DOI:
10.1039/C5RA19712K
(Paper)
RSC Adv., 2015,
5, 105339-105346
Remarkable flux effect of Li-codoping on highly enhanced luminescence of orthosilicate Ba2SiO4:Eu2+ phosphors for NUV-LEDs: autonomous impurity purification by eutectic Li2CO3 melts†
Received
24th September 2015
, Accepted 30th November 2015
First published on 1st December 2015
Abstract
We report large photoluminescence (PL) enhancement of green-emitting Ba2SiO4:Eu2+ phosphors prepared in an eutectic Li2CO3 melt as a flux. Among the phosphor materials synthesized using low-purity precursors (99%-pure BaCO3 and 99.6%-pure SiO2), the emission intensity of Ba2SiO4:(Li0.02,Eu0.02) (Li = 200% excess) is found to be 470% as high as that of Ba2SiO4:Eu0.02 (no-Li) and in fact is almost equivalent to that of Li-undoped Ba2SiO4:Eu2+ synthesized using ultrapure precursors (99.98%-pure BaCO3 and 99.995%-pure SiO2). In combination with the results from PL measurements and inductively coupled plasma mass spectrometry (ICP-MS), the elemental distribution of the products and melts found from time-of-flight secondary ion mass spectrometry (TOF-SIMS) directly indicates that the excess Li2CO3 autonomously removes the impurities that were originally contained in the low-purity precursors, in particular SiO2, and thus critically improves the photoluminescence efficiency of the material. The newly found Li flux effect on large PL enhancement may not only contribute to more economic production of phosphors but provide a platform for discovery of new efficient phosphors for solid state lighting.
1 Introduction
In search for highly efficient solid state lighting materials, Li-codoping has been very successful in improving luminescence properties of rare earth-doped phosphors.1–12 It is generally accepted that the Li+ ions doped in the host lattices play important roles in the enhanced luminescence properties of the phosphors. The enhanced luminescence of the Li-codoped phosphors has been explained as follows: (i) copresence of Li+ ions favorably modifies the local structure of the activator ions and their special distribution;1–4 (ii) codoping of Li+ ions creates oxygen vacancies which might act as a sensitizer for the energy transfer to activator ions;5,7,9,10 (iii) Li precursors such as Li2CO3 or LiF act as a flux which promotes incorporation of the activator ions into the host lattice and also enhances crystal growth during the firing process at high temperatures;5,6,12 and (iv) Li-codoping provides the product particles with a morphology that is more desirable in reducing internal reflection.5,6 Among these possible mechanisms of luminescence enhancement by Li-codoping, the flux effect (i.e., (iii)) has been found to play a minor role. In the studies of Li-codoping in Y2SiO5:Pr3+,12 for example, the flux effect was responsible to ∼5% of the total enhancement of the emission, while the structural effect including increased Pr3+ separation and phase change was accountable for the rest (95%). Herein we report that the flux effect can be much more significant via a newly discovered mechanism, that is, autonomous impurity purification, by using green-emitting (Ba,Sr)2SiO4:Eu2+ phosphor system. Green-emitting (Ba,Sr)2SiO4:Eu2+ phosphor is promising for UV-LEDs because of its short decay time and high luminescence characteristics under UV excitation. Furthermore, the chromaticity of the (Ba,Sr)2SiO4:Eu phosphor can be controlled from the near UV (NUV) to the red by changing the crystal field splitting or covalency of the host lattice through Li-codoping, for example.13,14 The electronic configuration of Eu2+ is [Xe]4f7, and its lowest excited state of 4f levels is located at 28 × 103 cm−1 and it is in higher energy than that of the 4f65d1 in