Syntheses, structures and luminescence of a series of coordination polymers constructed with 4-substituted 1,2,4-triazole and biscarboxylate co-ligands†
Shan-Shan Han,
Lu-Lu Shi,
Ke Li,
Shan Zhao,
Bao-Long Li* and
Bing Wu
State and Local Joint Engineering Laboratory for Functional Polymeric Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P.R. China. E-mail: libaolong@suda.edu.cn
First published on 7th December 2015
Abstract
Eight coordination polymers {[Cd3(btpm)2(1,4-bdc)3]·6H2O}n (1), {[Cd2(btpm)(mip)2(H2O)]·1.25H2O}n (2), {[Cd2(btpm)(nip)2(H2O)]·H2O}n (3), {[Cd4(btpm)3(H0.5suc)2(suc)2Cl(H2O)2]·9H2O}n (4), {[Cd2(btpm)(glu)2(H2O)2]·5H2O}n (5), {[Cd2(btpm)(mbda)2(H2O)]·3H2O}n (6), {[Zn(btpm)(1,4-bdc)](1,4-H2bdc)}n (7), {[Zn(btpm)(adc)]·3H2O}n (8) (btpm = bis(4-(1,2,4-triazol-4-yl)phenyl)methane, 1,4-bdc = 1,4-benzenedicarboxylate, mip = 5-methyl-isophthalate, nip = 5-nitro-isophthalate, suc = succinate, glu = glutarate, mbda = 1,3-benzenediacetate, adc = 1,3-adamantanedicarboxylate) were hydrothermally synthesized. 1 shows an unusual 8-connected three-dimensional network based on Cd3(COO)2 trimer clusters. 2 exhibits an unusual (3,7)-connected three-dimensional network based on Cd2(COO) dimers. 3 presents a (3,4,5)-connected 3D network. 4 shows the 6-connected three-dimensional pcu net based on Cd4Cl tetranuclear clusters. 5 exhibits a 2-fold interpenetrating three-dimensional pcu network based on Cd2(COO)2 dimers. 6 presents an unusual 2D network based on nanotube-like [Cd2(mbda)2] chains. 7 shows the intriguing 2D → 3D polythreaded network based on a special 2D (6,3) network, with six Zn(II) atoms at six corners and four 1,4-bdc and two double btpm at six edges. 8 exhibits an undulated 2D (4,4) network. 1–8 show structural diversity based on mono-metal, bi-, tri- and tetra-nuclear metal clusters. The coordination polymers can be tuned with different biscarboxylates and metal cations based on the semi-rigid 4-substituted 1,2,4-triazole ligand btpm. The luminescence and thermal stability were investigated.
Introduction
The coordination polymers (CPs) are of great interest not only due to their fascinating topologies but also their potential applications as functional materials for magnetism, gas storage, gas adsorption, molecular recognition, catalysis, luminescence and so on.1,2 The centre metal ions and organic bridging ligands are the key for the construction of the coordination polymers with intriguing topologies and interesting properties. The Cd(II) and Zn(II) complexes with their metal cations adopting the d10 configuration have attracted great interest because of attractive fluorescence properties and potential applications as new advanced luminescent materials.3The metal–organic frameworks such as 3, 4, 5, and 6-connected networks have been widely synthesized. However, it is still a great challenge to synthesize the development of high-connected frameworks, such as 7-, 8-, 9-, 10- and 12-connected, which can be partly attributed to the limited coordination numbers of single metal ions and steric hindrance of the most commonly used organic ligands.4
Meanwhile, metal-cluster-based organic frameworks (MCOFs) have attracted particular attention because of their unique frameworks and possible applications as functional materials for high-connected networks.5 The biscarboxylate ligands such as 1,4-benzenedicarboxylate,6 5-methyl-isophthalate,7 5-nitro-isophthalate,8 succinate,9 glutarate,10 1,3-benzenediacetate11 and 1,3-adamantanedicarboxylate12 present versatile coordination modes and are very effective building blocks for construction of MOFs. 1,2,4-Triazole and its derivatives are very interesting ligands because they combine the coordination geometry of both pyrazole and imidazole with regard to the arrangement of their three heteroatoms.13 The 1-substituted 1,2,4-triazole ligands have been widely synthesized and characterized.14,15 However the 4-substituted 1,2,4-triazole ligands have been less synthesized and characterized.16,17
In previous work, we synthesized many metal–organic frameworks using flexible 1-substituted bis(1,2,4-triazole) ligands such as 1,2-bis(1,2,4-triazol-1-yl)ethane (bte),18 1,3-bis(1,2,4-triazol-1-yl)propane (btp),19 1,4-bis(1,2,4-triazol-1-yl)butane (btb),20 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene (bbtz)21 and 1,4-bis(1,2,4-triazol-1-ylmethyl)-2,3,4,5-tetramethylbenzene (tmtz).22 For example, {[Cu9(OH)6(bte)2(sip)4(H2O)3]·6H2O}n shows a (3,14)-connected net based on the unprecedented enneanuclear copper(II) cluster [Cu9(μμ3-OH)4(μ2-OH)2] (sip = 5-sulfoisophthalate).18a {[Zn(btp)(1,4-bdc)][Zn(btp)(1,4-bdc)0.5Cl]H2O}n (1,4-bdc = 1,4-benzenedicarboxylate) represents a new type of entanglement that only a half of the loops of 2D networks are polythreading by 1D chains containing alternating rings and rods.19 [2D-Mn(btb)2(NCS)2][1D-Mn(btb)2(NCS)2]20a and [Cd3(bbtz)6(H2O)6](BF4)6·1.75H2O21a show 2D (4,4) networks and 1D ribbons of rings polycatenated in a 3D array.
Recently we synthesized some metal–organic frameworks using flexible 4-substituted bis(1,2,4-triazole) ligands such as 1,2-bis(1,2,4-triazol-4-yl)ethane (btre)23 and 1,4-bis(1,2,4-triazol-4-yl)benzene (btx).24 {[Cd(btre)Cl]·OH}n forms a 3D microporous cationic network (btre = 1,2-bis(1,2,4-triazol-4-yl)ethane). Whereas {[Cd(btre)Cl][CdCl(dca)2]·0.5H2O}n exhibits a polythreading coordination array formed from a 3D microporous cationic network and 1D anion ladders.23a {[Zn(btre)0.5(OH-bdc)(H2O)2]1.5H2O}n (OH-bdc = 5-hydrohy-isophthalate) exhibits an unusual 3D coordination network formed by parallel polythreading of twofold polycatenated (6,3) layers.23b {[Zn(btx)(1,4-bdc)]3H2O}n (1,4-bdc = 1,4-benzenedicarboxylate) shows a 5-fold interpenetrated three-dimensional diamondoid network. [Zn(btx)0.5(fum)(H2O)]n (fum = fumarate) displays a 2D → 3D inclined polycatenation motif consisting of two sets of equivalent 2D (6,3) layers.24
When the rigid bifunctional ligands are used as spacers to connect metal centers, the topology of the network is usually determined by the coordination geometry of the central metal preference. Contrary to the rigid spacers, the flexible ligand, which can adopt various conformations, may induce coordination polymers with novel topologies. In contrast to rigid ligands with fixed conformation and flexible ligands with changing configuration in the assembly of target coordination polymers, the semi-rigid ligands combine the advantages of both. Bis(4-(1,2,4-triazol-4-yl)phenyl)methane (btpm) is a long and semi-rigid 4-substituted 1,2,4-triazol ligand containing two phenyl rings, two 1,2,4-triazol rings and one CH2 group. The combination N-donor ligand (btpm) and biscarboxylate mixed ligand system can result in novel topologies and intriguing properties. In the present work, eight metal–organic frameworks {[Cd3(btpm)2(1,4-bdc)3]·6H2O}n (1), {[Cd2(btpm)(mip)2(H2O)]·1.25H2O}n (2), {[Cd2(btpm)(nip)2(H2O)]·H2O}n (3), {[Cd4(btpm)3(H0.5suc)2(suc)2Cl(H2O)2]·9H2O}n (4), {[Cd2(btpm)(glu)2(H2O)2]·5H2O}n (5), {[Cd2(btpm)(mbda)2(H2O)]·3H2O}n (6), {[Zn(btpm)(1,4-bdc)](1,4-H2bdc)}n (7), {[Zn(btpm)(adc)]·3H2O}n (8) (1,4-bdc = 1,4-benzenedicarboxylate, mip = 5-methyl-isophthalate, nip = 5-nitro-isophthalate, suc = succinate, glu = glutarate, mbda = 1,3-benzenediacetate, adc = 1,3-adamantanedicarboxylate) were hydrothermal synthesized (Scheme 1). 1–8 show the structural diversity based on mono-metal, bi-, tri- and tetra-nuclear metal clusters. The luminescence and thermal stability were investigated.
