11, 28–36 CrossRef CAS.
- J. W. Zhou, G. R. Whittell and I. Manners, Macromolecules, 2014, 47, 3529–3543 CrossRef CAS.
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176–188 CrossRef CAS PubMed.
- I. Manners, Science, 2001, 294, 1664–1666 CrossRef CAS PubMed.
- A. S. Abd-El-Aziz, C. Agatemor and N. Etkin, Macromol. Rapid Commun., 2014, 35, 513–559 CrossRef CAS PubMed.
- M. A. Vorotyntsev and S. V. Vasilyeva, Adv. Colloid Interface Sci., 2008, 139, 97–149 CrossRef CAS PubMed.
- A. S. Abd-El-Aziz, P. O. Shipman, B. N. Boden and W. S. McNeil, Prog. Polym. Sci., 2010, 35, 714–836 CrossRef CAS.
- S. J. Liu, Y. Chen, W. J. Xu, Q. Zhao and W. Huang, Macromol. Rapid Commun., 2012, 33, 461–480 CrossRef CAS PubMed.
- A. S. Abd-El-Aziz and E. A. Strohm, Polymer, 2012, 53, 4879–4921 CrossRef CAS.
- I. Korczagin, R. G. H. Lammertink, M. A. Hempenius, S. Golze and G. J. Vancso, Adv. Polym. Sci., 2006, 200, 91–117 CrossRef CAS.
- X. Y. Ling, C. Acikgoz, I. Y. Phang, M. A. Hempenius, D. N. Reinhoudt, G. J. Vancso and J. Huskens, Nanoscale, 2010, 2, 1455–1460 RSC.
- R. A. Krüger and T. Baumgartner, Dalton Trans., 2010, 39, 5759–5767 RSC.
- Z. R. Lin, Adv. Mater., 1999, 11, 1153–1154 CrossRef CAS.
- J. Song, D. Janczewski, Y. J. Ma, M. Hempenius, J. W. Xu and G. J. Vancso, J. Mater. Chem. B, 2013, 1, 828–834 RSC.
- S. Zou, I. Korczagin, M. A. Hempenius, H. Schönherr and G. J. Vancso, Polymer, 2006, 47, 2483–2492 CrossRef CAS.
- J. J. McDowell, N. S. Zacharia, D. Puzzo, I. Manners and G. A. Ozin, J. Am. Chem. Soc., 2010, 132, 3236–3237 CrossRef CAS PubMed.
- C. L. Ho and W. Y. Wong, Coord. Chem. Rev., 2013, 257, 1614–1649 CrossRef CAS.
- D. Coquiere, J. Bos, J. Beld and G. Roelfes, Angew. Chem., Int. Ed., 2009, 48, 5159–5162 CrossRef CAS PubMed.
- F. S. Arimoto and A. C. Haven, J. Am. Chem. Soc., 1955, 77, 6295–6297 CrossRef CAS.
- J. B. Heilmann, M. Scheibitz, Y. Qin, A. Sundararaman, F. Jäkle, T. Kretz, M. Bolte, H. W. Lerner, M. C. Holthausen and M. Wagner, Angew. Chem., Int. Ed., 2006, 45, 920–925 CrossRef CAS PubMed.
- T. Le Bouder, O. Maury, A. Bondon, K. Costuas, E. Amouyal, I. Ledoux, J. Zyss and H. le Bozec, J. Am. Chem. Soc., 2003, 125, 12284–12299 CrossRef CAS PubMed.
- D. A. Foucher, B. Z. Tang and I. Manners, J. Am. Chem. Soc., 1992, 114, 6246–6248 CrossRef CAS.
- D. Foucher, R. Ziembinski, R. Petersen, J. Pudelski, M. Edwards, Y. Z. Ni, J. Massey, C. R. Jaeger, G. J. Vancso and I. Manners, Macromolecules, 1994, 27, 3992–3999 CrossRef CAS.