most compounds, so that Eu2+ usually exhibits broad-band emission associated with f–d transitions. As the Eu2+ activator is incorporated in Ba2+ sites, a new interaction between [SiO4]4− and Eu2+ activator probably occurs, –[O–Si–O]–Eu2+–[O–Si–O]–. Thus, by Li+ codoping, the change of covalency as well as crystal field strength in the host lattice may give rise to the modification of the characteristic of Eu2+ emission, which results in the improved luminescent intensity of Ba2SiO4:Eu2+ phosphor. Additional Li-codoping effect has not been reported so far in enhancing the luminescent property of the phosphors or for Eu2+-activated phosphors in general, although such enhancement from a flux effect for Eu2+-activated phosphors have been discovered by one of the authors.5,6
2 Experimental
Li-codoped Ba2SiO4:Eu2+ materials were synthesized via a solid-state reaction with stoichiometric or excessive amounts of Li2CO3. The stoichiometric amounts of BaCO3 (Aldrich, 99% and 99.98%), SiO2 (Aldrich, 99.6% and 99.995%) Li2CO3 (Aldrich, 99.99%), and Eu2O3 (Aldrich, 99.9%) were mixed and pulverized, then heated at 1250 °C for 6 h in air using alumina boats for the regular syntheses. The obtained powders with white body color were fired at 1250 °C for 6 h in mild reducing atmosphere (4% H2/Ar). After the reduction of the material, the white colored powder was changed into a white-greenish powder. Additionally, in the weighing step, Li2CO3 was added excessively between 100 and 300% excess relative to the stoichiometric one. Thermogravimetry and differential scanning calorimetry (TG/DSC, SDT Q 600 model) were used to determine the weight loss of starting materials, such as BaCO3, SiO2, Eu2O3, and Li2CO3. A heating rate of 10 °C min−1 was used for measurements from room temperature to 1200 °C in air. The structures of Ba2SiO4:(Li,Eu) compounds were characterized using a X-ray diffractometer (XRD 6000 model, Shimadzu) using Cu-Kα radiation at 30 kV and 30 mA. The unit-cell parameters were obtained using a nonlinear least-squares cell refinement program (UnitCell). The luminescent spectra were measured at room temperature using a fluorescent spectrophotometer (Scinco, FluoroMate FS-2). The reflectance spectra of phosphors were recorded using UV-visible spectrophotometer (UV-2600, Shimadzu) with BaSO4 as a reference. Elemental analysis was performed using an inductively coupled plasma mass spectrometer (ICP-MS, ELAN DRC II model, Perkin Elmer). TOF-SIMS experiments were performed with a TOF-SIMS V (ION-TOF GmbH, Münster, Germany) instrument in KBSI, Busan center by using a pulsed 30 keV Bi+ primary beam with a current 1 pA. The analyzed area used in this work was a square of 100 μm × 100 μm and the data acquisition time was 100 s. In order to prepare SIMS samples, the high purity indium metal (99.995% purity) was cut and pressed loosely using a pelletizer (ϕ = 8 mm), and subsequently the powder sample was placed over the indium metal disc and pressed. The particulates were homogeneously embedded in the indium metal disc. Positive ion spectra were internally calibrated using H+, H2+, CH3+, C2H5+, and C3H7+ peaks and normalized to the respective secondary total ion yields. The chemical images of the analyzed area were recorded with 128 × 128 pixel resolution during the data acquisition. Charge effects were compensated by means of an interlaced pulsed electron flood gun (Ek = 20 eV). The brightness of phosphor materials was measured using a CHROMA METER CS-100A model (MINOLTA). PL quantum yields of Ba2SiO4:Eu0.02 phosphor materials before and after Li-doping were measured using a PL spectrometer with an integrating sphere (Hamamatsu Photonics: Quantum Yield Measurement system C9920-02) at room temperature.
