Organoamido zirconium(IV) and titanium(IV) complexes and their catalysis towards ethylene polymerization†
Meisu Zhou*,
Qiaokun Yang,
Hongbo Tong,
Lei Yan and
Xiaoyang Wang
Institute of Applied Chemistry, Shanxi University, Taiyuan 030006, People's Republic of China. E-mail: mszhou@sxu.edu.cn; Fax: +86-351-7011688; Tel: +86-351-7010722
First published on 8th December 2015
Abstract
The reaction of PhN(Li)SiMe3 with 2 equiv. of dimethylcyanamide with further addition of 2 equiv. of CpTiCl3 led via Me3SiCl elimination to dinuclear titanium(IV) compound 1. The reaction of DippN(Li)SiMe3 with 1 equiv. of dimethylcyanamide with further addition of 1 equiv. of CpTiCl3 afforded centrosymmetric dimeric titanium(IV) compound 2 and mononuclear 3 with the elimination of Me3SiCl. The zirconium(IV) compound 4 was obtained by the addition reaction of PhN(Li)SiMe3 with 2 equiv. of dimethylcyanamide and metathesis reaction with ZrCl4. X-ray diffraction studies of 1–4 establish the dinuclear titanium(IV) structure in 1, half-titanocene of 2 and 3, and a propeller-like complex fragment of 4. Complexes 2 and 4 show moderate or high activities in the polymerization of ethylene upon activation with methylaluminoxane (MAO).
Introduction
Amido-based ligands have played an important role in developing alternative ligands to cyclopentadienyl ligands in organometallic chemistry1–3 and their zirconium(IV) and titanium(IV) complexes have shown catalytic activity in α-olefin polymerization.4 Among which the tunable three atom, 4-electronic guanidinato and amidinato systems5 and the five atom, 6-electronic systems such as β-diketiminato6 and 1,3,5-triazapentadienato anions7,8 were developed. The β-diketiminato ligands and non-symmetric guanidinato and amidinato ligands were synthesized via the addition reactions of carbon minus and nitrogen minus to α-hydrogen-free carbonitriles, respectively, and their zirconium(IV) or hafnium(IV) complexes were found to be olefin polymerization catalysts.9 Guanidinate-supported titanium imido complexes could be used as a catalyst for alkyne hydroamination,10 and the zirconium–amido guanidinate as a potential precursor for the CVD of ZrO2 thin films.11 The 2,4-N,N′-disubstituted-1,3,5-triazapentadienato ligands were developed in our group via the insertion reactions of various nitriles to M–N bonds with the migrations of SiMe3 group from N → N′ and concomitant N–C bond formation,12 and used to stabilize main and transition metals.13 Because of their strong bond to the metals by the donor nitrogen atoms, tunable electronic and steric properties, and various bonding modes to metals, 1,3,5-triazapentadienato ligands might have considerable potential as spectator ligands especially in the area of catalysis. In addition, there is no precedent of 1,3,5-triazapentadienyl ligands in group 4 metals. Therefore the reaction of lithium trimethylsilylaniline with two equivalents of α-hydrogen-free dimethylcyanamide, and further with two equivalents of CpTiCl3, led to dinuclear titanium 1,3,5-triazapentadienate complex 1. The reaction of DippN(Li)SiMe3 with 1 equiv. of dimethylcyanamide, further addition of 1 equiv. of CpTiCl3 afforded a centrosymmetric dimeric titanium(IV) compound 2 and mononuclear 3 with the elimination of Me3SiCl. Propeller-like zirconium 1,3,5-triazapentadienate complex 4 was obtained via the reaction of lithium trimethylsilylaniline with two equivalents of dimethylcyanamide, and one third equivalents of ZrCl4. To our knowledge, this is a rare example of titanocene guanidinate system and the first example of 1,3,5-triazapentadienyl ancillary ligand used in group 4 metals. In this paper we report the synthesis and X-ray solid state structures of new titanocene and zirconium complexes 1–4 containing either guanidinato or 1,3,5-triazapentadienyl ligands, and also the use of 2 and 4 as catalyst precursors for the polymerization of ethylene.Experimental section