- V. Bellas and M. Rehahn, Angew. Chem., Int. Ed., 2007, 46, 5082–5104 CrossRef CAS PubMed.
- C. Friebe, M. D. Hager, A. Winter and U. S. Schubert, Adv. Mater., 2012, 24, 332–345 CrossRef CAS.
- A. B. Powell, C. W. Bielawski and A. H. Cowley, J. Am. Chem. Soc., 2010, 132, 10184–10194 CrossRef CAS PubMed.
- P. R. Andres and U. S. Schubert, Adv. Mater., 2004, 16, 1043–1068 CrossRef CAS.
- A. S. Abd-El-Aziz and E. K. Todd, Coord. Chem. Rev., 2003, 246, 3–52 CrossRef CAS.
- U. S. Schubert and C. Eschbaumer, Angew. Chem., Int. Ed., 2002, 41, 2893–2926 Search PubMed.
- P. Nguyen, P. Gomez-Elipe and I. Manners, Chem. Rev., 1999, 99, 1515–1548 CrossRef CAS PubMed.
- C. Weder, J. Inorg. Organomet. Polym. Mater., 2006, 16, 101–113 CrossRef CAS.
- M. O. Wolf, J. Inorg. Organomet. Polym. Mater., 2006, 16, 189–199 CrossRef CAS.
- B. J. Holliday and T. M. Swager, Chem. Commun., 2005, 23–36 RSC.
- I. Manners, Synthetic Metal-Containing Polymers, Wiley-VCH, Weinheim, 2004 Search PubMed.
- R. D. Archer, Inorganic and Organometallic Polymers, Wiley-VCH, Weinheim, 2001 Search PubMed.
- T. P. Russell, Science, 2002, 297, 964–967 CrossRef CAS PubMed.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- L. Zhai, Chem. Soc. Rev., 2013, 42, 7148–7160 RSC.
- M. E. A. Fegley, S. S. Pinnock, C. N. Malele and W. E. Jones, Inorg. Chim. Acta, 2012, 381, 78–84 CrossRef CAS PubMed.
- N. J. Ronkainen, H. B. Halsall and W. R. Heineman, Chem. Soc. Rev., 2010, 39, 1747–1763 RSC.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- L. M. Goldenberg, M. R. Bryce and M. C. Petty, J. Mater. Chem., 1999, 9, 1957–1974 RSC.
- Y. Yang, G. A. Turnbull and I. D. W. Samuel, Adv. Funct. Mater., 2010, 20, 2093–2097 CrossRef CAS.
- D. Grieshaber, R. MacKenzie, J. Voros and E. Reimhult, Sensors, 2008, 8, 1400–1458 CrossRef CAS.
- X. D. Wang and O. S. Wolfbeis, Chem. Soc. Rev., 2014, 43, 3666–3761 RSC.
- Z. Wang, A. R. McWilliams, C. E. B. Evans, X. Lu, S. Chung, M. A. Winnik and I. Manners, Adv. Funct. Mater., 2002, 12, 415–419 CrossRef CAS.
- A. Wild, A. Winter, M. D. Hager and U. S. Schubert, Analyst, 2012, 137, 2333–2337 RSC.
- C. J. Qin, W. Y. Wong and L. X. Wang, Macromolecules, 2011, 44, 483–489 CrossRef CAS.
- A. J. Lan, K. H. Li, H. H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. C. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334–2338 CrossRef CAS PubMed.
- D. W. R. Balkenende, S. Coulibaly, S. Balog, Y. C. Simon, G. L. Fiore and C. Weder, J. Am. Chem. Soc., 2014, 136, 10493–10498 CrossRef CAS PubMed.
- C. Caliendo, E. Verona, A. Damico, A. Furlani, G. Infante and M. V. Russo, Sens. Actuators, B, 1995, 25, 670–672 CrossRef CAS.
- C. Caliendo, I. Fratoddi, M. V. Russo and C. Lo Sterzo, J. Appl. Phys., 2003, 93, 10071–10077 CrossRef CAS.