3 Results and discussion
3.1 TG/DSC of Li-compounds as a Li+-ion source and structural characterization
TG and DSC were performed to examine the thermal behavior of the Li2CO3, LiOH, and LiF as a Li+-ion source as shown in Fig. 1. The additional TG/DSC curves of BaCO3, SiO2, and Eu2O3 are presented in Fig. S1 (ESI†). The weight loss of Li2CO3 begins at around 700 °C and is complete at 800 °C with the melting point at 713 °C in DSC curve and subsequently the additional weight loss restarts up to 1200 °C corresponding to the decarbonation of Li2CO3, in which the experimental weight loss (59.5%) is in good agreement with the theoretical value (59.6%). For Li2O, the continuous weight loss above 1000 °C may be due to slow evaporation of Li2O. LiOH dehydroxylates to form Li2O above ∼500 °C and the formed Li2O gradually evaporates at the high temperature region. However, the origin of the slight weight gain between 680 and 960 °C is not clear. The TG thermogram of LiF indicates that evaporation of LiF starts at ∼840 °C and is significant in our firing condition (at 1250 °C for 6 h in air). Based on the TG and DSC thermograms, therefore, Li2CO3 is the best candidate as a Li+-ion source in examining the Li-doping effect of the host materials. The X-ray diffraction (XRD) patterns of three products with different amounts of Li2CO3 are shown in Fig. 2 and they are consistent with the XRD pattern of Ba2SiO4 with a primitive orthorhombic cell (Pmcn).15 The refined unit cell parameters are as follows: for Ba2SiO4:Eu0.02 (no-Li), a = 5.815 Å, b = 10.211 Å, c = 7.510 Å; for Ba2SiO4:(Li0.02,Eu0.02) (nominal-Li), a = 5.807 Å, b = 10.200 Å, c = 7.503 Å; and for Ba2SiO4:(Li0.02,Eu0.02) (Li-300% excess), a = 5.815 Å, b = 10.216 Å, c = 7.512 Å. It is noted that the unit cell parameters of the Li doped compound, Ba2SiO4:(Li0.02,Eu0.02) (nominal-Li) are slightly reduced compared with those of Ba2SiO4:Eu0.02 (no-Li), which is probably due to the formation of the oxygen defects associated with the volatility of Li2O at high temperature. At a higher Li doping level (300% excess), the unit cell parameters are close to those of Ba2SiO4:Eu0.02 (no-Li) compound as shown in Fig. 3. SIMS studies were carried out in order to examine the content of Li as well as other elements present,16,17 and the depth profile of secondary ion intensities (for Ba, Si, Eu, Li, and O atom) of Ba2SiO4:(Lix,Eu0.02) (x = 0.02, 0.05 and 0.08) are shown in Fig. 4. The sample with a larger Li content shows a larger signal intensity in the Li channel and somewhat smaller intensity for the Ba while the rest of the elements (Si, and O) have the nearly the same intensities. Evidently, the SIMS intensity of Ba is gradually diminished with increasing Li-content because Li-ions occupy Ba sites in the Ba2SiO4 crystal lattice with an orthosilicate-type structure as depicted in Ba2−xLixSiO4 chemical formula. In addition, the signal intensities of the elements are constant from near the surface to the interior (ca. 800 nm) of the grains for all the samples, indicating that the individual particles are atomistically homogeneous throughout the body, as desired.
 |
| | Fig. 1 TG and DSC curves of Li2CO3, LiOH, and LiF. | |
 |
| | Fig. 2 XRD patterns of Ba2SiO4:(Li,Eu) phosphors. | |
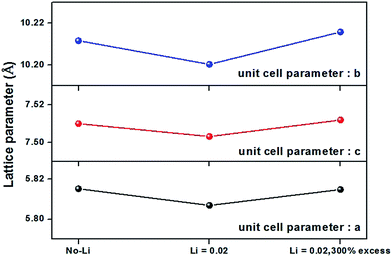 |
| | Fig. 3 Variation of unit cell parameters in Ba2SiO4:(Li,Eu) phosphors as a function of Li content. | |
 |
| | Fig. 4 Atomic intensity versus sputtered depth of Ba2SiO4:(Lix,Eu0.02) as a function of Li content. | |
3.2 Photoluminescence spectra of Li-doped Ba2SiO4:Eu0.02 materials
