Facile synthesis of viologen and its reversible lithium storage property in organic lithium-ion batteries†
Arnab Ghosh and
Sagar Mitra*
Department of Energy Science and Engineering, IIT Bombay, Powai, Mumbai 400076, India. E-mail: sagar.mitra@iitb.ac.in; Tel: + 91 22 2576 7849
First published on 8th December 2015
Abstract
The reversible lithium-ion storage property of viologen has been explored for the first time. The viologen exhibits a reversible capacity of 175 mA h g−1 against lithium at 50 mA g−1 discharge rate, long term cycling performance and good rate capability. The reversible lithiation–delithiation property of the viologen has been confirmed by ex situ NMR studies.
Due to high specific capacity and high energy density, rechargeable lithium ion battery technology1 is becoming the most promising alternative to lead-acid, Ni–Cd, and Ni–MH batteries. The conventional lithium ion batteries are based on inorganic insertion compounds (e.g., LiCoO2, LiMn2O4, LiFePO4, LiV3O8 etc.) as cathode materials2–5 and Si, Sn etc.6,7 as anode materials. However, large scale production of these materials using ceramic processes causes a serious concern about environmental issues. Additionally, extracting of these transition metals form their ores and transforming them into electro-active materials is more expensive and produce toxic by-products. Therefore, there is a surging demand for cost-effective, sustainable electrode materials.8,9 As an alternative to conventional inorganic materials, the redox active organic compounds offer the opportunity to develop low cost and sustainable “green batteries” thanks to their sustainability, structural variety, flexibility10 and no ecological footprint. Not only that, the charge carrying function of elemental ions can be substituted by other molecular ions in redox reactions of organic compounds as reported by M. Yao et al.11 very recently.
The organic electrode materials are not a new concept, since, the first organic cathode based on dichloroisocyanuric acid was proposed in 1969.12 After that, for a long time, organic electrode materials have received much less attention because of their less capacity, poor rate performance due to their solubility in electrolytes and high electronic resistivity. Recently, Tarascon and co-workers provided a solution to overcome those problems by proposing conjugated carboxylic acid salts.13 Thus after, several organic electrode materials14–17 using conjugated carbonyl compounds, organosulfur compounds, electrochemically active radical polymers18–20 etc. have been reported till the date.
In this paper, for the first time, a small organic compound of N,N-disubstituted-4,4′-bipyridine (commonly known as ‘viologen’) family, is described as an electrode material for lithium-ion batteries. Viologens are most often used as redox mediators,21,22 molecular electronics,23 and potential electrochromic materials24–27 in commercially available smart windows and rear view mirrors. The electrochromic behavior of the viologen monomers is based on reversible interconversion between two redox states via 2e− redox reaction, when externally negative electric potential is applied. Due to the excellent redox properties, the use of viologens are being shifted towards energy storage devices. Viologens have been used as charge carriers in a polymer based battery by Palmore et al.28 Recently, poly(vinyl-benzyl ethyl viologen), a polymeric viologen has been reported as redox active polymer for non-aqueous redox flow batteries (NRFBs) by Nagarjuna et al.29 Inspiring by these works, we synthesized a viologen monomer N,N-di(boron trifluoride)-4,4′-bipyridine [or boron trifluoride viologen] (BTFV), comprising of a 4,4′-bipyridine core, sandwiched between two BF3 molecules and used it as an electrode material for lithium ion battery. BTFV has a stable aromatic core, which offers a theoretical capacity of 183 mA h g−1 (calculation is shown in ESI†) with respect to lithium, by following the same electrochemistry as in electrochromic devices and redox flow batteries. Hence, it could be an alternative electrode material for sustainable and cost-effective lithium-ion batteries.
N,N-di-(boron trifluoride)-4,4′-bipyridine (BTFV) was prepared via following steps (Scheme 1): firstly, a suspension of 4,4′-bipyridine (1 mmol) was prepared in 10 mL diethyl ether. To this suspension, 2.2 mmol of boron trifluoride diethyl etherate BF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O(C2H5)2 was added (excess etherated boron trifluoride was added to avoid any mono-quaternization of 4,4′-bipyridine). Due to the lower electronegativity of nitrogen in comparison to oxygen, the ether molecules coordinately attached to BF3 substituted by 4,4′-bipyridine molecules and immediately a white colored precipitated was obtained due to instant Lewis acid–base adduct formation between BF3 and 4,4′-bipyridine. The colorless property of BTFV is due to absence of any charge transfer transition and could be beneficial for electrochromic applications. The precipitate was then filtrated and air-dried. The formation of product was confirmed by FTIR, 13C-NMR, 11B-NMR and 19F-NMR. X-ray diffraction studies were performed for both 4,4′-bipyridine and BTFV to understand the change of crystallinity of 4,4′-bipyridine upon adduct formation. The morphology and microstructure of the product was understood by scanning electron microscopy (SEM). Energy dispersive X-ray (EDX) analysis was carried out for elemental analysis of the product.