- C. Caliendo, I. Fratoddi and M. V. Russo, Appl. Phys. Lett., 2002, 80, 4849–4851 CrossRef CAS.
- C. Caliendo, G. Contini, I. Fratoddi, S. Irrera, P. Pertici, G. Scavia and M. V. Russo, Nanotechnology, 2007, 18, 125504 CrossRef.
- P. Sun, Y. D. Jiang, G. Z. Xie, J. S. Yu, X. S. Du and J. Hu, J. Appl. Polym. Sci., 2010, 116, 562–567 CrossRef CAS.
- G. J. Zhou, W. Y. Wong, C. Ye and Z. Y. Lin, Adv. Funct. Mater., 2007, 17, 963–975 CrossRef.
- H. D. Jirimali, R. K. Nagarale, J. M. Lee, D. Saravanakumar and W. Shin, ChemPhysChem, 2013, 14, 2232–2236 CrossRef CAS PubMed.
- P. Chadha and P. J. Ragogna, Chem. Commun., 2011, 47, 5301–5303 RSC.
- S. J. Payne, G. L. Fiore, C. L. Fraser and J. N. Demas, Anal. Chem., 2010, 82, 917–921 CrossRef CAS PubMed.
- C. Wang, D. M. Liu and W. B. Lin, J. Am. Chem. Soc., 2013, 135, 13222–13234 CrossRef CAS PubMed.
- Y. L. Wang, L. Salmon, J. Ruiz and D. Astruc, Nat. Commun., 2014, 5, 3489 Search PubMed.
- M. H. Yan, S. K. P. Velu, G. Royal and P. Terech, J. Colloid Interface Sci., 2013, 399, 6–12 CrossRef CAS PubMed.
- B. R. Eggins, Chemical sensors and biosensors, John Wiley & Sons, West, Sussex, England, 2002 Search PubMed.
- A. Malinauskas, in Encyclopedia of Surface and Colloid Science, Marcel Dekker, New York, 2002, pp. 753–773 Search PubMed.
- J. M. Zen, A. S. Kumar and D. M. Tsai, Electroanalysis, 2003, 15, 1073–1087 CrossRef CAS.
- J. L. Lutkenhaus and P. T. Hammond, Soft Matter, 2007, 3, 804–816 RSC.
- I. Tokarev, M. Motornov and S. Minko, J. Mater. Chem., 2009, 19, 6932–6948 RSC.
- I. Tokarev and S. Minko, Adv. Mater., 2009, 21, 241–247 CrossRef CAS.
- I. Tokarev and S. Minko, Soft Matter, 2009, 5, 511–524 RSC.
- F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2009, 93, 394–412 CrossRef CAS.
- D. Belanger and J. Pinson, Chem. Soc. Rev., 2011, 40, 3995–4048 RSC.
- Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, ed. G. Decher and J. B. Schlenoff, Wiley-VCH, Weinheim, 2012 Search PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 2nd edn, 2001 Search PubMed.
- T. J. Kealy and P. L. Pauson, Nature, 1951, 168, 1039–1040 CrossRef CAS.
- A. L. Eckermann, D. J. Feld, J. A. Shaw and T. J. Meade, Coord. Chem. Rev., 2010, 254, 1769–1802 CrossRef CAS PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- Y. Ohashi, Reactivity in Molecular Crystals, Wiley-VCH, Hoboken, 2008 Search PubMed.
- W. A. Amer, L. Wang, A. M. Amin, L. A. Ma and H. J. Yu, J. Inorg. Organomet. Polym. Mater., 2010, 20, 605–615 CrossRef CAS.
- B. Fabre, Acc. Chem. Res., 2010, 43, 1509–1518 CrossRef CAS PubMed.
- K. L. Robinson and N. S. Lawrence, Electroanalysis, 2006, 18, 677–683 CrossRef CAS.
- K. L. Robinson and N. S. Lawrence, Anal. Chem., 2006, 78, 2450–2455 CrossRef CAS PubMed.
