Thermal treatment of heavy oily sludge: resource recovery and potential utilization of residual asphalt-like emulsion as a stabilization/solidification material
Gang Li,
Shuhai Guo* and
Hanfeng Ye
Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, PR China. E-mail: shuhaiguo@iae.ac.cn
First published on 8th December 2015
Abstract
An innovative application of oily sludge via a distillation modification treatment has been proposed. Particular attention was paid to key parameters of the recovered light oil and residual emulsion, including the separation ratio of light oil, the change of chemical composition, values of penetration and softening point of the residual emulsion. In addition, leaching tests were conducted to investigate the effect of modified oily sludge as a material to solidify other hazardous waste on controlling the release of heavy metals. The results showed that the separated light oil was higher than 29.2% of the original dewatered oily sludge, such as 33.4% at 493 K and 39.2% at 573 K for 180 min. In an appropriate range of thermal treatment parameters (distillation temperature 493–533 K and time 2–3 h), the research achieved more desirable results for the residual emulsion. For example, the content of resin and asphaltene in the residual emulsion was increased from 29.1% to 47.5% at 493 K for 180 min. Furthermore, it was found that the values of penetration and softening point of the residual emulsion were 88 and 48.5 °C, respectively. And this modification enhanced its bond capacity. When this asphalt-like emulsion was used as a solidifying or embedding material, an ideal ratio was achieved at 0.5 (m/m) for controlling the release of heavy metals in this study. The results contribute to the development of new technologies relating to the utilization of oily sludge.
1. Introduction
Oily sludge is co-produced during the process of petroleum exploration and production, and is considered as a special type of hazardous waste from the flotation cell or settling tank in oilfields, in terms of continuing to pose significant risks to human health.1,2 Incineration or landfill is usually adopted after recycling the light oil, but some valuable uses of oily sludge are often neglected. Generally, oily sludge contains different concentrations of water (40–70 wt%), petroleum hydrocarbons (15–25 wt%), and mineral particles (10–20 wt%).3,4 If the oily sludge is dewatered, a valuable substance remains that contains approximately 70% hydrocarbons (mostly paraffins and asphaltenes), together with clay, sand, inorganic matter and heavy metals. Perhaps the most effective approach is to utilize the sludge intact, for example in building or embedding materials, as this can completely avoid the technical problems otherwise experienced during hydrocarbon extraction processes, such as demulsification, desorption and separation.5,6 Hassan et al.7,8 investigated the potential uses of petroleum-contaminated soil (PCS) in highway construction. The results indicated that the use of PCS in an asphalt-concrete mixture application would pose no immediate or long-term threat to the environment; the concentrations of metals and organic compounds did not exceed the maximum contaminant levels set for TCLP (toxicity characteristic leaching procedure) extracts. Therefore, the benefits of recovering and reusing oily sludge include not only a saving of vast quantities of petroleum, but also an absolute decrease in hydrocarbon and heavy metal pollution.Some studies have suggested that the oily sludge can be treated by low temperature distillation. Producing a residue of greater value in an alternative fuels program or a asphalt-like emulsion reuse were well-documented and oft-repeated.9,10 Ayen and Swanstrom evaluated low temperature thermal treatment of filter cakes using laboratory and pilot-scale equipment. Considering the content of cyanides within the acceptable limit and combined with stabilization of heavy metals in the treatment residues, the process was successfully designed and commercialized. In addition, the paper reported the sludge stream carries the same waste codes as the original waste feed, and it can be filtered. The most likely use of this stream would be as fuel to a cement kiln. However, it did not mention the detailed steps for removal the clay minerals. Obviously, single mechanical filtration can not achieve desired separation effect for viscous sludge with lots of minerals. Kuriakose et al.11 reported that the waste sludge from a refiner plant can be converted into different grades of industrial bitumen; approximately 17% of lighter oils and industrial bitumen of 90/15 grade were obtained by vacuum distillation. The usefulness of the industrial bitumen produced was tested in the preparation of bituminous paints. Thermal treatment of oily sludge can irreversibly change the content of heavy components, and enhance its bond capacity. However, compared to refiner sludge, there are more low-molecular hydrocarbon components of petroleum in oily sludge from flotation cell or settling tank during crude oil production.