Experimental section
Materials and physical measurements
All reagents were of analytical grade and used without further purification. Elemental analyses for C, H and N were performed on a Perkin-Elmer 240C analyser. IR spectra were obtained for KBr pellets on a Nicolet 170SX FT-IR spectrophotometer in the 4000–400 cm−1 region. XPRD were performed on a D/MAX-3C diffractometer with the CuKα radiation (λ = 1.5406) at room temperature. The luminescence measurements were carried out in the solid state at room temperature and the spectra were collected with a Perkin-Elmer LS50B spectrofluorimeter. TGA was carried out using a Thermal Analyst 2100 TA Instrument and SDT 2960 Simultaneous TGA-DTA Instrument in flowing dinitrogen at a heating rate of 10 °C min−1.Synthesis of {[Cd3(btpm)2(1,4-bdc)3]·6H2O}n (1)
A solution of 1,4-H2bdc (0.2 mmol) in 5 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.2 mmol) in 8 mL MeOH and Cd(NO3)2·4H2O (0.2 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 130 °C for 4 days and then cooled to room temperature to give the pale yellow crystals 1 in 52% yield. Anal. calc for C58H52Cd3N12O18 (1): C, 45.17; H, 3.40; N, 10.90; found: C, 45.13; H, 3.38; N, 10.81%. IR (cm−1, KBr): 3398m, 3097w, 1636s, 1580s, 1542m, 1387s, 1329m, 1247m, 1097w, 1049w, 1018w, 884w, 844w, 817m, 792w, 748s, 642w.Synthesis of {[Cd2(btpm)(mip)2(H2O)]·1.25H2O}n (2)
A solution of H2mip (0.2 mmol) in 6 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.1 mmol) in 5 mL MeOH and Cd(NO3)2·4H2O (0.2 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 110 °C for 3 days and then cooled to room temperature to give the pale yellow crystals 2 in 47% yield. Anal. Calc. for C35H30.50Cd2N6O10.25 (2): C, 45.50; H, 3.33; N, 9.10; found: C, 45.38; H, 3.29; N, 9.02%. IR (cm−1, KBr): 3357m, 3152w, 3103w, 1613m, 1559s, 1533vs, 1425w, 1368s, 1245m, 1129w, 1096m, 1040w, 1014w, 982w, 865w, 820w, 793m, 776s, 730m, 707w, 637w.Synthesis of {[Cd2(btpm)(nip)2(H2O)]·H2O}n (3)
A solution of H2nip (0.2 mmol) in 6 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.1 mmol) in 5 mL MeOH and Cd(NO3)2·4H2O (0.2 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 130 °C for 1 day and then cooled to room temperature to give the yellow crystals 3 in 62% yield. Anal. calc for C33H24Cd2N8O14 (3): C, 40.39; H, 2.47; N, 11.42; found: C, 40.32; H, 2.43; N, 11.35%. IR (cm−1, KBr): 3449m, 3102m, 1608vs, 1536vs, 1453s, 1371vs, 1336s, 1293w, 1241m, 1199w, 1100m, 1059w, 1041w, 1017w, 923w, 864w, 791w, 730s, 647w, 526w.Synthesis of {[Cd4(btpm)3(H0.5suc)2(suc)2Cl(H2O)2]·9H2O}n (4)
A solution of H2suc (0.1 mmol) in 5 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.1 mmol) in 6 mL MeOH and CdCl2·2H2O (0.1 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 90 °C for 3 days and then cooled to room temperature to give the pale yellow crystals 4 in 36% yield. Anal. calc for C67H81Cd4ClN18O27 (4): C, 39.15; H, 3.97; N, 12.27; found: C, 39.21; H, 3.93; N, 12.21%. IR (cm−1, KBr): 3429s, 3111m, 1655w, 1545vs, 1464w, 1402s, 1272w, 1250m, 1149w, 1102m, 1051m, 1019w, 985w, 865w, 793w, 642w.Synthesis of {[Cd2(btpm)(glu)2(H2O)2]·5H2O}n (5)
A solution of H2glu (0.2 mmol) in 6 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.1 mmol) in 5 mL MeOH and Cd(NO3)2·4H2O (0.2 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 110 °C for 3 days and then cooled to room temperature to give the yellow crystals 5 in 38% yield. Anal. calc for C27H40Cd2N6O15 (5): C, 35.50; H, 4.41; N, 9.20; found: C, 35.52; H, 4.38; N, 9.15%. IR (cm−1, KBr): 3442s, 3123m, 1554vs, 1407s, 1317w, 1272w, 1244m, 1099m, 1051w, 1016w, 865w, 822w, 794m, 652w.Synthesis of {[Cd2(btpm)(mbda)2(H2O)]·3H2O}n (6)
A solution of H2mbda (0.2 mmol) in 5 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.1 mmol) in 6 mL MeOH and Cd(NO3)2·4H2O (0.2 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 110 °C for 3 days and then cooled to room temperature to give the yellow crystals 6 in 58% yield. Anal. calc for C37H38Cd2N6O12 (6): C, 45.18; H, 3.89; N, 8.55; found: C, 45.23; H, 3.82; N, 8.51%. IR (cm−1, KBr): 3447s, 3130m, 1551vs, 1335vs, 1290w, 1243w, 1100m, 1047w, 1019w, 812w, 794w, 745w, 720w, 669w, 625w, 523w.Synthesis of {[Zn(btpm)(1,4-bdc)](1,4-H2bdc)}n (7)
A solution of 1,4-H2bdc (0.2 mmol) in 5 mL H2O was adjusted to pH 4 with dilute NaOH solution. Then btpm (0.1 mmol) in 8 mL MeOH and Zn(NO3)2·6H2O (0.1 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 130 °C for 4 days and then cooled to room temperature to give the yellow crystals 7 in 34% yield. Anal. calc for C33H24N6O8Zn (7): C, 56.79; H, 3.47; N, 12.04; found: C, 56.71; H, 3.39; N, 12.11%. IR (cm−1, KBr): 3128m, 2931w, 1710s, 1695m, 1604s, 1560s, 1541s, 1501m, 1390m, 1370m, 1249s, 1119w, 1096w, 1049w, 1017w, 989w, 892w, 867w, 831w, 820w, 792m, 745s, 630w.Synthesis of {[Zn(btpm)(adc)]·3H2O}n (8)