- N. S. Lawrence and K. L. Robinson, Talanta, 2007, 74, 365–369 CrossRef CAS PubMed.
- S. X. Zhang, Y. Q. Fu and C. Q. Sun, Electroanalysis, 2003, 15, 739–746 CrossRef CAS.
- J. Wang, Chem. Rev., 2008, 108, 814–825 CrossRef CAS PubMed.
- T. Saito and M. Watanabe, React. Funct. Polym., 1998, 37, 263–269 CrossRef CAS.
- S. Koide and K. Yokoyama, J. Electroanal. Chem., 1999, 468, 193–201 CrossRef CAS.
- M. Senel, E. Cevik and M. F. Abasiyanik, Sens. Actuators, B, 2010, 145, 444–450 CrossRef CAS.
- W. W. Yang, H. Zhou and C. Q. Sun, Macromol. Rapid Commun., 2007, 28, 265–270 CrossRef CAS.
- K. Sirkar and M. V. Pishko, Anal. Chem., 1998, 70, 2888–2894 CrossRef CAS.
- S. A. Merchant, D. T. Glatzhofer and D. W. Schmidtke, Langmuir, 2007, 23, 11295–11302 CrossRef CAS PubMed.
- S. A. Merchant, T. O. Tran, M. T. Meredith, T. C. Cline, D. T. Glatzhofer and D. W. Schmidtke, Langmuir, 2009, 25, 7736–7742 CrossRef CAS PubMed.
- C. Bunte, O. Prucker, T. König and J. Rühe, Langmuir, 2010, 26, 6019–6027 CrossRef CAS PubMed.
- B. Nagel, A. Warsinke and M. Katterle, Langmuir, 2007, 23, 6807–6811 CrossRef CAS PubMed.
- Z. B. Zhang, S. J. Yuan, X. L. Zhu, K. G. Neoh and E. T. Kang, Biosens. Bioelectron., 2010, 25, 1102–1108 CrossRef CAS PubMed.
- L. Yuan, W. Wei and S. Q. Liu, Biosens. Bioelectron., 2012, 38, 79–85 CrossRef CAS PubMed.
- A. A. Reitinger, N. A. Hutter, A. Donner, M. Steenackers, O. A. Williams, M. Stutzmann, R. Jordan and J. A. Garrido, Adv. Funct. Mater., 2013, 23, 2979–2986 CrossRef CAS.
- Y. F. Wu, S. Q. Liu and L. He, Anal. Chem., 2009, 81, 7015–7021 CrossRef CAS PubMed.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- Y. M. Yu, M. Z. Yin, K. Müllen and W. Knoll, J. Mater. Chem., 2012, 22, 7880–7886 RSC.
- J. F. Quinn, A. P. R. Johnston, G. K. Such, A. N. Zelikin and F. Caruso, Chem. Soc. Rev., 2007, 36, 707–718 RSC.
- S. X. Zhang, W. W. Yang, Y. M. Niu and C. Q. Sun, Sens. Actuators, B, 2004, 101, 387–393 CrossRef CAS.
- N. Palomera, J. L. Vera, E. Melendez, J. E. Ramirez-Vick, M. S. Tomar, S. K. Arya and S. P. Singh, J. Electroanal. Chem., 2011, 658, 33–37 CrossRef CAS.
- Y. J. Ma, W. F. Dong, M. A. Hempenius, H. Möhwald and G. J. Vancso, Nat. Mater., 2006, 5, 724–729 CrossRef CAS PubMed.
- M. A. Hempenius, C. Cirmi, J. Song and G. J. Vancso, Macromolecules, 2009, 42, 2324–2326 CrossRef CAS.
- X. F. Sui, L. van Ingen, M. A. Hempenius and G. J. Vancso, Macromol. Rapid Commun., 2010, 31, 2059–2063 CrossRef CAS PubMed.
- I. Manners, Chem. Commun., 1999, 857–865 RSC.