O(C2H5)2 was added (excess etherated boron trifluoride was added to avoid any mono-quaternization of 4,4′-bipyridine). Due to the lower electronegativity of nitrogen in comparison to oxygen, the ether molecules coordinately attached to BF3 substituted by 4,4′-bipyridine molecules and immediately a white colored precipitated was obtained due to instant Lewis acid–base adduct formation between BF3 and 4,4′-bipyridine. The colorless property of BTFV is due to absence of any charge transfer transition and could be beneficial for electrochromic applications. The precipitate was then filtrated and air-dried. The formation of product was confirmed by FTIR, 13C-NMR, 11B-NMR and 19F-NMR. X-ray diffraction studies were performed for both 4,4′-bipyridine and BTFV to understand the change of crystallinity of 4,4′-bipyridine upon adduct formation. The morphology and microstructure of the product was understood by scanning electron microscopy (SEM). Energy dispersive X-ray (EDX) analysis was carried out for elemental analysis of the product.
The reversible redox property of BTFV was confirmed by cyclic voltammetry, carried out on an Autolab PGSTAT 302N electrochemical workstation, using three-electrode configuration with fluorine doped tin oxide (FTO) coated glass as working electrode, Pt-rod as counter electrode and Ag/AgCl/KCl half-cell as reference electrode. The cyclic voltammetry experiment was performed with 20 mM solution of BTFV prepared in a 15 mL liquid electrolyte comprise of N,N-dimethylformamide (DMF) and 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide ionic liquid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at a scan rate of 5 mV s−1. Furthermore, to investigate the preliminary battery performance of the viologen, another cyclic voltammetry was carried out against lithium metal in presence of lithium electrolyte. Working electrode was prepared using BTFV as active material, super P as conductive additive and polyethylene oxide (PEO) as binder with an overall ratio of 60
1 v/v) at a scan rate of 5 mV s−1. Furthermore, to investigate the preliminary battery performance of the viologen, another cyclic voltammetry was carried out against lithium metal in presence of lithium electrolyte. Working electrode was prepared using BTFV as active material, super P as conductive additive and polyethylene oxide (PEO) as binder with an overall ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (by wt). The slurry was prepared in acetonitrile and then blade cast onto a carbon coated copper foil. The active material loading was about 0.45 mg cm−2. Swagelok-type cells are prepared using this organic electrode as working electrode with lithium foil as reference/counter electrode and borosilicate glass fiber sheet as a separator soaked with 50 μL of electrolyte comprising of 1 M LiPF6 in 1
10 (by wt). The slurry was prepared in acetonitrile and then blade cast onto a carbon coated copper foil. The active material loading was about 0.45 mg cm−2. Swagelok-type cells are prepared using this organic electrode as working electrode with lithium foil as reference/counter electrode and borosilicate glass fiber sheet as a separator soaked with 50 μL of electrolyte comprising of 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethylene carbonate (EC) and dimethyl carbonate (DMC). Cyclic voltammetry was carried out at a scan rate of 50 μV s−1 within the potential window of 0.01–3.0 V (vs. Li/Li+) using Biologic VMP-3. The Galvanostatic charge–discharge experiments were performed using an Arbin BT-2000 within two different potential ranges of 0.01–3.0 V and 0.8–2.8 V vs. Li/Li+. All capacities were calculated based on the mass of BTFV in electrode.
1 (v/v) ethylene carbonate (EC) and dimethyl carbonate (DMC). Cyclic voltammetry was carried out at a scan rate of 50 μV s−1 within the potential window of 0.01–3.0 V (vs. Li/Li+) using Biologic VMP-3. The Galvanostatic charge–discharge experiments were performed using an Arbin BT-2000 within two different potential ranges of 0.01–3.0 V and 0.8–2.8 V vs. Li/Li+. All capacities were calculated based on the mass of BTFV in electrode.