Fig. 5 shows the PL spectra and emission spectra (λex = 370 nm) of Ba2SiO4:Eu0.02 and its Li-codoped samples synthesized using BaCO3 (99% purity), SiO2 (99.6% purity), Eu2O3 (99.9% purity) and Li2CO3 (99.99% purity), in which x is the nominal amount of Li (x = 0.02, 0.05 and 0.08). All the samples exhibit a broad absorption band from 220 to 470 nm, which may be ascribed to the spin allowed 4f7(8S2/7)–4f65d transitions of Eu2+.18–22 The emission spectra show symmetric bands centered at 503 nm, associated with a green-emission. The emission intensity is increased with higher Li+ concentration, and the maximum intensity at Li = 0.02 is observed, indicating that the optimal concentration of Li+-ion is 0.02. It should be mentioned that the defect-related luminescent materials have been prepared and characterized, such as nanocrystal SiO2,23 MoS2,24 Gd2O3:Dy,25 ZnO quantum dots,26 Y2O3:Eu27 etc. As shown in Table 1, the considerable amounts of Li-defects are observed due to the volatility of Li2O in the firing step at 1250 °C. It is presumed that the cationic defects induced by the Li+-ions introduced into the Ba2+-sites have an effect on the effective energy transfer from the host-lattice (Ba2SiO4) to the Eu2+-activator ions as revealed in the diffuse reflectance spectra (Fig. 6). For the Ba2SiO4:(Lix,Eu0.02) phosphor materials, the maximum emission intensity corresponds to the optimal concentration of Li+-ion (x = 0.02). It is remarkable that the emission intensity of Ba2SiO4:(Li0.02,Eu0.02) phosphor is about 3.2 times higher than that of Ba2SiO4:Eu0.02 phosphor even in very small quantity of Li. Fig. 6 presents the diffuse reflectance spectra of Ba2SiO4, Ba2SiO4:Eu0.02, and Li-codoped Ba2SiO4:Eu0.02 phosphors. As the Eu2+ ions are doped into the Ba2SiO4 host lattice, the broad absorption bands are manifested in the range of 300–450 nm. It might be assured that the broad absorption bands around 300 and 450 nm in Ba2SiO4:Eu2+ phosphor are associated with the 4f → 5d transition of Eu2+ because the absorption band of the Ba2SiO4 host material is absent in this wavelength range. Furthermore, the absorption bands of the Li-doped Ba2SiO4:Eu0.02 are still stronger than that of Ba2SiO4:Eu0.02 without Li-doping, which is well coincident with the excitation spectra as previously presented in Fig. 5.
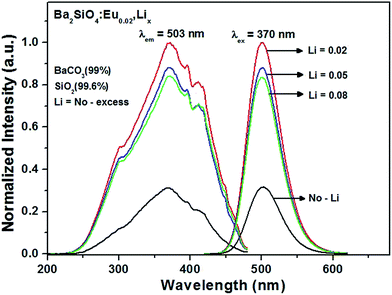 |
| | Fig. 5 PL spectra of Ba2SiO4:(Lix,Eu0.02) as a function of Li content. | |
Table 1 Li contents of Ba2SiO4:(Lix,Eu0.02) determined by ICP
| Li (x) |
Li content, ppm (nominal composition, ppm) |
Li defect (×10−2) |
| 0.02 |
1.06 (380.93) |
1.99 |
| 0.05 |
41.49 (962.6) |
4.75 |
| 0.08 |
155.63 (1557.16) |
7.20 |
 |
| | Fig. 6 Diffuse reflectance spectra of Ba2SiO4:(Lix,Eu0.02) as a function of Li content. | |
The remarkable PL enhancement by Li-codoping warrants further examination of the materials in search for its origin. One may presume that the Li+ ions introduced into the Ba2+-sites in Ba2SiO4:Eu0.02 crystal lattice create the cation vacancies as well as the oxygen vacancies for charge balance, which might act as a sensitizer for the effective energy transfer from the host lattice (Ba2SiO4) to the activator ions (Eu2+). As shown in Table 1, ICP results indicate that the materials do have a considerable amount of Li defects probably due to the volatility of Li2O in the firing step, at 1250 °C for 12 h in air and 4% H2/Ar. In contrast to our expectation, however, the increase in the nominal content of Li improves the PL of Ba2SiO4:(Li0.02,Eu0.02) synthesized using the excess Li2CO3 (100%, 200%, and 300%) as shown in Fig. 7. Namely, the PL intensity is much higher when the samples were prepared