A solution of H2adc (0.2 mmol) in 5 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then btpm (0.2 mmol) in 8 mL MeOH and Zn(NO3)2·6H2O (0.2 mmol) in 5 mL H2O were added with stirring. The mixture was added to a 25 mL Teflon-lined stainless autoclave and this was sealed and heated to 110 °C for 4 days and then cooled to room temperature to give the yellow crystals 8 in 54% yield. Anal. calc for C29H34N6O7Zn (8): C, 54.09; H, 5.32; N, 13.05; found: C, 53.87; H, 5.24; N, 13.01%. IR (cm−1, KBr): 3082m, 2940w, 2893w, 1593m, 1536vs, 1450w, 1364m, 1334m, 1312m, 1240w, 1096m, 1019w, 1005w, 981w, 865w, 793w, 772m, 753s, 675w, 641w.X-ray crystallography
Suitable single crystals of 1–8 were carefully selected under an optical microscope and glued to thin glass fibers. The diffraction data were collected on the Rigaku Mercury or Saturn CCD or Agilent Gemini Atlas CCD diffractometer with graphite monochromated MoKα or Cu Kα radiation. Intensities were collected by the ω scan technique. The structures were solved by direct methods and refined with full-matrix least-squares technique (SHELXTL-97).25 The parameters of the crystal data collection and refinement of 1–8 are given in Tables 1 and 2. Selected bond lengths and bond angles are listed in Table S1 in the ESI.†| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Formula | C58H52Cd3N12O18 | C35H30.50Cd2N6O10.25 | C33H24Cd2N8O14 | C67H81Cd4ClN18O27 |
| Fw | 1542.32 | 923.95 | 981.40 | 2055.55 |
| T/K | 293(2) | 293(2) | 223(2) | 223(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P21/a | P21/a | C2/c |
| a/Å | 16.6815(17) | 16.095(3) | 15.964(4) | 24.5296(6) |
| b/Å | 21.985(2) | 16.492(3) | 16.319(3) | 19.2262(6) |
| c/Å | 17.129(2) | 16.308(3) | 16.578(4) | 16.5962(4) |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 96.646(4) | 118.020(5) | 118.020(4) | 94.361(3) |
| γ (°) | 90 | 90 | 90 | 90 |
| V/Å3 | 6239.7(11) | 3821.4(13) | 3812.5(15) | 7804.3(4) |
| F(000) | 3088 | 1842 | 1944 | 4136 |
| Z | 4 | 4 | 4 | 4 |
| ρcalcd (g cm−3) | 1.642 | 1.606 | 1.710 | 1.749 |
| μ (mm−1) | 1.094 | 1.176 | 1.193 | 9.720 |
| Reflections collected | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 328 328 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 577 577 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 965 965 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
| Unique reflections | 7089 [R(int) = 0.0406] | 6890 [R(int) = 0.0757] | 8684 [R(int) = 0.0518] | 6975 [R(int) = 0.0518] |
| Parameter | 420 | 503 | 548 | 500 |
| Goodness of fit | 1.049 | 1.132 | 1.093 | 1.050 |
| R1 [I > 2σ(I)] | 0.0482 | 0.0658 | 0.0626 | 0.0520 |
| wR2 (all data) | 0.1452 | 0.1686 | 0.1595 | 0.1532 |
| 5 | 6 | 7 | 8 | |
|---|---|---|---|---|
| Formula | C27H40Cd2N6O15 | C37H38Cd2N6O12 | C33H24N6O8Zn | C29H34N6O7Zn |
| Fw | 913.45 | 983.53 | 697.95 | 643.99 |
| T/K | 293(2) | 293(2) | 293(2) | 293(2) |
| Crystal system | Monoclinic | Triclinic | Triclinic | Triclinic |
| Space group | P21/n | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 14.877(3) | 10.4052(14) | 9.6296(15) | 9.135(3) |
| b/Å | 16.998(3) | 12.1291(15) | 12.8670(16) | 10.723(4) |
| c/Å | 16.095(3) | 16.213(2) | 13.3979(17) | 16.636(6) |
| α (°) | 90 | 84.763(6) | 71.781(13) | 94.855(6) |
| β (°) | 112.498(3) | 83.142(5) | 75.362(15) | 102.944(6) |
| γ (°) | 90 | 89.783(6) | 74.395(15) | 107.147(4) |
| V/Å3 | 3760.1(12) | 2023.0(4) | 1492.7(4) | 1497.6(9) |
| F(000) | 1840 | 988 | 716 | 672 |
| Z | 4 | 2 | 2 | 2 |
| ρcalcd (g cm−3) | 1.614 | 1.615 | 1.553 | 1.428 |
| μ (mm−1) | 1.202 | 1.119 | 0.89 | 0.876 |
| Reflections collected | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712 712 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 689 689 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 032 032 |
| Unique reflections | 8541 [R(int) = 0.0295] | 7374 [R(int) = 0.0408] | 5442 [R(int) = 0.0553] | 6144 [R(int) = 0.0456] |
| Parameter | 494 | 523 | 440 | 428 |
| Goodness of fit | 1.047 | 1.087 | 1.056 | 1.014 |
| R1 [I > 2σ(I)] | 0.0414 | 0.0604 | 0.0650 | 0.0771 |
| wR2 (all data) | 0.1431 | 0.1751 | 0.1515 | 0.1657 |
Results and discussion
Crystal structure of {[Cd3(btpm)2(1,4-bdc)3]·6H2O}n (1)
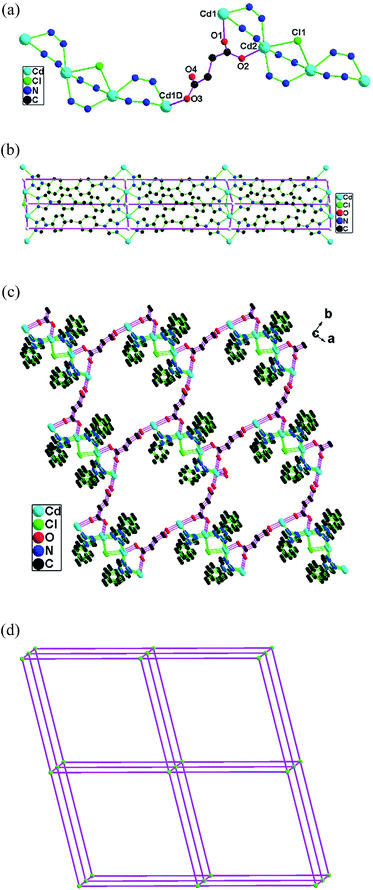
1 shows an unusual 8-connected three-dimensional network based on the Cd3(COO)2 trimer clusters. The asymmetric unit of 1 consists of one Cd(II) atom (Cd1), one half Cd(II) atom (Cd2), one btpm (N1–N6), one 1,4-bdc (O1–O4), one half 1,4-bdc (O5O6) and disordered lattice water molecules. The Cd1 atom is coordinated by five carboxylate oxygen atoms from three 1,4-bdc ligands and two triazole nitrogen atoms from two btpm ligands in the distorted pentagonal-bipyramidal geometry (Fig. S1 in the ESI†). The Cd2 atom is six-coordinated with two carboxylate oxygen atoms from two 1,4-bdc ligands and four triazole nitrogen atoms from four btpm ligands in a distorted octahedral geometry.The oxygen atom O1 of carboxylate group of 1,4-bdc ligand exhibits the bidentate bridging mode and connect two Cd(II) atoms (Fig. 1a). The carboxylate groups (O3O4, O5O6) of 1,4-bdc ligands act as bidentate chelating mode. Therefore one kind of 1,4-bdc ligands (O1–O4) show the tridentate bridging and link three Cd(II) atoms. One kind of 1,4-bdc ligands (O5O6) exhibit the 2-connected sticks and link two Cd(II) atoms.