- X. F. Sui, X. L. Feng, J. Song, M. A. Hempenius and G. J. Vancso, J. Mater. Chem., 2012, 22, 11261–11267 RSC.
- X. L. Feng, A. Curnurcu, X. F. Sui, J. Song, M. A. Hernpenius and G. J. Vancso, Langmuir, 2013, 29, 7257–7265 CrossRef CAS PubMed.
- J. Song, D. Janczewski, Y. J. Ma, L. van Ingen, C. E. Sim, Q. L. Goh, J. W. Xu and G. J. Vancso, Eur. Polym. J., 2013, 49, 2477–2484 CrossRef CAS.
- J. Song, D. Janczewski, Y. J. Ma, M. Hempenius, J. W. Xu and G. J. Vancso, J. Colloid Interface Sci., 2013, 405, 256–261 CrossRef CAS PubMed.
- X. Feng, X. Sui, M. A. Hempenius and G. J. Vancso, J. Am. Chem. Soc., 2014, 136, 7865–7868 CrossRef CAS PubMed.
- J. Lee, H. Ahn, I. Choi, M. Boese and M. J. Park, Macromolecules, 2012, 45, 3121–3128 CrossRef CAS.
- G. R. Newkome, E. F. He and C. N. Moorefield, Chem. Rev., 1999, 99, 1689–1746 CrossRef CAS PubMed.
- M. P. G. Armada, J. Losada, M. Zamora, B. Alonso, I. Cuadrado and C. M. Casado, Bioelectrochemistry, 2006, 69, 65–73 CrossRef CAS PubMed.
- M. C. Daniel, F. Ba, J. R. Aranzaes and D. Astruc, Inorg. Chem., 2004, 43, 8649–8657 CrossRef CAS PubMed.
- D. Astruc, M. C. Daniel and J. Ruiz, Chem. Commun., 2004, 2637–2649 RSC.
- C. Ornelas, J. R. Aranzaes, E. Cloutet, S. Alves and D. Astruc, Angew. Chem., Int. Ed., 2007, 46, 872–877 CrossRef CAS PubMed.
- D. Astruc, C. Ornelas and J. R. Aranzaes, J. Inorg. Organomet. Polym. Mater., 2008, 18, 4–17 CrossRef CAS.
- J. Camponovo, J. Ruiz, E. Cloutet and D. Astruc, Chem.–Eur. J., 2009, 15, 2990–3002 CrossRef CAS PubMed.
- R. Djeda, A. Rapakousiou, L. Y. Liang, N. Guidolin, J. Ruiz and D. Astruc, Angew. Chem., Int. Ed., 2010, 49, 8152–8156 CrossRef CAS PubMed.
- S. R. Miller, D. A. Gustowski, Z. H. Chen, G. W. Gokel, L. Echegoyen and A. E. Kaifer, Anal. Chem., 1988, 60, 2021–2024 CrossRef CAS.
- D. A. Guschin, J. Castillo, N. Dimcheva and W. Schuhmann, Anal. Bioanal. Chem., 2010, 398, 1661–1673 CrossRef CAS PubMed.
- S. C. Barton, J. Gallaway and P. Atanassov, Chem. Rev., 2004, 104, 4867–4886 CrossRef CAS PubMed.
- P. O. Conghaile, S. Poller, D. MacAodha, W. Schuhmann and D. Leech, Biosens. Bioelectron., 2013, 43, 30–37 CrossRef PubMed.
- N. Havens, P. Trihn, D. Kim, M. Luna, A. K. Wanekaya and A. Mugweru, Electrochim. Acta, 2010, 55, 2186–2190 CrossRef CAS.
- A. A. J. Torriero, E. Salinas, F. Battaglini and J. Raba, Anal. Chim. Acta, 2003, 498, 155–163 CrossRef CAS.
- T. M. Park, Anal. Lett., 1999, 32, 287–298 CrossRef CAS.
- H. F. Cui, Y. H. Cui, Y. L. Sun, K. Zhang and W. D. Zhang, Nanotechnology, 2010, 21, 215601 CrossRef PubMed.