with an excess amount of Li2CO3, indicating that the presence of the Li defects is not the critical cause for the PL enhancement. In understanding the origin of the PL enhancement, a critical clue is evident in TOF-SIMS results for the Ba2SiO4:(Li0.02,Eu0.02) (Li = 300% excess) product which was synthesized using SiO2 (99.6% purity), BaCO3 (99% purity), Li2CO3 (99.99% purity), and Eu2O3 (99.9% purity). After firing, two different samples were collected in the zirconia crucible, in first the interior of the cohesive powder-lump, in second the fused part remained at the bottom of the zirconia crucible. XRD pattern of the powder in the interior of the powder-lump shows that of Ba2SiO4 with a pure single phase, whereas the impurity phases such as BaZrO3, LiAlSiO4, KAlSiO4, and ZrO2 are manifested at the bottom of zirconia crucible as presented in Fig. 8. The PL properties between two samples are compared in Fig. 9. The excitation and emission spectra of the impurity phases at the bottom of zirconia crucible are quite different from those of Ba2SiO4:Eu2+ and its intensities are very low. Fig. 10 presents TOF-SIMS positive secondary ion mass spectra of the two samples. Interestingly, three main impurities determined by ICP-MS (see Table S1†) are present much more significantly in the sample from the bottom. Moreover, the Zr+ secondary ion is present only in the sample from the bottom, whereas there is no Zr+ ion in the sample from the upper part. Fig. 11 shows the images of the Al+, K+, Fe+, and Zr+ secondary ion in which the specific elemental topography of each ion in the samples are presented in a linear color scale bar with color range from black to white, where black corresponds to the zero counts and white to the maximum intensity (e.g. 2.972 × 103 counts for Al+ at the left in the top in Fig. 11). Images of TOF-SIMS collected from the different positions of the powder sample coincide well with the intensities of the secondary cations as presented in Fig. 11. It should be noted that the intensities of the secondary cation in the sample collected from the bottom of the crucible are still stronger than those from the upper part. Since the target product particles remain in the upper part of the crucible, it is reasonable to say that the products have impurities much less than what we would expect from the amount of the impurities of the starting precursors and that the added Li2CO3 as a Li+ ion source effectively removes the impurities in the precursors. This is supported by the fact that in Fig. 7 all the products prepared with excess Li2CO3 (100, 200 and 300%) show nearly the same PL intensity. It should be noted that the liquid-phase sintering is usually considered to occur in three stages which are rearrangement, solution precipitation, and Ostwald ripening.28 As the temperature is raised above 710 °C, the Li2CO3–Li2O mixture melts and reacts with BaCO3, SiO2, and Eu2O3 to form a eutectic liquid. Assuming that there is good wetting between the liquid and the particulate solids, the impure solids dissolve at the solid/liquid interfaces, diffuse through the liquid and precipitate on the particles at another site which is the bottom of the zirconia crucible in our system. In essence, the excess amount of Li2CO3 as a Li+-source plays an important role as a molten flux medium that effectively traps impurities in the melt and thus result in a low impurity level in the phosphor product. This proposition is further substantiated when we compare the PL intensities of the Li-codoped products with another set of products prepared without Li-codoping but instead with high-purity precursors. Fig. 12 shows the excitation and emission spectra of Ba2SiO4:Eu0.02 products with precursors of varied purities. From the trend in the PL intensity, it is evident that the PL efficiency is strongly dependent of the chemicals purities of the precursors, with the maximum emission intensity from the highest-purity BaCO3 (99.98%) and SiO2 (99.995%) precursors being 5.2 times higher than that from the lowest-purity BaCO3 (99%) and SiO2 (99.6%) precursors. The emission intensity from BaCO3 (99%) and SiO2 (99.995%) precursors is 4.7 times higher than that from BaCO3 (99%) and SiO2 (99.6%), indicating that the emission intensity is more sensitive to the purity of SiO2 than that of BaCO3. A Ba2SiO4:Eu0.02 product prepared with the aqua regia-purified SiO2 and BaCO3 (99%) shows a PL intensity 2.5 times higher than that of Ba2SiO4:Eu0.02 using BaCO3 (99%) and the as-received SiO2 (99.6%). Remarkably, Fig. 13 shows that the PL intensities are quite comparable between Li-codoped Ba2SiO4:(Li0.02,Eu0.02) synthesized using the lowest-purity BaCO3 (99%) and SiO2 (99.6%) but with 200%-excess Li2CO3, and Ba2SiO4:Eu0.02 synthesized using the highest-purity BaCO3 (99.98%) and SiO2 (99.995%) precursors. As shown in Table S1,† further ICP-MS analysis showed that the low-purity SiO2 (Aldrich, 99.6% purity) contain three main impurities: Al (3891 ppm), K (1312 ppm), and Fe (478 ppm). After treating the SiO2 with a hot aqua regia, the concentrations of three main impurities are also reduced: Al (2288 ppm), K (186 ppm), and Fe (102 ppm). Fig. 14 shows the relative brightness monitored under 365 nm UV-light, which is in good agreement with PL results as presented in Fig. 7 and 13. Luminescent properties obtained from the emission spectra and quantum yields of Ba2SiO4:Eu0.02 phosphor materials before and after Li-doping are summarized in Table 2. The photoluminescence quantum yield (QY), which is the probability that a chromophore emits a photon upon its return from the excited to the ground state, can be represented as the ratio of its radiative to the non-radiative rate,29–32
| | |
Φ = krad/(krad + knrad)
| (1) |
where
krad and
knrad are the rate constant for radiative emission and non-radiative deexcitation to the ground state, respectively. As presented in
Table 2, the luminescent properties (maximum
Iem ratio, emission peak area ratio, and relative brightness) associated with the radiative process have the similar values, while the QY values (related with radiative and non-radiative process) are somewhat different,
i.e., the smaller differences compared to those of radiative process. Evidently, the discrepancy between three-radiative and QY values may be attributed to the energy losses due to the non-radiative process.
 |
| | Fig. 7 PL spectra of Ba2SiO4:(Li0.02,Eu0.02) synthesized with Li-excess. | |
 |
| | Fig. 8 XRD patterns of Ba2SiO4:(Li0.02,Eu0.02) phosphor (Li = 300% excess) synthesized using zirconia crucible. | |
 |
| | Fig. 9 Excitation and emission spectra of Ba2SiO4:(Li0.02,Eu0.02) phosphor (Li = 300% excess) synthesized using zirconia crucible. | |
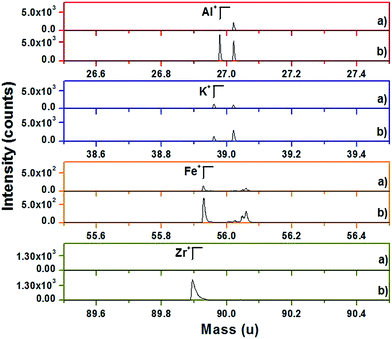 |
| | Fig. 10 TOF-SIMS mass spectra of Ba2SiO4:(Li0.02,Eu0.02) synthesized using Li-300% excess. In each secondary ion, the spectrum in the upper part (a) corresponds to the powder sample and that in the lower part (b) corresponds to the bottom of the zirconia crucible. | |
 |
| | Fig. 11 TOF-SIMS images of Ba2SiO4:(Li0.02,Eu0.02) synthesized using Li-300% excess. The images in the upper part correspond to the powder sample and those in the lower part correspond to the bottom of the zirconia crucible. | |