The btpm ligands exhibit the μ4-tetradentate coordination mode through its N1/N2 and N4/N5 couples (Fig. 1b). The Cd2 atom links two Cd1 atoms through double 1 and 2 position triazole nitrogen atoms and carboxylate O1 atom to form the Cd3(COO)2 trimer cluster. The Cd4 atom links two Cd3 atoms through double 1 and 2 position triazole nitrogen atoms and carboxylate O1 atom to form the Cd3(COO)2 trimer cluster.
Each Cd3(COO)2 trimer cluster connects eight adjacent Cd3(COO)2 trimer clusters through two double btpm and six 1,4-bdc bridges (Fig. 1c), leading to an unusual three-dimensional network (Fig. 1d).
To simplify the structure of 1, the Cd3(COO)2 trimer clusters are simplified as one nodes which are 8-connected. The double btpm and 1,4-bdc ligands ligands exhibit 2-connected nodes. The three-dimensional motif in 1 can be simplified as a 8-connected network with the point symbol of (36·418·53·6) (Fig. 1e).26 The most networks are 3, 4, 5 and 6-connected network. The high-connected (7- and 8-connected) networks are relative few.4,5
Crystal structure of {[Cd2(btpm)(mip)2(H2O)]·1.25H2O}n (2)
2 exhibits an unusual (3,7)-connected three-dimensional network based on the Cd2(COO) dimers. The asymmetric unit of 2 consists of two Cd(II) atoms (Cd1, Cd2), one btpm (N1–N6), two mip (O1–O4, O5–O8) ligands and disordered lattice water molecules. The Cd1 atom is coordinated by four carboxylate oxygen atoms from three mip ligands and two triazole nitrogen atoms from two btpm ligands in the distorted octahedral geometry (Fig. S2 in the ESI†). The Cd2 atom is six-coordinated with three carboxylate oxygen atoms from three mip ligands, one oxygen atom from the coordination water and two triazole nitrogen atoms from two btpm ligands in a distorted octahedral geometry.There are two kinds of mip ligands. The O1 oxygen atom of carboxylate group of one kind of mip ligand (O1–O4) exhibits the bridging mode and connects two Cd(II) atoms (Cd1, Cd2) and forms the Cd2(COO) dimer (Fig. 2a). The carboxylate oxygen atoms O2 and O4 both monodentate coordinate one Cd(II) atom. This kind of mip ligands (O1–O4) act as 2-connected bridges and link two Cd2(COO) dimers. The other kind of mip ligands show the 3-connected bridges through three carboxylate oxygen atoms (O5, O6, O8) and connect three Cd2(COO) dimers (Fig. 2b). The btpm ligands exhibit the μ4-tetradentate coordination mode through its N1/N2, N4/N5 couple (Fig. 2c).
Each Cd2(COO) dimer connects two btpm, two 2-connected mip and three 3-connected ligands (Fig. 2d), leading to an unusual three-dimensional network (Fig. 2e).
To simplify the structure of 2, the Cd2(COO) dimers are simplified as one nodes which are 7-connected. The btpm and one kind of mip ligands (O1–O4) exhibit 2-connected nodes. The other kind of mip ligands (O5–O8) are 3-connected. The three-dimensional motif in 2 can be simplified as the (3,7)-connected network with the Schläfli symbol of (4·62)3(43·611·87) (Fig. 2f).26 The high-connected (3,7)-connected networks are few and unusual.4,5
{[Cd2(btpm)(nip)2(H2O)]·H2O}n (3)
3 presents the (3,4,5)-connected 3D network. The asymmetric unit of 3 consists of two Cd(II) atoms (Cd1, Cd2), one btpm (N1–N6), two nip (O1–O6, O7–O12) ligands, one coordination water (O13) and disordered lattice water molecules. The Cd1 atom is coordinated by four carboxylate oxygen atoms from three nip ligands, one oxygen atom from the coordination water and two triazole nitrogen atoms from two btpm ligands in the distorted octahedral geometry (Fig. S3 in the ESI†). The Cd2 atom is five-coordinated with three carboxylate oxygen atoms from three nip ligands and two triazole nitrogen atoms from two btpm ligands in a distorted trigonal-bipyramidal geometry.The carboxylate groups (O1O2, O7O8) of two nip ligands shows bidentate bridging mode and connect two Cd(II) atoms. The carboxylate groups (O3O4, O9O10) of two nip ligands shows monodentate bridging mode. All nip ligands exhibit the tridentate modes and connect three Cd(II) atoms (Fig. 3a). The btpm ligands exhibit the μ4-tetradentate coordination mode through its N1/N2, N4/N5 couple (Fig. 3b). Each Cd(II) atom (Cd1 or Cd2) connects three nip and two btpm ligands and extend to lead to an unusual three-dimensional network (Fig. 3c).
Topologically, the Cd(II) atoms are 5-connected. The nip ligands act as the 3-connected nodes. The btpm ligands show the 4-connected nodes. The three-dimensional motif in 3 can be simplified as the (3,4,5)-connected network with the Schläfli symbol of (4·82)2(42·82·102) (43·611·87) (Fig. 3d).26 Most of MOFs are 2-nodes. The MOFs with 3-node are relative few and unusual.
Crystal structure of {[Cd4(btpm)3(H0.5suc)2(suc)2Cl(H2O)2]·9H2O}n (4)
4 shows the 6-connected three-dimensional pcu net based on the Cd4Cl tetranuclear clusters. The asymmetric unit of 4 consists of two Cd(II) atoms (Cd1, Cd2), one suc (O1–O4) and one H0.5suc (O5–O8, part protonization suc ligand), one and half btpm (N1–N6, N7–N9), one Cl−, one coordinated water (O9) and the lattice water molecules. The Cd1 atom is coordinated by three carboxylate oxygen atoms from one H0.5suc and two suc ligands, one oxygen atom from the coordinated water and two triazole nitrogen atoms from two btpm ligands in the distorted octahedral geometry (Fig. S4 in the ESI†). The Cd2 atom is six-coordinated with one carboxylate oxygen atom from one suc, one Cl− and four triazole nitrogen atoms from four btpm ligands in a distorted octahedral geometry. The btpm ligands exhibit the μ4-tetradentate coordination mode through its (N1/N2, N4/N5, N7/N8) couples.The Cd(II) atoms links other Cd(II) atoms through double 1 and 2 position triazole nitrogen atoms from btpm ligands, the carboxylate group (O1O2) and Cl− bridge to form the Cd4Cl tetra-nuclear cluster (Fig. 4a). One carboxylate group (O1O2) of suc ligand shows the bridges and connects two Cd(II) atoms. The other carboxylate (O3O4) exhibits the monodentate. One Cd4Cl tetranuclear cluster links two Cd4Cl tetranuclear clusters through triple btpm ligands to construct one-dimensional ladder with three legs (Fig. 4b). Each one-dimensional ladder connects four one-dimensional ladders through suc ligands and forms unusual three-dimensional network (Fig. 4c).
To simplify the structure of 4, the Cd4Cl tetranuclear clusters are simplified as one nodes which are 6-connected. The three-dimensional motif in 4 can be simplified as the 6-connected three-dimensional pcu net (Fig. 4d).
Crystal structure of {[Cd2(btpm)(glu)2(H2O)2]·5H2O}n (5)
5 exhibits the 2-fold interpenetrating three-dimensional pcu network based on Cd2(COO)2 dimers. The asymmetric unit of 5 consists of two Cd(II) atoms (Cd1, Cd2), one btpm (N1–N6), two glu (O1–O4, O5–O8) ligands, two coordination water and disordered lattice water molecules. The Cd1 and Cd2 atoms are coordinated by four carboxylate oxygen atoms from three glu ligands, one oxygen from one coordination water and two triazole nitrogen atoms from two btpm ligands in the distorted pentahedral-bipyramidal geometry (Fig. S5 in the ESI†).There are two kinds of glu ligands. The carboxylate group (O1O2, O5O6) of the glu ligand exhibits the chelating mode. The other carboxylate (O3O4) of one glu ligand shows the monodentate mode. The glu ligand (O1–O4) acts as 2-connected bridge and connects two Cd(II) atoms. The other carboxylate (O7O8) of the glu ligand presents the tridentate mode and connects two Cd(II) atoms and forms Cd2(COO)2 dimer with the Cd⋯Cd distance of 3.9429(7). Two kinds of glu ligands link the Cd(II) atoms and construct the [Cd2(bda)2]n two-dimensional network (Fig. 5a). The adjacent [Cd2(bda)2]n two-dimensional networks are connected by btpm ligands and construct the three-dimensional network (Fig. 5b).
Topologically, the Cd2(COO)2 dimer is 6-connected if it is simplified as one node. The glu and btpm ligands are 2-connected. The 3D network can be described as a 6-connected 3D pcu network based on the Cd2(COO)2 dimer (Fig. 5c). Moreover, two identical 3D networks are still mutually self-interpenetrated to generate the 2-fold interpenetrating 3D pcu network because of the porous nature of the single network (Fig. 5d).
Crystal structure of {[Cd2(btpm)(mbda)2(H2O)]·3H2O}n (6)
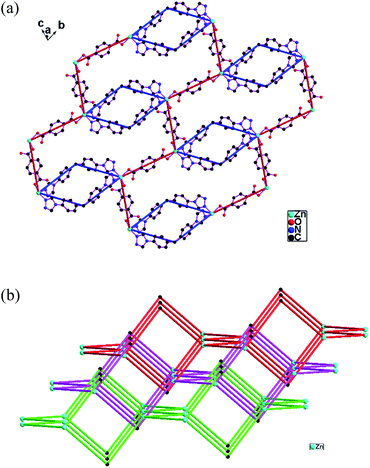
6 presents unusual 2D network based on the nanotube-like [Cd2(mbda)2] chains. The asymmetric unit of 6 consists of two Cd(II) atoms (Cd1, Cd2), one btpm (N1–N6), two mbda (O1–O4, O5–O8) ligands, one coordination water and disordered lattice water molecules. The Cd1 atom is coordinated by five carboxylate oxygen atoms from three mbda ligands and two triazole nitrogen atoms from two btpm ligands in the distorted pentagonal-bipyramidal geometry. The Cd2 atom is coordinated by five carboxylate oxygen atoms from three mbda ligands, one oxygen from one coordination water and one triazole nitrogen atom from one btpm ligand in the distorted pentagonal-bipyramidal geometry (Fig. S6 in the ESI†).There are two kinds of mbda ligands. The carboxylate groups (O1O2, O3O4, O5O6) of the mbda ligands exhibit the chelating mode. The other carboxylate (O7O8) of one mbda ligand shows tridentate bridging mode. Two mbda ligands (O5O6, O7O8) connect four Cd(II) atoms (Cd1,Cd2, Cd1C, Cd2C) to form the [Cd4(mbda)2] ring. The adjacent the [Cd4(mbda)2] rings are further linked by the other mbda ligands (O1O2, O3O4) to construct the nanotube-like chain [Cd2(mbda)2]n (Fig. 6a and b). The nanotube-like chains [Cd2(mbda)2]n are connected by the btpm ligands to form the unusual 2D network (Fig. 6c).
 | ||
| Fig. 6 (a) The nanotube-like chain [Cd2(mbda)2]n in 6. (b) Viewing the nanotube-like chain [Cd2(mbda)2]n along the a direction in 6. (c) The unusual 2D network in 6. | ||
Crystal structure of {[Zn(btpm)(1,4-bdc)](1,4-H2bdc)}n (7)
7 shows the intriguing 2D → 3D polythreaded network based on the special 2D (6,3) network. The asymmetric unit of 7 consists of one Zn(II) atom (Zn1), one btpm (N1–N6), two halves 1,4-bdc ligand (O1O2, O3O4) water and two halves free 1,4-H2bdc ligand (O5O6, O7O8). The Zn(II) atom is coordinated by two carboxylate oxygen atoms from two 1,4-bdc ligands and two triazole nitrogen atoms from two btpm ligands in the distorted tetrahedral geometry (Fig. S7 in the ESI†). The bdc ligand exhibits the bidentate coordination mode and bridges two Zn(II) atoms. The distances of Zn⋯Zn separation by bdc are 10.8825(17) and 11.0318(15). The btpm ligand shows the bidentate coordination mode. Two btpm ligands double-bridge two Zn(II) atoms to form [Zn2(btpm)2] 32-membered rings with the Zn⋯Zn distance of 14.5345(25). The whole network is a special 2D (6,3) network, with six Zn(II) atoms at six corners and four 1,4-bdc and two double btpm at six edges (Fig. 7a).The most striking structural feature of 7 is that the adjacent 2D (6,3) networks parallel polythread each other to form the 2D → 3D polythreaded network (Fig. 7b). The centeriod-to-centeriod, perpendicular distances and dihedral angle of the benzene ring C22–C24/C22–C24 (−x + 1, −y + 2, −z + 1) of 1,4-bdc from one 2D network and the triazole ring N1–N3/C14/C15 (x + 1, y, z) of btpm from the polythreaded 2D network are 3.802, 3.496 and 14.8°, showing the obvious π–π interactions between the benzene and triazole rings from adjacent polythreaded 2D networks. There are hydrogen bonding interactions (O(6)–H(1W)⋯O(4) (x, y − 1, z) 2.643(5), O(8)–H(2W)⋯N(5) (−x, −y + 3, −z) 2.831(5)) between the free 1,4-H2bdc and the adjacent 2D (6,3) networks (Table S2 in the ESI†). These hydrogen bonding interactions improve the stability of the 2D → 3D polythreaded network. The 2D → 3D polythreaded networks are few and unusual.27,23b
Crystal structure of {[Zn(btpm)(adc)]·3H2O}n (8)
8 shows an undulated 2D (4,4) network. The asymmetry unit consists of one Zn(II) atom, one btpm, one adc and lattice water molecules. Each Zn(II) atom displays a distorted trigonal-bipyramidal coordination geometry, coordinated by three carboxylate oxygen atoms from two adc and two triazole nitrogen atoms from two btpm (Fig. S8 in the ESI†). One carboxylate group (O1O2) of the adc ligand acts the monodentate mode. The other carboxylate group (O3O4) of the adc ligand acts the chelating mode.Each Zn(II) atom is bridged by two btpm and two adc ligands to form an undulated 2D (4,4) network (Fig. 8). Four Zn(II) atoms, two btpm and two adc ligands forms a [Zn4(bib)2(adc)2] unit with the Zn⋯Zn distances of 9.1349(32) and 16.6365(55) for adc and btpm bridging, respectively. Two adjacent undulated 2D (4,4) networks tightly stack so that the convex adc ligands of one network extend into the concave of the adjacent network (Fig. S9 in the ESI†). The centeriod-to-centeriod and perpendicular distances of the triazole ring N4–N6/C16/C17 of btpm from one 2D network with the symmetry triazole ring (−x, −y, −z + 2) of btpm from adjacent 2D network are 4.138 and 3.201, showing the obvious π–π interactions between the triazole rings from adjacent 2D networks. The hydrogen bonding interactions between the carboxylate oxygen atoms and lattice water molecules, and intra the lattice water molecules improve the stability of the network.
Effect of biscarboxylate ligands and Cd(II) or Zn(II) on the structures of 1–8
In compounds 1–6, Cd(II) is the metal center, the semi-rigid 4-substituted 1,4-triazole N-ligand btpm was used as the main ligand and biscarboxylate ligands were used as the auxiliary ligands (Scheme 1), aiming at exploring the effect of biscarboxylates on the assembly and structure of target compounds. Initially, we chose aromatic biscarboxylate 1,4-bdc as an ancillary ligand, thus the intriguing high-connected metal-cluster-based organic framework 1 was obtained which shows the 8-connected three-dimensional network based on the Cd3(COO)2 trimer clusters. If the 1,4-bdc is replaced by aromatic biscarboxylate mip, the high-connected metal-cluster-based organic framework 2 was synthesized which exhibits an unusual (3,7)-connected three-dimensional network based on the Cd2(COO) dimers. 3 was obtained when aromatic biscarboxylate nip was used which shows the (3,4,5)-connected network with the Schläfli symbol of (4·82)2(42·82·102) (43·611·87). If flexible aliphatic biscarboxylate sum is used. The metal-cluster-based organic framework 4 was synthesized which shows the 6-connected three-dimensional pcu net based on the Cd4Cl tetranuclear clusters. A 2-fold interpenetrating three-dimensional pcu network 5 based on Cd2(COO)2 dimers was synthesized if the flexible aliphatic biscarboxylate glu was used. 6 presents an unusual 2D network based on the nanotube-like [Cd2(mbda)2] chains when flexible aromatic biscarboxylate mbda was used.In 7 and 8, Zn(II) is the metal center. If the metal center Cd(II) in 1 was replaced by Zn(II), the interesting polythreaded network 7 was obtained. 7 shows the intriguing 2D → 3D polythreaded network based on the special 2D (6,3) network, with six Zn(II) atoms at six corners and four bdc and two double btpm at six edges. 8 exhibits the undulated 2D (4,4) network when the rigid adc was used.
1–8 show the structural diversity based on mono-metal, bi-, tri- and tetra-nuclear metal clusters. The metal–organic frameworks can be tuned with the different biscarboxylates and metal cations based on the semi-rigid 4-substituted 1,4-triazole ligand btpm.
PXRD and photoluminescence properties
The measured and simulated PXRDs confirm the purity of 1–8 (Fig. S10–S17 in ESI†). Photoluminescence complexes with d10 electronic configuration have attracted much attention owing to their ability to enhance, shift, and quench luminescent emission of organic ligands by metal coordination. Hence, the solid state luminescence spectra of 1–8 and free btpm ligand at room temperature were investigated (Fig. 9). The free btpm ligand in the solid state shows the emission band maxima approximately at 332 nm upon excitation at 273 nm. The free 1,4-H2bdc,6a H2mip7a and H2mbda11a exhibit the emissions at 390, 380 and 307 nm, respectively. The free H2suc,9a H2glu10a and H2adc12a present no obvious emission at the same condition. 1 exhibits the strong emission band maxima approximate at 438 nm and a peak at 333 nm upon excitation at 310 nm. 2 shows the strong emission band maxima approximate at 424 nm upon excitation at 310 nm. 3 and 7 have no emission at the same condition. 4, 5 and 6 present the emissions bands maxima approximate at 300, 302 and 306 nm, upon excitation at same 273 nm. 8 has a strong emission at 557 nm upon excitation at 380 nm. Because the Zn(II) and Cd(II) ions are difficult to oxidize or to reduce due to the d10 configuration, the emissions are neither metal-to-ligand charge transfer (MLCT) nor ligand-to-metal charge transfer (LMCT). The emissions of 1, 2 and 8 can be tentatively attributed to the mixed intra-ligand btpm and rigid biscarboxylate 1,4-bdc, mip or adc charge transitions. The emissions of 4, 5 and 6 can be tentatively attributed to the intra-ligand btpm charge transition because 4, 5 and 6 show the similar emission bands unrelated to the biscarboxylate ligands suc, glu and mbda.6–12Thermogravimetric analysis
To characterize the MOFs more fully in terms of thermal stability, the thermal behaviors of 1–8 were examined by TGA. The experiments were performed on samples consisting of numerous single crystals with a heating rate of 10 °C min−1 (Fig. 10). In the TG curve of 1, the lattice water molecules were completely lost at 140 °C (calcd: 7.01%, found: 7.23%). The network was thermally stable upon heating to 285 °C. Then the decomposition happened and did not end until 800 °C. The main residue should be CdO (calcd: 24.98%, found: 27.61%). The coordination and lattice water molecules of 2, 3, 4, 5 and 6 were completely lost at 128 °C, 176 °C, 135 °C, 150 °C and 125 °C, respectively (calcd: 4.39%, found: 4.43% for 2; calcd: 3.67%, found: 3.56% for 3; calcd: 9.64%, found: 9.96% for 4; calcd: 13.81%, found: 13.96% for 5; calcd: 7.33%, found: 7.48% for 6). The anhydrous matters of 2, 3, 4, 5 and 6 were thermally stable upon heating to 295 °C, 320 °C, 248 °C, 255 °C and 268 °C, respectively. Then the weight loss continuously occurred, which did not end until 800 °C. The main residues at 800 °C were consistent with CdO (Calcd: 27.80%, found: 28.31% for 2; calcd: 26.17%, found: 27.58% for 3; calcd: 24.99%, found: 26.64% for 4; calcd: 28.11%, found: 29.30% for 5; calcd: 26.11%, found: 28.44% for 6). 7 was stable up to 320 °C. Then 7 exhibited the continuous weight loss, which did not end until 800 °C. The main residue should be ZnO (calcd: 11.66%, found: 13.51%). For 8, the lattice water molecules were released from 40 to 120 °C (calcd: 8.39%, found: 8.58%). The remaining substance was stable upon heating to 240 °C. Then the weight decrease continuously happened. The main residue should be ZnO (calcd: 12.64%, found: 14.38%).Conclusions
In summary, eight metal–organic frameworks based on the semi-rigid 4-substituted 1,4-triazole ligand btpm and biscarboxylate ligands were synthesized by the hydrothermal method. 1–8 show the structural diversity based on mono-metal, bi-, tri- and tetra-nuclear metal clusters. The luminescence and thermal stability were investigated. The results show that the metal–organic frameworks can be tuned with the different biscarboxylates and metal cations based on the semi-rigid 4-substituted 1,4-triazole ligand btpm. The metal–organic frameworks (1, 2, 8 and 4–6) should be good candidates for potential photoactive materials. This work provides new information regarding not only metal–organic frameworks (MOFs) but also metal-cluster-based organic frameworks (MCOFs).Acknowledgements
This work is supported by the Natural Science Foundation of China (No. 21171126), the Priority Academic Program Development of Jiangsu Higher Education Institutions and Key laboratory of Organic Synthesis of Jiangsu Province and the Foundation for Young Talents in College of Anhui Province (No. gxyqZD2016372).References
- (a) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS PubMed; (b) P. Q. Liao, D. D. Zhou, A. X. Zhu, L. Jiang, R. B. Lin, J. P. Zhang and X. M. Chen, J. Am. Chem. Soc., 2012, 134, 17380 CrossRef CAS; (c) H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (d) M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282 CrossRef CAS; (e) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (f) J. R. Li, R. J. Kuppler and H. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- (a) Z. Z. Lu, R. Zhang, Y. Z. Li, Z. J. Guo and H. G. Zheng, J. Am. Chem. Soc., 2011, 133, 4172 CrossRef CAS PubMed; (b) A. Laguna, T. Lasanta, J. M. Lopezde-Luzuriaga, M. Monge, P. Naumov and M. E. Olmos, J. Am. Chem. Soc., 2010, 132, 456 CrossRef CAS PubMed; (c) H. Wu, H. Y. Liu, Y. Y. Liu, J. Yang, B. Liu and J. F. Ma, Chem. Commun., 2011, 47, 1818 RSC; (d) L. L. Wen, J. B. Zhao, K. L. Lv, Y. H. Wu, K. J. Deng, X. K. Leng and D. F. Li, Cryst. Growth Des., 2012, 12, 1603 CrossRef CAS; (e) H. Xu, W. W. Bao, Y. Xu, X. L. Liu, X. Shen and D. R. Zhu, CrystEngComm, 2012, 14, 5720 RSC; (f) P. Yang, X. He, M. X. Li, Q. Ye, J. Z. Ge, Z. X. Wang, S. R. Zhu, M. Shao and H. L. Cai, J. Mater. Chem., 2012, 22, 2398 RSC; (g) F. Y. Yi, S. Dang, W. T. Yang and Z. M. Sun, CrystEngComm, 2013, 15, 8320 RSC; (h) H. B. Zhou, J. H. Yao, X. P. Shen, H. Zhou and A. H. Yuan, RSC Adv., 2014, 4, 61 RSC; (i) Y. X. Tan, Y. P. He, H. Wang and J. Zhang, RSC Adv., 2014, 4, 1480 RSC.