- H. L. Kang, R. G. Liu, H. F. Sun, J. M. Zhen, Q. M. Li and Y. Huang, J. Phys. Chem. B, 2012, 116, 55–62 CrossRef CAS PubMed.
- S. C. Barton, H. H. Kim, G. Binyamin, Y. C. Zhang and A. Heller, J. Am. Chem. Soc., 2001, 123, 5802–5803 CrossRef CAS.
- N. Mano, H. H. Kim, Y. C. Zhang and A. Heller, J. Am. Chem. Soc., 2002, 124, 6480–6486 CrossRef CAS PubMed.
- T. Chen, S. C. Barton, G. Binyamin, Z. Q. Gao, Y. C. Zhang, H. H. Kim and A. Heller, J. Am. Chem. Soc., 2001, 123, 8630–8631 CrossRef CAS PubMed.
- H. H. Kim, N. Mano, X. C. Zhang and A. Heller, J. Electrochem. Soc., 2003, 150, A209–A213 CrossRef CAS.
- N. Mano and A. Heller, J. Electrochem. Soc., 2003, 150, A1136–A1138 CrossRef CAS.
- N. Mano, F. Mao and A. Heller, J. Am. Chem. Soc., 2002, 124, 12962–12963 CrossRef CAS PubMed.
- X. F. Sui, X. L. Feng, M. A. Hempenius and G. J. Vancso, J. Mater. Chem. B, 2013, 1, 1658–1672 RSC.
- E. Suraniti, S. Vives, S. Tsujimura and N. Mano, J. Electrochem. Soc., 2013, 160, G79–G82 CrossRef CAS.
- H. M. Liu, C. X. Liu, L. Y. Jiang, J. Liu, Q. D. Yang, Z. H. Guo and X. X. Cai, Electroanalysis, 2008, 20, 170–177 CrossRef CAS.
- T. J. Ohara, R. Rajagopalan and A. Heller, Anal. Chem., 1994, 66, 2451–2457 CrossRef CAS PubMed.
- M. Vreeke, R. Maidan and A. Heller, Anal. Chem., 1992, 64, 3084–3090 CrossRef CAS.
- E. Baldini, V. C. Dall'Orto, C. Danilowicz, I. Rezzano and E. J. Calvo, Electroanalysis, 2002, 14, 1157–1164 CrossRef CAS.
- A. Heller, Curr. Opin. Chem. Biol., 2006, 10, 664–672 CrossRef CAS PubMed.
- M. N. Zafar, X. J. Wang, C. Sygmund, R. Ludwig, D. Leech and L. Gorton, Anal. Chem., 2012, 84, 334–341 CrossRef CAS PubMed.
- F. Mao, N. Mano and A. Heller, J. Am. Chem. Soc., 2003, 125, 4951–4957 CrossRef CAS PubMed.
- Z. Q. Gao, G. Binyamin, H. H. Kim, S. C. Barton, Y. C. Zhang and A. Heller, Angew. Chem., Int. Ed., 2002, 41, 810–813 CrossRef CAS.
- P. P. Joshi, S. A. Merchant, Y. D. Wang and D. W. Schmidtke, Anal. Chem., 2005, 77, 3183–3188 CrossRef CAS PubMed.
- Y. P. Sun, J. Q. Sun, X. Zhang, C. Q. Sun, Y. Wang and J. C. Shen, Thin Solid Films, 1998, 327, 730–733 CrossRef.
- T. K. Tam, J. Zhou, M. Pita, M. Ornatska, S. Minko and E. Katz, J. Am. Chem. Soc., 2008, 130, 10888–10889 CrossRef CAS PubMed.
- T. K. Tam, M. Ornatska, M. Pita, S. Minko and E. Katz, J. Phys. Chem. C, 2008, 112, 8438–8445 CAS.
- D. J. Caruana and A. Heller, J. Am. Chem. Soc., 1999, 121, 769–774 CrossRef CAS.