FTIR spectra of N,N-di-(boron trifluoride)-4,4′-bipyridine (BTFV) is shown in ESI (Fig. S1†). A characteristic peak is observed at 1050 cm−1 which is related to formation of nitrogen-boron co-ordination bond (N → B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N+ – B−), while the peak intensity at around 1300 cm−1 (due to B–O bond) is found to be decreased, indicating the replacement of coordinated diethyl ether molecules by 4,4′-bipyridine. A medium and broad peak is observed around 1476 cm−1 corresponding to the aromatic C
N+ – B−), while the peak intensity at around 1300 cm−1 (due to B–O bond) is found to be decreased, indicating the replacement of coordinated diethyl ether molecules by 4,4′-bipyridine. A medium and broad peak is observed around 1476 cm−1 corresponding to the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond stretching. The band at 3199 cm−1 is due to aromatic hydrogens (Ar–H) attached to the bi-pyridine ring. The band at 781 cm−1 is assigned to aromatic C–H bending vibration.
C bond stretching. The band at 3199 cm−1 is due to aromatic hydrogens (Ar–H) attached to the bi-pyridine ring. The band at 781 cm−1 is assigned to aromatic C–H bending vibration.
13C-NMR was taken (Fig. 1a) by dissolving the product (BTFV) in CD3CN. The peaks at 1.57 ppm and 118.5 ppm are corresponding to the carbons present in –CD3 and –CN groups. The peaks at 15.04 ppm (–OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3) and 66.99 ppm (–O
H3) and 66.99 ppm (–O![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2CH3) are because of residual amount of ether present in the product. The characteristic peaks of the product are assigned as follows: 127.9 ppm (the aromatic carbons next to the carbon directly attached with nitrogen), 143.85 ppm (aromatic carbons directly attached with nitrogen) and 153.58 ppm (the aromatic carbons opposite to nitrogen).
H2CH3) are because of residual amount of ether present in the product. The characteristic peaks of the product are assigned as follows: 127.9 ppm (the aromatic carbons next to the carbon directly attached with nitrogen), 143.85 ppm (aromatic carbons directly attached with nitrogen) and 153.58 ppm (the aromatic carbons opposite to nitrogen).
Boron NMR was taken to further confirm the formation of viologen. Fig. 1b shows the 11B-NMR spectra of BTFV. It can be observed from the spectra that there is a significant peak shift from 0 ppm (for pure diethyl etherated BF3 (ref. 30)) to 4.58 ppm, confirming the replacement of diethyl ether molecules and adduct formation between BF3 and 4,4′-bipyridine.
Fig. 1c shows the fluorine NMR spectrum of BTFV. Boron has two naturally occurring NMR active nuclei 10B and 11B. Hence, two peaks were observed at −148.7 and −148.6 ppm, respectively. The strong peak at −148.7 ppm is due to the fluorine attached to 11B (more sensitive) atom and the weak one at −148.6 ppm representing the fluorine attached to 10B. The significant peak shift from −153.2 ppm (pure diethyl etherated BF3(ref. 30)) to −148.7 indicates the replacement of coordinately bonded diethyl ether molecules by 4,4′-bipyridine molecules. The XRD patterns of 4,4′-bipyridine and BTFV showed a remarkable difference in crystallinity of 4,4′-bipyridine upon adduct formation with boron trifluoride (Fig. S2†).