 |
| | Fig. 12 PL spectra of Ba2SiO4:Eu0.02 synthesized using the starting chemicals with the high and low purity. | |
 |
| | Fig. 13 Comparison of PL property between Ba2SiO4:Eu0.02 (using the high purity) and Ba2SiO4:(Li0.02,Eu0.02) (using the low purity and Li-200% excess). | |
 |
| | Fig. 14 Relative brightness of phosphor materials; Ba2SiO4:Eu0.02 (a), Ba2SiO4:(Li0.02,Eu0.02) (b), Ba2SiO4:(Li0.02,Eu0.02) (c), Ba2SiO4:(Li0.02,Eu0.02) (d), Ba2SiO4:(Li0.02,Eu0.02) (e), Ba2SiO4:Eu0.02 (f). Brightness of Ba2SiO4:Eu0.02 (a) is located to 100%. | |
Table 2 Luminescent properties of Ba2SiO4:(Li0.02,Eu0.02) phosphor materials
| Starting material |
Maximum Iem ratioa (%) |
Emission peak area ratioa (%) |
Relative brightness, cd m−2a (%) |
Quantum yield (%) |
| Property of Ba2SiO4:Eu0.02 material synthesized with BaCO3(H) and SiO2(H) is located to 100%. BaCO3(H) corresponds to BaCO3(99.98% purity), SiO2(H) to SiO2(99.995% purity), BaCO3(L) to BaCO3(99% purity), and SiO2(L) to SiO2(99.6% purity). |
| BaCO3(H)b, SiO2(H)b no-Li addition |
100 |
100 |
100 |
63.9 |
| BaCO3(L)b, SiO2(L)b Li: 200% excess |
90.8 |
91.4 |
92.6 |
69.0 |
| BaCO3(L)b, SiO2(L)b Li: 300% excess |
88.4 |
88.8 |
91.3 |
63.2 |
| BaCO3(L)b, SiO2(L)b Li: no-excess |
60.9 |
61.7 |
63.2 |
53.9 |
| BaCO3(L)b, SiO2(L)b no-Li addition |
19.3 |
19.5 |
22.3 |
31.5 |
4 Conclusions
Large photoluminescence enhancement up to 470% has been achieved for the green emitting (Ba,Sr)2SiO4:Eu2+ phosphor material via Li-codoping using Li2CO3 as a flux, when low-purity precursors were employed. By examining the elemental distribution in the reaction products and flux after the Li-codoping experiment, it was discovered that the excess Li condition results in extraction of Al, K and Fe impurities from the low-purity precursors, hence decreasing impurity concentrations in the phosphor product. This effect was further verified by comparing the photoluminescence efficiency of the Li-codoped phosphors with that of Li-undoped phosphors that were prepared with high-purity precursors. This newly-discovered mechanism of photoluminescence enhancement upon Li-codoping may lead to more economical adoption of solid state lighting for energy efficiency. This also provides an efficient synthetic route aiming alleviation of the undesired luminescent quenching by impurities and thus allows us to explore new phosphors that are particularly susceptible to such impurity effect.
Acknowledgements
This research was financially supported by the Ministry of Education (MOE) and National Research Foundation of Korea (NRF) through the Human Resource Training Project for Regional Innovation (2014H1C1A1066859).
References
- G. Chen, H. Liu, H. Liang, G. Somesfalean and Z. Zhang, J. Phys. Chem. C, 2008, 112, 12030–12036 CAS
 .
. - D. Li, Y. Wang, X. Zhang, H. Dong, L. Liu, G. Shi and Y. Song, J. Appl. Phys., 2012, 112, 094701–094705 CrossRef
 .
. - Q. Sun, X. Chen, Z. Liu, F. Wang, Z. Jiang and C. Wang, J. Alloys Compd., 2011, 509, 5336–5340 CrossRef CAS
 .
. - Y. Wang, N. Can and P. D. Townsend, J. Lumin., 2011, 131, 1864–1868 CrossRef CAS
 .
. - J. C. Park, H. K. Moon, D. K. Kim, S. H. Byeon, B. C. Kim and K. S. Suh, Appl. Phys. Lett., 2000, 77, 2162–2164 CrossRef CAS
 .
. - S. H. Byeon, M. G. Ko, J. C. Park and D. K. Kim, Chem. Mater., 2002, 14, 603–608 CrossRef CAS
 .
. - F. Gu, S. F. Wang, M. K. Lu, G. J. Zhou, D. Xu and D. R. Yuan, Langmuir, 2004, 20, 3528–3531 CrossRef CAS PubMed
 .
. - S. H. Shin, J. H. Kang, D. Y. Jeon and D. S. Zang, J. Lumin., 2005, 114, 275–280 CrossRef CAS
 .
. - W. Li and J. Lee, J. Phys. Chem. C, 2008, 112, 11679–11684 CAS
 .