- C. N. Campbell, D. Gal, N. Cristler, C. Banditrat and A. Heller, Anal. Chem., 2002, 74, 158–162 CrossRef CAS PubMed.
- Y. C. Zhang, H. H. Kim and A. Heller, Anal. Chem., 2003, 75, 3267–3269 CrossRef CAS PubMed.
- H. Xie, C. Y. Zhang and Z. Q. Gao, Anal. Chem., 2004, 76, 1611–1617 CrossRef CAS PubMed.
- T. Shioya and T. M. Swager, Chem. Commun., 2002, 1364–1365 RSC.
- B. J. Holliday, T. B. Stanford and T. M. Swager, Chem. Mater., 2006, 18, 5649–5651 CrossRef CAS.
- N. Haddour, J. Chauvin, C. Gondran and S. Cosnier, J. Am. Chem. Soc., 2006, 128, 9693–9698 CrossRef CAS PubMed.
- W. J. Yao, A. Le Goff, N. Spinelli, M. Holzinger, G. W. Diao, D. Shan, E. Defrancq and S. Cosnier, Biosens. Bioelectron., 2013, 42, 556–562 CrossRef CAS PubMed.
- A. Le Goff and S. Cosnier, J. Mater. Chem., 2011, 21, 3910–3915 RSC.
- Y. J. Qu, X. L. Liu, X. W. Zheng and Z. H. Guo, Anal. Sci., 2012, 28, 571–576 CrossRef CAS PubMed.
- W. W. Zhao, J. J. Xu and H. Y. Chen, Chem. Rev., 2014, 114, 7421–7441 CrossRef CAS PubMed.
- Q. L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- A. U. Czaja, N. Trukhan and U. Müller, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- J. P. Lei, R. C. Qian, P. H. Ling, L. Cui and H. X. Ju, TrAC, Trends Anal. Chem., 2014, 58, 71–78 CrossRef CAS.
- B. Q. Yuan, R. C. Zhang, X. X. Jiao, J. Li, H. Z. Shi and D. J. Zhang, Electrochem. Commun., 2014, 40, 92–95 CrossRef CAS.
- H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. Tadjarodi and A. R. Fakhari, Biosens. Bioelectron., 2013, 42, 426–429 CrossRef CAS PubMed.
- H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. R. Fakhari and M. M. Amini, Electrochim. Acta, 2013, 88, 301–309 CrossRef CAS.
- Y. F. Zhang, X. J. Bo, C. Luhana, H. Wang, M. Li and L. P. Guo, Chem. Commun., 2013, 49, 6885–6887 RSC.
- X. Wang, Q. X. Wang, Q. H. Wang, F. Gao, Y. Z. Yang and H. X. Guo, ACS Appl. Mater. Interfaces, 2014, 6, 11573–11580 CAS.
- Y. C. Fu, P. H. Li, L. J. Bu, T. Wang, Q. J. Xie, J. H. Chen and S. Z. Yao, Anal. Chem., 2011, 83, 6511–6517 CrossRef CAS PubMed.
- W. J. Ma, Q. Jiang, P. Yu, L. F. Yang and L. Q. Mao, Anal. Chem., 2013, 85, 7550–7557 CrossRef CAS PubMed.
- Y. Wang, Y. C. Wu, J. Xie, H. L. Ge and X. Y. Hu, Analyst, 2013, 138, 5113–5120 RSC.
- Y. Wang, H. L. Ge, Y. C. Wu, G. Q. Ye, H. H. Chen and X. Y. Hu, Talanta, 2014, 129, 100–105 CrossRef CAS PubMed.
- S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Sensors, 2009, 9, 1574–1589 CrossRef CAS PubMed.
- J. J. Gassensmith, J. Y. Kim, J. M. Holcroft, O. K. Farha, J. F. Stoddart, J. T. Hupp and N. C. Jeong, J. Am. Chem. Soc., 2014, 136, 8277–8282 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |