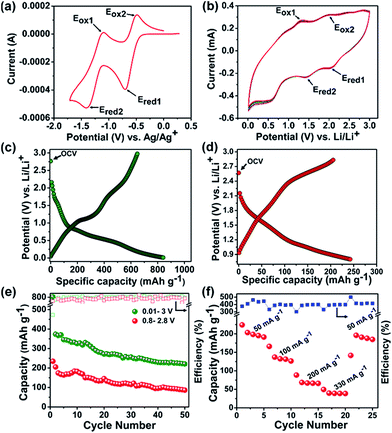
The BTFV showed a very good redox property as it is clear from cyclic voltammogram (Fig. 2a) with respect to Ag/AgCl/KCl reference electrode (EAg+/Ag = 0.20 V vs. SHE). The first reduction peak in the cathodic sweep is obtained at −0.71 V which is attributed to the reduction of the neutral N,N-di-(boron trifluoride)-4,4′-bipyridine (BTFV0) to its corresponding radical-anion (BTFV˙−) form. Another peak is seen at relatively higher potential of −1.40 V, which corresponds to the further reduction leading to formation of di-anionic species (BTFV2−). On changing the sweep direction, the first and second anodic peaks were obtained at around −1.11 V and −0.47 V, respectively. The first anodic peak at −1.11 V indicates the oxidation of di-anionic viologen (BTFV2−) to radical-anion (BTFV˙−) and the second one implies the further oxidation to the neutral form (BTFV0). Furthermore, two pairs of approximately symmetrical sized cathodic and anodic peaks have almost same areas, suggesting good lithiation/de-lithiation reversibility for this organic electrode material. Following the same redox chemistry, a reversible two steps lithiation and delithiation process in BTFV is also observed in CV experiment against Li/Li+ (Fig. 2b). During reduction process, three prominent peaks are obtained. The peaks at around 2.15 V is related to the conversion of neutral BTFV to its radical-anion form and the following peak at 1.5 V is due to further reduction of radical-cation to di-anionic viologen as shown in ESI (Fig. S4†). The peak at around 0.7–0.5 V is because of reversible insertion of Li+ ions into the carbon black.
The first cycle charge–discharge profile of BTFV electrode within the potential range of 0.01–3.0 V vs. Li/Li+ at the scan rate of 50 mA g−1 is shown in Fig. 2c. The discharge profile exhibits three plateaus at around 2.15 V, 1.55 V and 0.7–0.5 V. This is in good agreement with the three reduction peaks in the CV operated between 0.01–3.0 V. The first cycle charge–discharge profile of BTFV electrode within the potential range of 0.8–2.8 V (vs. Li/Li+) at 50 mA g−1 current rate exhibited only two discharge plateaus at around 2.15 V and 1.55 V (Fig. 2d). From Fig. 2e it can be observed that the specific capacity obtained from cycling performance operated between 0.01–3.0 V is much higher than that between 0.8–2.8 V. Since, the theoretical capacity of BTFV is 183 mA h g−1, at least 160 mA h g−1 comes from the reversible intercalation of Li+ ions into carbon at lower potential (0.5–0.7 V) and it is possible that at most 300 mA h g−1 (= 600 mA h g−1 × 30 wt% of super P/60 wt% BTFV) contribution from super P carbon as reported by C. W. Lee et al.31 Furthermore, during cycling between 0.01–3.0 V some irreversible capacity in the first discharge may be attributed to the rapid decomposition of electrolyte and formation of solid electrolyte interphase (SEI) at lower potential.
To further interrogate the electrochemical properties of the viologen, additional experiments to examine rate capability (Fig. 2f) and extended cycling performance (Fig. S7†) were carried out. The rate performance has been carried out at 50, 100, 200 and 330 mA h g−1 discharge rates and results are shown in Fig. 2f. The capacities at different rates found to be stable.
To interpret the reversible lithium storage property of this viologen, ex situ NMR characterizations have been carried out. Two separate Swagelok cells are prepared with BTFV as the working electrode. First cell has been discharged to 0.8 V (vs. Li/Li+) and for the second cell first discharge to 0.8 V and then recharged to 2.8 V. Both the electrodes are washed properly in order to remove the electrolyte salt, if any. Then electrode materials are taken out from the current collector and dried at room temperature. All of these steps are carried out inside the argon-filled glove box. The electrode materials are then separately centrifuged in acetonitrile-d3 (CD3CN). A little volume of supernatant liquid was taken for NMR characterizations. Fig. 3 shows 7Li-NMR spectrum for both the electrode materials. The electrode material discharged to 0.8 V exhibits a strong peak at 1.94 ppm (Fig. 3a) corresponds to lithiated BTFV. While for the electrode material which is first discharged to 0.8 V and then recharged to 2.8 V, the peak at 1.94 ppm gets disappeared and a very weak peak is observed at 0.83 ppm (Fig. 3b) due to the minute amount of unwashed LiPF6 salt. The disappearance of lithium peak at 1.94 ppm indicates the de-lithiation of BTFV.
In summary, an organic material of N,N-disubstituted-4,4′-bipyridine (viologen) category synthesized in a very simple way. The viologen shows a good capacity retention and good rate capability at different current rates with respect to lithium and hence it can be an alternative electrode material for sustainable organic lithium ion batteries.
Acknowledgements
This work is financially supported by National Centre for Photovoltaic Research and Education (NCPRE), IIT Bombay. The authors are thankful to SAIF (IIT Bombay) for instrument facility. Author A. Ghosh would like to thank Sudeep Sarkar and Ananta Sarkar for their help to get instrumental facilities.Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928 CrossRef CAS PubMed.
- C. M. Julien, A. Mauger, K. Zaghib and H. Groult, Inorganics, 2014, 2, 132 CrossRef CAS.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419 CrossRef.
- S. Sarkar, H. Banda and S. Mitra, Electrochim. Acta, 2013, 99, 242 CrossRef CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- A. M. Tripathi and S. Mitra, ChemElectroChem, 2014, 1, 1327 CrossRef CAS.
- D. Larcher and J. M. Tarascon, Nat. Chem., 2014, 7, 19 CrossRef PubMed.
- Z. Song and H. Zhou, Energy Environ. Sci., 2013, 6, 2280 CAS.
- A. L. Goodwin, Nat. Mater., 2010, 9, 7 CrossRef CAS.
- M. Yao, H. Sano, H. Ando and T. Kiyobayashi, Sci. Rep., 2015, 5, 10962 CrossRef CAS PubMed.
- D. L. Williams, J. J. Byrne and J. S. Driscoll, J. Electrochem. Soc., 1969, 116, 2 CrossRef CAS.
- M. Armand, S. Grugeon, H. Vezin, S. Laruelle, P. Ribière, P. Poizot and J.-M. Tarascon, Nat. Mater., 2009, 8, 120 CrossRef CAS PubMed.
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742 CrossRef CAS.
- L. M. Zhu, A. W. Lei, Y. L. Cao, X. P. Ai and H. X. Yang, Chem. Commun., 2013, 49, 567 RSC.
- H. Chen, M. Armand, G. Demailly, F. Dolhem, P. Poizot and J.-M. Tarascon, ChemSusChem, 2008, 1, 348 CrossRef CAS PubMed.
- S. Goriparti, M. N. K. Harish and S. Sampath, Chem. Commun., 2013, 49, 7234 RSC.
- P. Novák, K. Müller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207 CrossRef.
- X. Han, C. Chang, L. Yuan, T. Sun and J. Sun, Adv. Mater., 2007, 19, 1616 CrossRef CAS.
- T. Janoschka, M. D. Hager and U. S. Schubert, Adv. Mater., 2012, 24, 6397 CrossRef CAS.
- D. M. Scott, T. H. Tsang, L. Chetty, S. Aloi and B. Y. Liaw, J. Power Sources, 2011, 196, 10556 CrossRef CAS.
- M. J. Lacey, J. T. Frith and J. R. Owen, Electrochem. Commun., 2013, 26, 74 CrossRef CAS.
- P. M. S. Monk, The Viologens: Physicochemical Properties, Synthesis and Applications of the Salts of 4,4′-Bipyridine, J. Wiley & Sons, 1998 Search PubMed.
- R. J. Mortimer, Electrochim. Acta, 1999, 44, 2971 CrossRef CAS.
- R. J. Mortimer, A. L. Dyer and J. R. Reynolds, Displays, 2006, 27, 2 CrossRef CAS.
- R. J. Mortimer and T. S. Varley, Sol. Energy Mater. Sol. Cells, 2012, 99, 213 CrossRef CAS.
- R. Sydam, A. Ghosh and M. Deepa, Org. Electron., 2015, 17, 33 CrossRef CAS.
- S. Sen, J. Saraidaridis, S. Y. Kim and G. T. R. Palmore, ACS Appl. Mater. Interfaces, 2013, 5, 7825 CAS.
- G. Nagarjuna, J. Hui, K. J. Cheng, T. Lichtenstein, M. Shen, J. S. Moore and J. R. Loópez, J. Am. Chem. Soc., 2014, 136, 16309 CrossRef CAS PubMed.
- E. L. Myers, C. P. Butts and V. K. Aggarwal, Chem. Commun., 2006, 4434 RSC.
- R. M. Gnanamuthu and C. W. Lee, Mater. Chem. Phys., 2011, 130, 831 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra20301e |
| This journal is © The Royal Society of Chemistry 2015 |