. - C. Zhang, H. Lian, D. Kong, S. Huang and J. Lin, J. Phys. Chem. C, 2009, 113, 1580–1588 CAS
 .
. - H. K. Yang, J. W. Chung, B. K. Moon, B. C. Choi, J. H. Jeong, J. S. Bae and K. H. Kim, Solid State Sci., 2011, 13, 1420–1423 CrossRef CAS
 .
. - E. L. Cates, A. P. Wilkinson and J. H. Kim, J. Phys. Chem. C, 2012, 116, 12772–12778 CAS
 .
. - S. Shionoya and W. Yen, Phosphor Handbook, CRC Press, London, New York, 1999, p. 192 Search PubMed
 .
. - H. S. Kang, S. K. Hong, Y. C. Kang, K. Y. Jung, Y. G. Shul and S. B. Park, J. Alloys Compd., 2005, 402, 246–250 CrossRef CAS
 .
. - H. P. Grosse and E. Tillmanns, Cryst. Struct. Commun., 1974, 3, 599–601 CAS
 .
. - A. V. Walker, Anal. Chem., 2008, 80, 8865–8870 CrossRef CAS PubMed
 .
. - S. Seki, H. Tamura and H. Sumiya, Appl. Surf. Sci., 1999, 147, 14–18 CrossRef CAS
 .
. - S. Zhang, Y. Nakai, T. Tsuboi, Y. Huang and H. J. Seo, Chem. Mater., 2011, 23, 1216–1224 CrossRef CAS
 .
. - W. B. Im, Y. I. Kim, H. S. Yoo and D. Y. Jeon, Inorg. Chem., 2009, 48, 557–564 CrossRef CAS PubMed
 .
. - K. Inoue, N. Hirosaki, R. J. Xie and T. Takeda, J. Phys. Chem. C, 2009, 113, 9392–9397 CAS
 .
. - D. Kim, J. Jang, S. I. Ahn, S. H. Kim and J. C. Park, J. Mater. Chem. C, 2014, 2, 2799–2805 RSC
 .
. - D. Kim, S. Park, S. Kim, S. G. Kang and J. C. Park, Inorg. Chem., 2014, 53, 11966–11973 CrossRef CAS PubMed
 .
. - K. S. Min, K. V. Shcheglov, C. M. Yang, H. A. Atwater, M. L. Brongersma and A. Polman, Appl. Phys. Lett., 1996, 69, 2033–2035 CrossRef CAS
 .
. - P. K. Chow, R. B. Jacobs-Gedrim, J. Gao, T. M. Lu, B. Yu, H. Terrones and N. Koratkar, ACS Nano, 2015, 9, 1520–1527 CrossRef CAS PubMed
 .
. - T. Selvalakshmi, S. Sellaiyan, A. Uedono and A. C. Bose, RSC Adv., 2014, 4, 34257–34266 RSC
 .
. - A. Asok, A. R. Kulkarni and M. N. Gandhi, J. Mater. Chem. C, 2014, 2, 1691–1697 RSC
 .
. - H. Huang, X. Sun, S. Wang, Y. Liu, X. Li, J. Liu, Z. Kang and S. T. Lee, Dalton Trans., 2011, 40, 11362–11366 RSC
 .
. - M. N. Rahaman, Ceramic Processing and Sintering, Marcel Dekker, New York, 1995 Search PubMed
 .
. - J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed
 .
. - M. Yorulmaz, S. Khatua, P. Zijlstra, A. Gaiduk and M. Orrit, Nano Lett., 2012, 12, 4385–4391 CrossRef CAS PubMed
 .
. - J. Zhao, O. Chen, D. B. Strasfeld and M. G. Bawendi, Nano Lett., 2012, 12, 4477–4483 CrossRef CAS PubMed
 .
. - J. Hoy, P. J. Morrison, L. K. Steinberg, W. E. Buhro and R. A. Loomis, J. Phys. Chem. Lett., 2013, 4, 2053–2060 CrossRef CAS PubMed
 .
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19712k |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 



.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.