- (a) H. H. Li, Z. Niu, L. Chen, H. B. Jiang, Y. P. Wang and P. Cheng, CrystEngComm, 2015, 17, 5101 RSC; (b) L. Yang, C. Qin, B. Q. Song, X. Li, C. G. Wang and Z. M. Su, CrystEngComm, 2015, 17, 4517 RSC; (c) Y. P. Xia, Y. W. Li, D. C. Li, Q. X. Yao, Y. C. Du and J. M. Dou, CrystEngComm, 2015, 17, 2459 RSC; (d) J. M. Hao, B. Y. Yu, K. V. Hecke and G. H. Cui, CrystEngComm, 2015, 17, 2279 RSC; (e) Z. Z. Xue, T. L. Sheng, Y. L. Wang, S. M. Hu, Y. H. Wen, Y. W. Wang, H. R. Li, R. B. Fu and X. T. Wu, CrystEngComm, 2015, 17, 2004 RSC; (f) Q. P. Li and J. J. Qian, RSC Adv., 2014, 4, 32391 RSC.
- (a) Y. Q. Lan, S. L. Li, K. Z. Shao, X. L. Wang, D. Y. Du, Z. M. Su and D. J. Wang, Cryst. Growth Des., 2008, 8, 3490 CrossRef CAS; (b) D. Xiao, H. Chen, G. Zhang, D. Sun, J. He, R. Yuan and E. Wang, CrystEngComm, 2011, 13, 433 RSC; (c) K. H. He, W. C. Song, Y. W. Li, Y. Q. Chen and X. H. Bu, Cryst. Growth Des., 2012, 12, 1064 CrossRef CAS; (d) Q. Chen, W. Xue, J. B. Lin, R. B. Lin, M. H. Zeng and X. M. Chen, Dalton Trans., 2012, 41, 4199 RSC; (e) Q. Chen, W. Xue, B. Y. Wang, M. H. Zeng and X. M. Chen, CrystEngComm, 2012, 14, 2009 RSC; (f) S. M. Fang, M. Chen, X. G. Yang, C. Wang, M. Du and C. S. Liu, CrystEngComm, 2012, 14, 5299 RSC; (g) Z. Zhang, S. Xiang, Q. Zheng, X. Rao, J. U. Mondal, H. D. Arman, G. Qian and B. Chen, Cryst. Growth Des., 2010, 10, 2372 CrossRef CAS.
- (a) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed; (b) U. Schubert, Chem. Soc. Rev., 2011, 40, 575 RSC; (c) X. Z. Song, S. Y. Song, S. N. Zhao, Z. M. Hao, M. Zhu, X. Meng and H. J. Zhang, Dalton Trans., 2013, 42, 8183 RSC; (d) D. S. Li, J. Zhao, Y. P. Wu, B. Liu, L. Bai, K. Zou and M. Du, Inorg. Chem., 2013, 52, 8091 CrossRef CAS PubMed; (e) X. Li, H. L. Sun, X. S. Wu, X. Qiu and M. Du, Inorg. Chem., 2010, 49, 1865 CrossRef CAS PubMed; (f) Y. Q. Lan, S. L. Li, K. Z. Shao, X. L. Wang, D. Y. Du, Z. M. Su and D. J. Wang, Cryst. Growth Des., 2008, 8, 3490 CrossRef CAS.
- (a) W. Q. Kan, J. Yang, Y. Y. Liu and J. F. Ma, CrystEngComm, 2012, 14, 6271 RSC; (b) H. J. Hao, F. J. Liu, H. F. Su, Z. H. Wang, D. F. Wang, R. B. Huang and L. S. Zheng, CrystEngComm, 2012, 14, 6726 RSC; (c) L. Zhou, Y. S. Xue, Y. Xu, J. Zhang and H. B. Du, CrystEngComm, 2013, 15, 7315 RSC.
- (a) M. L. Han, J. G. Wang, L. F. Ma, H. Guo and L. Y. Wang, CrystEngComm, 2012, 14, 2691 RSC; (b) J. Chen, C. P. Li and M. Du, CrystEngComm, 2011, 13, 1885 RSC; (c) M. Dai, W. Y. Yan, Z. G. Ren, H. F. Wang, W. J. Gong, F. L. Li, X. Zhao, H. X. Li and J. P. Lang, CrystEngComm, 2012, 14, 6230 RSC.
- (a) Y. Ge, N. Y. Li, X. Y. Ji, J. F. Wang, D. Liu and X. Y. Tang, CrystEngComm, 2014, 16, 6621 RSC; (b) X. Y. Lu, J. W. Ye, W. Li, W. T. Gong, L. J. Yang, Y. Lin and G. L. Ning, CrystEngComm, 2012, 14, 1337 RSC; (c) M. Du, Z. H. Zhang, Y. P. You and X. J. Zhao, CrystEngComm, 2008, 10, 306 RSC.
- (a) J. W. Uebler, J. A. Wilson and R. L. LaDuca, CrystEngComm, 2013, 15, 1586 RSC; (b) B. Bhattacharya, D. Saha, D. K. Maity, R. Dey and D. Ghoshal, CrystEngComm, 2014, 16, 4783 RSC; (c) Y. T. Liu, Y. Q. Du, X. Wu, Z. P. Zheng, X. M. Lin, L. C. Zhu and Y. P. Cai, CrystEngComm, 2014, 16, 6797 RSC.
- (a) D. Sun, Y. H. Li, H. J. Hao, F. J. Liu, Y. Zhao, R. B. Huang and L. S. Zheng, CrystEngComm, 2011, 13, 6431 RSC; (b) K. L. Hou, F. Y. Bai, Y. H. Xing, J. L. Wang and Z. Shi, CrystEngComm, 2011, 13, 3884 RSC; (c) E. M. Low and R. L. LaDuca, Inorg. Chim. Acta, 2015, 425, 221 CrossRef CAS.
- (a) G. P. Yang, Y. Y. Wang, W. H. Zhang, A. Y. Fu and R. T. Liu, CrystEngComm, 2010, 12, 1509 RSC; (b) L. K. Sposato, J. A. Nettleman and R. L. LaDuca, CrystEngComm, 2010, 12, 2374 RSC.
- (a) J. C. Jin, Y. Y. Wang, P. Liu, R. T. Liu, C. Ren and Q. Z. Shi, Cryst. Growth Des., 2010, 10, 2029 CrossRef CAS; (b) J. Xia, Z. D. Su, L. Yang, C. H. Zhang, S. Wang, S. H. Shi, L. Wang and Y. Fan, Inorg. Chim. Acta, 2014, 418, 106 CrossRef CAS; (c) X. Li, D. Y. Wei, S. J. Huang and Y. Q. Zheng, J. Solid State Chem., 2009, 182, 95 CrossRef CAS.
- J. G. Haasnoot, Coord. Chem. Rev., 2000, 200–202, 131 CrossRef CAS.
- G. Aromí, L. A. Barrios, O. Roubeau and P. Gamez, Coord. Chem. Rev., 2011, 255, 485 CrossRef.
- (a) Y. Garcia, C. Bravic, C. Gieck, D. Chasseau, W. Tremel and P. Gutlich, Inorg. Chem., 2005, 44, 9723 CrossRef CAS PubMed; (b) J. P. Zhang and X. M. Chen, Chem. Commun., 2006, 1689 RSC; (c) X. Zhu, Y. F. Feng, M. Li, B. L. Li and Y. Zhang, CrystEngComm, 2012, 14, 79 RSC; (d) N. Wang, J. G. Ma, W. Shi and P. Cheng, CrystEngComm, 2012, 14, 5198 RSC; (e) J. Zhao, D. S. Li, X. J. Ke, B. Liu, K. Zou and H. M. Hu, Dalton Trans., 2012, 41, 2560 RSC; (f) X. R. Meng, Y. L. Song, H. W. Hou, H. Y. Han, B. Xiao, Y. T. Fan and Y. Zhu, Inorg. Chem., 2004, 43, 3528 CrossRef CAS PubMed.
- (a) Y. Garcia, P. J. van Koningsbruggen, H. Kooijman, A. L. Spek, J. G. Haasnoot and O. Kahn, Eur. J. Inorg. Chem., 2000, 307 CrossRef; (b) H. A. Habib, J. Sanchiz and C. Janiak, Dalton Trans., 2008, 1734 RSC; (c) N. Liang, J. Wang, D. Y. Yuan, B. L. Li and H. Y. Li, Inorg. Chem. Commun., 2010, 13, 844 CrossRef CAS; (d) N. Liang, Y. F. Cui, D. Y. Yuan, B. L. Li and H. Y. Li, Inorg. Chim. Acta, 2011, 376, 612 CrossRef CAS; (e) H. A. Habib, A. Hoffmann, H. A. Hoppe, G. Steinfeld and C. Janiak, Inorg. Chem., 2009, 48, 2166 CrossRef CAS PubMed; (f) H. A. Habib, J. Sanchiz and C. Janiak, Inorg. Chim. Acta, 2009, 362, 2452 CrossRef CAS; (g) H. A. Habib, A. Hoffmann, H. A. Hoppe and C. Janiak, Dalton Trans., 2009, 1742 RSC.
- (a) N. Wan, Y. C. Feng, W. Shi, B. Zhao, P. Cheng, D. Z. Liao and S. P. Yan, CrystEngComm, 2012, 14, 2769 RSC; (b) B. Ding, P. Yang, Y. Y. Liu, Y. Wang and G. X. Du, CrystEngComm, 2013, 15, 2490 RSC; (c) B. Ding, Y. Y. Wang, S. X. Liu, X. X. Wu, Z. Z. Zhu, J. Z. Huo and Y. Y. Liu, CrystEngComm, 2015, 17, 5396 RSC.
- (a) X. Zhu, S. Zhao, Y. F. Peng, B. L. Li and B. Wu, CrystEngComm, 2013, 15, 9154 RSC; (b) X. Zhu, Q. Chen, Z. Yang, B. L. Li and H. Y. Li, CrystEngComm, 2013, 15, 471 RSC; (c) X. Y. Wang, B. L. Li, X. Zhu and S. Gao, Eur. J. Inorg. Chem., 2005, 3277 CrossRef CAS.
- J. Wang, X. Zhu, Y. F. Cui, B. L. Li and H. Y. Li, CrystEngComm, 2011, 13, 3342 RSC.
- (a) X. Zhu, X. G. Liu, B. L. Li and Y. Zhang, CrystEngComm, 2009, 11, 997 RSC; (b) L. Y. Wang, Y. Yang, K. Liu, B. L. Li and Y. Zhang, Cryst. Growth Des., 2008, 8, 3902 CrossRef CAS; (c) X. G. Liu, L. Y. Wang, X. Zhu, B. L. Li and Y. Zhang, Cryst. Growth Des., 2009, 9, 3997 CrossRef CAS.
- (a) B. L. Li, Y. F. Peng, B. Z. Li and Y. Zhang, Chem. Commun., 2005, 2333 RSC; (b) X. Zhu, P. P. Sun, J. G. Ding, B. L. Li and H. Y. Li, Cryst. Growth Des., 2012, 12, 3992 CrossRef CAS; (c) J. G. Ding, C. Yin, L. Y. Zheng, S. S. Han, B. L. Li and B. Wu, RSC Adv., 2014, 4, 24594 RSC.
- (a) M. Li, S. Zhao, Y. F. Peng, B. L. Li and H. Y. Li, Dalton Trans., 2013, 42, 9771 RSC; (b) M. Li, Q. Ling, Z. Yang, B. L. Li and H. Y. Li, CrystEngComm, 2013, 15, 3630 RSC.
- (a) Y. F. Cui, X. Qian, Q. Chen, B. L. Li and H. Y. Li, CrystEngComm, 2012, 14, 1201 RSC; (b) Y. F. Cui, P. P. Sun, Q. Chen, B. L. Li and H. Y. Li, CrystEngComm, 2012, 14, 4161 RSC; (c) Y. F. Peng, S. Zhao, K. Li, L. Liu, B. L. Li and B. Wu, CrystEngComm, 2015, 17, 2544 RSC.
- Y. F. Peng, L. Y. Zheng, S. S. Han, B. L. Li and H. Y. Li, Inorg. Chem. Commun., 2014, 44, 41 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- (a) V. A. Blatov, M. O'keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44 RSC; (b) E. V. Alexandrov, V. A. Blatov, A. V. Kochetkova and D. M. Proserpio, CrystEngComm, 2011, 13, 3947 RSC; (c) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed; (d) V. A. Blatov, A. P. Shevchenko and V. N. Serezhkin, J. Appl. Crystallogr., 2000, 33, 1193 CrossRef CAS; (e) V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CrossRef CAS; (f) See also: http://www.topospro.com.
- (a) C. Qin, X. Wang, L. Carlucci, M. Tong, E. Wang, C. Hu and L. Xu, Chem. Commun., 2004, 1876 RSC; (b) X. Wang, C. Qin, E. Wang, Y. Li, Z. Su, L. Xu and L. Carlucci, Angew. Chem., Int. Ed., 2005, 44, 5824 CrossRef CAS PubMed; (c) B. Xu, J. Lu and R. Cao, Cryst. Growth Des., 2009, 9, 3003 CrossRef CAS; (d) M. Du, X. G. Wang, Z. H. Zhang, L. F. Tang and X. J. Zhao, CrystEngComm, 2006, 8, 788 RSC; (e) M. Du, Z. H. Zhang, X. G. Wang, L. F. Tang and X. J. Zhao, CrystEngComm, 2008, 10, 1855 RSC; (f) G. L. Wen, Y. Y. Wang, Y. N. Zhang, G. P. Yang, A. Y. Fu and Q. Z. Shi, CrystEngComm, 2009, 11, 1519 RSC; (g) H. D. Guo, D. F. Qiu, X. M. Guo, G. L. Zheng, X. Wang, S. Dang and H. J. Zhang, CrystEngComm, 2009, 11, 2425 RSC; (h) Y. Y. Liu, Z. H. Wang, J. Yang, B. Liu, Y. Y. Liu and J. F. Ma, CrystEngComm, 2011, 13, 3811 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Selected bond lengths and angles, and additional figures for the crystal structures and PXRD. CCDC 1420646–1420653. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra19936k |
| This journal is © The Royal Society of Chemistry 2015 |