Thermal treatment of oily sludge involves torrefaction, direct distillation, pyrolysis and carbonization process. Temperature is the most important parameter in an experimental design. Deng et al.12 performed experimental and modeling study of the long cylindrical oily sludge drying process. The study presented a Boltzmann drying model, and predicted the air drying behavior of the long cylindrical oily sludge at 105–250 °C. Conesa et al.13 determined the pyrolysis of sludge from wastewater treatment plant of an oil refinery at 350, 400, 470 and 530 °C in nitrogen atmosphere. The study showed that in the liquids, the light hydrocarbon yield increased within increasing temperature, whereas the aromatic compounds diminished. The decomposition of the solid fraction proceeded through the pyrolysis of the char and later combustion of the residue formed. Furthermore, the reactivity of the chars vs. the oxygen was very high despite less of the conditions they were produced. Chang et al.14 investigated the pyrolysis of oil sludge at the temperature range 378–873 K. They concluded that the pyrolytic reaction was complex and significant in the range 450–800 K. The residues of oily sludge pyrolysis exhibited very high viscous form below 623 K. Andrade et al.15 designed a set of heat-treated at different temperatures (400, 500, 600, 700 and 900 °C) and obtained conductive carbon-clay nanocomposites. In a word, analysis of all the products obtained (gases, liquids and chars) usually were investigated and characterized during the complex thermal treatment process. However, two problems need to be solved: the change of oily sludge during thermal modification is required, and a suitable approach to the application of the modified residual solid (asphalt-like emulsion) must be identified.
Distillation is probably the most often used process to produce asphalt from heavy crude oil.16,17 Also, it has been used in studies on the fuel recovery for oily sludge treamtent.18,19 Reduce of the light hydrocarbons composition will be beneficial to optimize the properties of asphalt-like emulsion. Accordingly, the increase of the resin and asphaltenes content by thermal treatment is necessary. In addition, oxygen plays an important role in determining the properties of asphalt.20 The interaction of oxygen and hydrocarbons can generate oxygen-containing compounds, which contained carboxylic acids, phenols, ketones and esters etc. Among, esters are main component. The ester groups can connect two different molecules to produce a new material with higher molecular weight. This process enhanced the content of asphalt in the hydrocarbons, and changed the colloidal structure and chemical composition of asphalt.21 It has been reported that the softening point-penetration ratio of the residual emulsion has been bringing about appreciable variation during the distillation process.22 However, there is lack of analysis regarding the light oil and heavy components of oily sludge after thermal treatment.
Because of its highly hydrophobic and extraordinarily stable chemical and biological features, asphalt emulsion can be applied to control and decrease the release of hazardous material with a variety of structural and compositional characteristics. For example, PCS, galvanic sludge, incinerator bottom ash, and heavy metals contaminated soil have all been treated by asphalt emulsions to control the release of hazardous materials.23–27 In addition, previous results have also indicated that asphalt can decrease the leaching rates of inorganic pollutants during stabilization/solidification (S/S), because of its high content of resin and asphaltene.28,29 Meanwhile, applying asphalt in waste S/S prevented the risk of salinity and inorganic anions causing interference in the concrete hardening process.7,30 However, as petroleum resources decline, it is important to look for alternatives to bitumen. This is the reason why many researchers have focused on the use of cheaper materials for S/S. From this point of view, a modified residual solid (asphalt-like emulsion) appears to be an ideal candidate material for S/S treatment process.
The present study provides useful information on the modification of heavy oily sludge and its potential use as a stabilization/solidification material. The purpose of the research was to investigate the effect of thermal treatment on the properties of the residual asphalt-like emulsion. In addition, the modified residual emulsion was test during potential solidification process, and an attempt case was also made to gain an insight into the leaching behavior of heavy metals in the mixture of the bottom ash with the modified oily sludge.
2. Materials and methods
2.1. Materials
| Cu | Zn | Pb | Cd | Ni | Cr | |
|---|---|---|---|---|---|---|
| Bottom ash (mg kg−1) | 151.47 | 657.33 | 25.60 | 5.95 | 83.7 | 128.33 |
| TCLP of bottom ash (mg L−1) | 24.6 | 134.5 | 3.48 | 1.25 | 6.43 | 18.34 |
| GB5085.3-2007 (mg L−1) | 100 | 100 | 5.0 | 1.0 | 5.0 | 15.0 |
| Limit value (EPA) (mg L−1) | — | — | 5.0 | 1.0 | — | 5.0 |
2.2. Experiment design
A distillation thermal treatment system was designed and manufactured to carry out the oily sludge modification experiment. A schematic representation of the system is shown in Fig. 1. Basically, the reactor of the system had a cylindrical shape whose inner diameter was 22 cm and effective chamber length was 40 cm. It included an electric heater, which could be used to heat the sludge up to 850 K. The electric heater contained an electrical resistance heater, and a voltage controller. An agitator was employed for blending the oily sludge to obtain uniform temperature distribution in the reactor. A thermocouple was placed in the middle of the reactor to reflect the heating temperature. In addition, an air pump was set to supply air to the reactor. Concentrated H2SO4 was used to absorb moisture in the air. The final component of the system was the condenser unit, in which a water-cooled condenser (condenser tube, 0.6 m) was used to condense the vaporized light oil. The temperature of circulating cooling water is 18 °C.Before adding the oily sludge to the distillation reactor, it was dried at 105 °C for about 30 min, to separate the water content. Then, 2 kg of the oily sludge was added into the reactor. The thermochemical process of the samples was performed in the presence of O2 (the air flow rate: 2 L min−1), with continuous mixing. Four distillation temperatures (453, 493, 533 and 573 K) were designed. The sludge was heated at 10 K min−1 from original temperature. When it was reached at desired temperature, a temperature-controlled system was used for keeping the variation less than 3 °C. Rapid condensation of light oil was collected in a container. Experimental replicates were done using the same lot sludge for three times. The oily sludge was treated in the actor for three individual distillation processes by the same experimental parameters. Then the recovered light oil and the asphalt-like emulsion from three experiments were collected to test the data through homogeneous mixing. Quantitative analysis of hydrocarbons was carried out to compare the recovery rate of light oil. After distillation treatment, the residual emulsion was collected for analyzing the softening point, penetration and other characteristics.
The procedure used to mix the bottom ash with the modified oily sludge was based on empirical findings of the S/S of ash and salt from a waste incinerator,28 and of noncombustible industrial waste.32 The process involved mixing the bottom ash with the modified oily sludge at the chosen ratio (0.2–0.6) and homogenizing the mixture for approximately 15 min. This process kept the temperature about 120–140 °C. The viscous residual sludge was stirred continuously, and the bottom ash was thrown into the actor uniformly. The mixture was subsequently put into a solidification pattern. It was compacted under a pressure of about 0.4 MPa, and then pushed out. The solidification pattern was a rectangle of 10 cm width and 15 cm length. The thickness of the compacted mixture in each pattern was 1 cm.
The leaching tests were conducted using a horizontal vibration extraction procedure. The aim was to study the potential use in controlling the release of heavy metals from the composite mixture. The leaching solution was prepared using a standard leaching test, and the heavy metal content in the leachate was determined (TCLP, EPA method 1311). Then it was compared with threshold limits required in identification standard for hazardous wastes – identification for extraction procedure toxicity (Chinese national standard GB 5085.3-2007).
2.3. Analysis methods
The content of the oil and moisture was determined according to existing procedures,33 as was the content of the saturated compounds, the aromatics, the resins and asphaltenes of the modified oily sludge.34 The value of pH was monitored using a pH meter (PHS-3B, Shanghai). The characteristics of the modified sludge were analyzed following previously used methods,35 and the mass of distilled light oil was determined by weighting.3. Results and discussion
3.1. Modification of heavy oily sludge
 | ||
| Fig. 2 Rate of light oil recovery at various distillation temperatures (experimental conditions: heating rate of 10 K min−1, stirring speed of 120 rpm, air volume of 2 L min−1). | ||
| Item | Value |
|---|---|
| Iodine value (gI per 100 g) | 36.72 |
| Oxidation stability (mg per 100 mL) | 1.1 |
| Freezing point (°C) | 1.0 |
| Flash point (°C) | 105.0 |
| Acidity (mg KOH per 100 mL) | 21.42 |
| Existent gum (mg per 100 mL) | 22.6 |
| Total sulfur (wt%) | 0.0461 |
| Ash content (wt%) | 0.004 |
| Cold filter plugging point (°C) | 3.0 |
| Cetane number | 51.2 |
| Density (20°C kg−1 m−3) | 0.8206 |
The ratio of light oil recovery after distillation in the present study was not in agreement with previous results.36,37 These studies focused on utilizing thermal treatment of oil sludge to enhance the rates of pyrolysis and oxidative reactions in the oily sludge, which was taken from the crude oil storage tank of a typical petroleum refinery plant. The crude oil tank bottom sludge contained more solid content (i.e. 15%) than that of the dewatered DAF sludge used in our work (i.e. 11.7%), especially more clay found in DAF sludges. In addition, the object of the oily sludge treatment was different. The recycling and reuse of residual emulsion was considered as the most important aspect in our study. Therefore, much light oil was recovered mainly through direct distillation, rather than promoting the pyrolysis of heavy components through increasing temperature. In fact, when the distillation temperature was lower than 550 K, it did not cause the decomposition of heavy petroleum hydrocarbons. Liu et al.38 reported that the pyrolysis process of oily sludge can be divided into three main stages within the temperature range for all heating rates. A second stage of decreasing mass is observed between 393 and 805 K and involves a very important weight loss (around 18 wt% of the original weight) mainly related to the volatilization and decomposition of organic matter in the oily sludge. As a result, it was observed that the total petroleum hydrocarbon concentration in the separated aqueous phase in triangular flask for the distillation method was much lower (i.e. 200 mg L−1) than that for pyrolysis (i.e. 1550 mg L−1). This was in agreement with previous studies showing that the distillation method was effective for separating oil from the aqueous phase.19 In addition, if the temperature was enhanced, pyrolysis of the oil was obvious. Previous paper reported that in pyrolysis process, about 80% of total organic carbon content in oily sludge converted into hydrocarbons and an important hydrocarbon yield occurred at temperatures between 327 °C and 450 °C.38
Two important parameters of the residual asphalt-like emulsion, penetration and softening point, which determined the binder and embedding characteristics, were analyzed to investigate the impact of oxygen with distillation process. As previous reports, the relationship of penetration and softening point was significant during evaluating the properties of asphalt.39,40 The results (Fig. 4 and 5) show that the value of penetration decreased, and the softening point enhanced with distillation time. They were clearly influenced by temperature. After 180 min, the two values varied slightly with distillation time. Compared to the findings of Kuriakose and Manjooran,11 the modified oily sludge was different from industrial bitumen produced by vacuum distillation with catalysts. The main reason was that the aim of our research was to investigate the feasibility of oily sludge modification and its potential use in the S/S of other hazardous wastes by simple distillation without separating clay. Also, the aforementioned study described how the residual sludge, after the removal of lighter oils, was converted into different grades of industrial bitumen via heat treatment at temperatures ranging from 200 to 250 °C, with AlCl3 as the catalyst, for time periods ranging from 2 to 3 h.11 Certainly, regardless of the cost, the addition of A1C13 as the catalyst can convert the oily sludge into some useful grades of industrial bitumen. However, this needs further testing on optimized parameters and strict catalyst condition in future research. In addition, a higher temperature of 573 K, as well as a lower temperature of 493 K, probably should not yield a better softening point-penetration relationship.
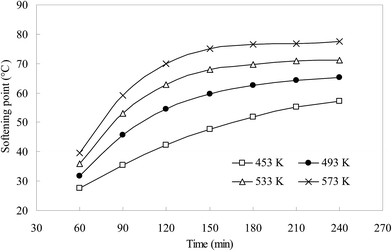 | ||
| Fig. 5 Effect of different modification temperatures on softening point properties of residual sludge. | ||
Besides the ratios of compounds, some parameters are usually measured to compare with the standard of industrial bitumen. After distillation at the four temperatures, residual oily sludge parameters such as penetration and softening point were determined, and the results revealed distinct differences in the penetration for the different samples; higher distillation temperatures yield lower values of the residual emulsion. During the modification process, penetration is perhaps the best indicator for the thermal treatment of oily sludge. In this study, the temperature of 493 K and duration time of 2.5 h were considered as the optimal conditions for preparing grades of industrial bitumen of lower penetration and higher softening point. The values of penetration and softening point of the modified oily sludge were 88 and 48.5, which fall within the requirements of bitumen 100# (pavement petroleum asphalt, SH0522, China).
Next, selected physicochemical properties of the residual emulsion after distillation at 493 K were characterized and summarized (Table 3). Compared with its parent oily sludge, the residual viscous asphalt-like emulsion contained higher concentrations of polar macromolecules (e.g., asphaltenes), which was in agreement with their higher molecular weight. It is accepted that heteroatoms are primarily concentrated in heavy oil components. The differences between the residual sludge and its corresponding parent oily sludge suggested that the increase of resins and asphaltenes caused the enhancement of cohesiveness. After distillation treatment for 180 min, the residual emulsion was highly viscous. This is consistent with previous reports showing that the pyrolysis residues of oily sludge exhibited highly viscous forms below 623 K (pyrolysis temperature), while less viscous or solid forms above 713 K.14 These variations in properties reflect the potential solidification characteristics of oily sludge (Fig. 6).
| Item | Value |
|---|---|
| Pour point (°C) | 42 |
| Wax (wt%) | 6.0 |
| Asphaltenes (wt%) | 8.9 |
| Acidity (mg KOH per g−1) | 4.3 |
| Flash point (°C) | 200 |
| Kinematic viscosity (cst, 100 °C) | 30.33 |
| Total sulfur (wt%) | 3.43 |
| Ash content (wt%) | 4.8 |
3.2. Leaching of heavy metals in bottom ash after mixing with modified oily sludge
The results of the leaching tests are presented in Table 4. For the TCLP test, solidification based on modified oily sludge reduced all the metals in the leachate from the solidified matrices. These results are in agreement with several other studies,41,42 and can be attributed to the strong detention capacity. An ideal ratio was achieved at 0.5 for controlling the release of heavy metals. When the ratio of modified oily sludge is less than this ratio, the release of several heavy metals will improve. Of course, among the six kinds of heavy metals detected, the environmental toxicological effects of Cd and Cr are the strongest. When the modified oily sludge was added in sufficient quantity to immobilize the ash completely, the concentration of heavy metal leaching was reduced. Therefore, a higher concentration of the modified sludge in the solidified products will result in lower leachability. Certainly, if the proportion of the modified sludge was decreased, coating the bottom ash would be an ideal way to control the release of heavy metals. A previous study reported how an asphalt coating was produced to form an immobilizing barrier against pollutant leaching, and the results showed that the quantity of emulsion needed to prepare an asphalt coating is relatively low.32 Based on comprehensive consideration of the compressive strength and leaching concentration of heavy metal ions of the modified sludge, it is confirmed that under the premise of non-hazardous to the environment, it represents an effective way to improve the disposal and utilization of oily sludge in controlling the release of heavy metals.| Mresidual solid/Mbottom ash+residual solid | Concentration of metals in leachate (ppm) | |||||
|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Cd | Ni | Cr | |
| 0.2 | 10.3 | 92.12 | 2.17 | 0.87 | 4.12 | 14.84 |
| 0.3 | 8.34 | 64.34 | 1.86 | 0.64 | 2.71 | 10.25 |
| 0.4 | 7.02 | 46.32 | 1.74 | 0.42 | 0.85 | 7.37 |
| 0.5 | 2.68 | 18.62 | 1.16 | 0.20 | 0.41 | 4.46 |
| 0.6 | 1.35 | 7.53 | 1.04 | 0.15 | 0.33 | 3.22 |
| GB5085.3-2007 | 100 | 100 | 5.0 | 1.0 | 5.0 | 15.0 |
| Limit value (EPA) | — | — | 5.0 | 1.0 | — | 5.0 |
4. Conclusions
This study investigated the feasibility of heavy oily sludge modification and its application in controlling the release of heavy metals as a stabilization/solidification material. From the results, the following main conclusions can be drawn:(1) The changes of the heavy oily sludge were the increase of heavy components ratio and the change of penetration and softening point. Among, the temperature of 493 K and duration time of 2.5 h were considered as the optimal conditions for preparing grades of industrial bitumen of lower penetration and higher softening point. The main physicochemical properties of the asphalt-like emulsion were in accordance with bitumen 100#.
(2) An ideal ratio was achieved at 0.5 for controlling the release of heavy metals during solidification. In addition, the increase of modified oily sludge ratio or coating method can achieve an acceptable performance in the leaching test, meaning the modified oily sludge demonstrated improvement in terms of the S/S of heavy metals.
(3) This study has important environmental engineering significance. Future studies could include, for example, the ratio of oxygen, the application of a catalyst, and the use of a better reactor, etc. In addition, the best ratio of embedded and long-term monitoring of the solidification based on modified oily sludge should be studied.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 Program) (grant no. 2012AA063401), and the National Natural Science Foundation of China (grant no. 21207138).References
- A. Al-Futaisi, A. Jamrah, B. Yaghi and R. Taha, J. Hazard. Mater., 2007, 141, 557–564 CrossRef CAS PubMed.
- G. D. Ji, T. H. Sun and J. N. Ni, Ecol. Eng., 2007, 29, 272–279 CrossRef.
- S. H. Guo, G. Li, J. H. Qu and X. L. Liu, Chem. Eng. J., 2011, 168, 746–751 CrossRef CAS.
- L. Yang, G. Nakhla and A. Bassi, J. Hazard. Mater., 2005, 125, 130–140 CrossRef CAS.
- E. A. Taiwo and J. A. Otolorin, Pet. Sci. Technol., 2009, 27, 836–844 CrossRef.
- M. A. Avila-Chavez, R. Eustaquio-Rincon, J. Reza and A. Trejo, Sep. Sci. Technol., 2007, 42, 2327–2345 CrossRef CAS.
- H. F. Hassan, R. Taha, A. A. Rawas, B. A. Shandoudi, K. A. Gheithi and A. M. A. Baram, Construct. Build. Mater., 2005, 19, 646–652 CrossRef.
- H. F. Hassan, A. A. Rawas, A. W. Hago, A. Jamrah, A. Al-Futaisi and T. Al-Sabqi, Construct. Build. Mater., 2008, 22, 1239–1246 CrossRef.
- K. Zhang, J. H. Zhu and Y. Zhou, Chem. Eng. Res. Des., 2014, 92, 2396–2403 CrossRef CAS.
- R. J. Ayen and C. P. Swanstrom, Environ. Prog., 1992, 11, 127–133 CrossRef CAS.
- A. P. Kuriakose and S. K. B. Manjooran, Energy Fuels, 1994, 8, 788–792 CrossRef CAS.
- S. H. Deng, X. B. Wang, H. Z. Ta, H. Mikulcic, Z. F. Li, R. J. Cao, Z. Wang and M. Vujanovi, Appl. Therm. Eng., 2015, 91, 354–362 CrossRef.
- J. A. Conesa, J. Moltó, J. Ariza, M. Ariza and A. García-Barneto, J. Anal. Appl. Pyrolysis, 2014, 107, 101–106 CrossRef CAS.
- C. Y. Chang, J. L. Shie, J. P. Lin, C. H. Wu, D. J. Lee and C. F. Chang, Energy Fuels, 2000, 14, 1176–1183 CrossRef CAS.
- P. F. Andrade, T. F. Azevedo, I. F. Gimenez, A. G. S. Filho and L. S. Barreto, J. Hazard. Mater., 2009, 167, 879–884 CrossRef CAS PubMed.
- I. B. Grudnikov, E. V. Ippolitov and Y. I. Grudnikova, Chem. Technol. Fuels Oils, 2004, 40, 370–381 CrossRef CAS.
- M. S. Kim, J. S. Hwang and H. R. Kim, J. Environ. Sci. Health, Part A: Environ. Sci. Eng. Toxic Hazard. Subst. Control, 1997, 32, 1013–1024 CrossRef.
- P. Punnaruttanakun, V. Meeyoo, C. Kalambaheti, P. Rangsunvigit, T. Rirksomboon and B. Kitiyanan, J. Anal. Appl. Pyrolysis, 2003, 68–69, 547–560 CrossRef CAS.
- H. Schmidt and W. Kaminsky, Chemosphere, 2001, 45, 285–290 CrossRef CAS.
- R. R. Zakieva, I. I. Gussamov, R. M. Gadel'shin, S. M. Petrov, D. A. Ibragimova and L. R. Baibekova, Chem. Technol. Fuels Oils, 2015, 51, 339–344 CrossRef CAS.
- S. Caro, A. Diaz, D. Rojas and H. Nuñez, Construct. Build. Mater., 2014, 61, 181–190 CrossRef.
- P. R. Herrington and G. F. A. Ball, Fuel, 1996, 75, 1129–1131 CrossRef CAS.
- J. N. Meegoda, Pract. Period. Hazard., Toxic, Radioact. Waste Manage., 1999, 3, 46–55 CrossRef CAS.
- V. Bednarik, M. Vondruska and M. Koutny, J. Hazard. Mater., 2005, 122, 139–145 CrossRef CAS PubMed.
- C. M. Huang, C. T. Chiu, K. C. Li and W. F. Yang, J. Hazard. Mater., 2006, 137, 1742–1749 CrossRef CAS PubMed.
- M. A. Arocha, B. J. McCoy and A. P. Jackman, J. Hazard. Mater., 1996, 51, 131–149 CrossRef CAS.
- J. S. Shon, S. H. Lee, G. N. Kim, I. T. Kim and H. S. Shin, Environ. Technol., 2000, 21, 407–415 CrossRef CAS.
- M. Vondruska, M. Sild, M. Koutny and V. Bednarik, Environ. Prot. Eng., 2002, 28, 129–142 CAS.
- K. Sawasa, H. Matsuda and M. Mizutani, J. Chem. Eng. Jpn., 2001, 34, 878–883 CrossRef.
- A. R. Pasandín and I. Pérez, Construct. Build. Mater., 2014, 55, 350–358 CrossRef.
- J. L. Zhang, J. G. Liu, C. Li, Y. Y. Jin and Y. F. Nie, Environ. Sci., 2008, 29, 1138–1142 Search PubMed.
- M. Sild, M. Vondruska, M. Koutny and V. Bednarik, Pract. Period. Hazard., Toxic, Radioact. Waste Manage., 2004, 8, 2–6 CrossRef CAS.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Water Works Association and Water Environment Federation, 21st edn, 2005 Search PubMed.
- EPA Method 3611B, Alumina Column Cleanup And Separation of Petroleum Wastes.
- ASTM D5, Standard Test Method for Penetration of Bituminous Materials; ASTM D36, Standard Test Method for Softening Point of Bitumen (Ring-and-Ball Apparatus).
- J. L. Shie, C. Y. Chang, J. P. Lin, C. H. Wu and D. J. Lee, J. Chem. Technol. Biotechnol., 2000, 75, 443–450 CrossRef CAS.
- J. L. Shie, J. P. Lin, C. Y. Chang, C. H. Wu, D. J. Lee, C. F. Chang and Y. H. Chen, Energy Fuels, 2004, 18, 1272–1281 CrossRef CAS.
- J. G. Liu, X. M. Jiang, L. S. Zhou, X. X. Han and Z. G. Cui, J. Hazard. Mater., 2009, 161, 1208–1215 CrossRef CAS PubMed.
- B. Sengoz and J. Oylumluoglu, Construct. Build. Mater., 2013, 45, 173–183 CrossRef.
- S. E. Zoorob, J. P. Castro-Gomes and L. A. Pereira Oliveira, Construct. Build. Mater., 2012, 27, 357–367 CrossRef.
- K. Anastasiadou, K. Christopoulos, E. Mousios and E. Gidarakos, J. Hazard. Mater., 2012, 207–208, 165–170 CrossRef CAS PubMed.
- D. Gress, X. Zhang, S. Tarr, I. Pazienza and T. Eighmy, in Transportation Research Record No. 1345, TRB, National Research Council, Washington, D.C., 1992, pp. 10–18 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