Materials and procedures
All manipulations were carried out under an atmosphere of argon using standard Schlenk techniques. Solvents were purchased from commercial sources. Deuterated CDCl3 was dried over activated molecular sieves (4 Å) and vacuum transferred before use. Diethyl ether and THF were dried and distilled from sodium/benzophenone and stored over a sodium mirror under argon. Dichloromethane was distilled from activated molecular sieves (4 Å) or CaH2. Glassware was oven-dried at 150 °C overnight. The NMR spectra were recorded on a Bruker DRX-300 instrument, and solvent resonances were used as the internal references for 1H spectra and 13C spectra. Melting points were measured in sealed capillaries and are uncorrected. Elemental analyses were carried out using a Vario EL-III analyzer (Germany).[{PhNC(NMe2)NC(NMe2)N}{TiCpCl2}2] (1)
(CH3)2NCN (0.36 mL, 4.52 mmol) was added to a solution of PhN(Li)SiMe3 (0.39 g, 2.26 mmol) in THF (30 mL) at −78 °C. The resulting mixture was warmed to ca. 25 °C and stirred overnight. CpTiCl3 (0.99 g, 4.52 mmol) was added at −78 °C. The resulting mixture was warmed to ca. 25 °C and stirred for 24 h. The volatiles were removed in vacuo, and the residue was extracted with 15 mL of dichloromethane and filtered. The filtrate was concentrated in vacuo to ca. 10 mL and left at room temperature for 3 d to give red crystals of 1 (0.53 g, 34.5%). 1H NMR (CDCl3): δ 2.52–3.20 (m, 12H, N(CH3)2), 6.57, 6.58, 6.69, 6.70 (2d, 10H, Cp), 6.89–7.51 (m, 5H, Ph). 13C NMR (CDCl3): δ 41.1, 41.8, 43.4 (N(CH3)), 119.3, 124.9 (Cp), 128.1–150.9 (Ph), 161.3, 162.2 (N–C–N). Anal. calcd for C22H27Cl4N5Ti2(CH2Cl2): C, 40.39; H, 4.27; N, 10.24. Found: C, 40.51; H, 4.26; N, 10.18.[{DippNC(NMe2)N}TiCpCl]2 (2) and [{DippN(H)C(NMe2)}TiCpCl2] (3)
(CH3)2NCN (0.25 mL, 3.04 mmol) was added to a solution of DippN(Li)SiMe3 (0.78 g, 3.04 mmol) in THF (30 mL) at −78 °C. The resulting mixture was warmed to ca. 25 °C and stirred overnight. CpTiCl3 (0.67 g, 3.04 mmol) was added at −78 °C. The resulting mixture was warmed to ca. 25 °C and stirred for 48 h. The volatiles were removed in vacuo, and the residue was extracted with 20 mL of dichloromethane and filtered. The filtrate was concentrated in vacuo to ca. 15 mL and left at room temperature for 6 d to give a first crop of deep red block crystals of 2 (0.104 g, 13%); filtered and the filtrate was stored at −25 °C for 10 days to give a second crop of minor red needles 3, which were separated manually, only adequate for structural determination. 2: 1H NMR (CDCl3): δ 1.18–1.44 (m, 24H, CH(CH3)2), 3.15–3.26 (m, 12H, N(CH3)2), 3.33–3.42 (m, 4H, CH(CH3)2), 5.82, 5.98, 5.99, 6.23, 6.41, 6.64, 6.67 (s, 10H, C5H5), 7.28–7.65 (m, 6H, Ph). 13C NMR (CDCl3): δ 22.2, 24.7 (CH(CH3)2), 28.3 (CH(CH3)2), 39.4, 40.0, 41.1 (N(CH3)2), 115.6, 116.1, 116.8, 116.9, 119.2 (C5H5), 123.7, 125.6, 129.2, 130.3, 132.1 (Ph), 147.2, 148.0 (Cipso), 149.2 (NCN).[{PhNC(NMe2)NC(NMe2)N(SiMe3)}3ZrCl] (4)
(CH3)2NCN (0.34 mL, 4.12 mmol) was added to a solution of PhN(Li)SiMe3 (0.35 g, 2.06 mmol) in Et2O (30 mL) at −78 °C. The resulting mixture was warmed to ca. 25 °C and stirred overnight. ZrCl4 (0.160 g, 0.69 mmol) was added at −78 °C. The resulting mixture was warmed to ca. 25 °C and stirred for 24 h, then filtered. The filtrate was concentrated in vacuo to ca. 10 mL and stored at −25 °C for several days, yielding colorless crystals of 4 (0.33 g, 40%). Mp: 73–75 °C. 1H NMR (CDCl3): δ −0.08–0.22 (m, 27H, SiMe3), 2.31–3.04 (m, 36H, N(CH3)2), 1.12–1.16, 3.40–3.42 (Et2O), 6.61–7.19 (m, 15H, Ph). 13C NMR (CDCl3): δ −3.62, −1.53, 0.65 (3s, SiMe3), 38.05, 39.91 (2s, (N(CH3)), 15.5, 66.1 (Et2O), 122.2, 123.1, 123.9, 125.9, 128.7, 148.6 (Ph), 160.8, 164.1 (N–C–N). Found: C, 53.43; H, 8.11; N, 17.72. C53H98ClN15O2Si3Zr requires C, 53.57; H, 8.31; N, 17.68%.General procedure for ethylene polymerization
Ethylene polymerization was carried out in a 500 mL autoclave stainless steel reactor equipped with a mechanical stirrer and a temperature controller. Briefly, toluene, the desired amount of cocatalyst, and a toluene solution of the catalytic precursor (the total volume was 100 mL) were added to the reactor in this order under an ethylene atmosphere. When the desired reaction temperature was reached, ethylene at 10 atm pressure was introduced to start the reaction, and the ethylene pressure was maintained by constant feeding of ethylene. After 30 min, the reaction was stopped. The solution was quenched with HCl–acidified ethanol (5%), and the precipitated polyethylene was filtered, washed with ethanol, and dried in vacuum at 60 °C to constant weight.X-ray crystallography
Data collection of 1–4 was performed with Mo-Kα radiation (λ = 0.71073 Å) on a Bruker Smart Apex CCD diffractometer using the omega scan mode yielding a total of N reflections. Crystals were coated in oil and then directly mounted on the diffractometer under a stream of cold nitrogen gas. Corrections were applied for Lorentz and polarization effects as well as absorption using multi-scans (SADABS).14 The structure was solved by direct method (SHELXS-97).15 The remaining non-hydrogen atoms were obtained from the successive difference Fourier map. All non-H atoms were refined with anisotropic displacement parameters, while the H atoms were constrained to parent sites, using a riding mode (SHELXTL).16 Crystal data and details of data collection and refinements for 1–4 are summarized in Table 1. CCDC (reference numbers 1424470–1424472) contain the supplementary crystallographic data for this paper.†| Compound | 1 | 3 | 4 |
|---|---|---|---|
| Formula | C23H29Cl6N5Ti2 | C20H29Cl2N3Ti | C53H98ClN15O2Si3Zr |
| M | 684.01 | 430.26 | 1188.40 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/n (no. 14) | P21/n (no. 14) | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) (no. 2) |
| a/Å | 11.095(5) | 8.966(9) | 12.6863(19) |
| b/Å | 19.886(9) | 14.723(17) | 14.732(2) |
| c/Å | 13.656(6) | 16.566(17) | 18.976(3) |
| α/° | 90.00 | 96.910(17) | 87.593(4) |
| β/° | 98.13(3) | 100.41(7) | 76.510(3) |
| γ/° | 90.00 | 90.190(19) | 82.086(4) |
| U/Å3 | 2983(2) | 2151(4) | 3415.7(9) |
| Z | 4 | 4 | 2 |
| Absorption coeff. (mm−1) | 1.095 | 0.655 | 0.299 |
| Unique reflections, Rint | 5255, 0.0385 | 3773, 0.0660 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078, 0.0425 078, 0.0425 |
| Reflections with I > 2σ(I) | 4611 | 3252 | 7669 |
| Final R indices [I > 2σ(I) ] R1, wR2 | 0.0736, 0.0858 | 0.1122, 0.1294 | 0.0534, 0.1000 |
| R indices (all data) R1, wR2 | 0.1495, 0.1553 | 0.2322, 0.2397 | 0.0997, 0.1201 |
Results and discussion
The reaction of PhN(Li)SiMe3 with two equivalents of Me2NCN and further with two equivalents of CpTiCl3 in the presence of THF afforded compound 1 in a Me3SiCl elimination reaction according to eqn (1). 1H and 13C NMR spectra are in accordance with the formula and elemental analysis is consistent.
 | (1) |
Suitable crystals for X-ray diffraction studies of 1 were obtained from the saturated dichloromethane solution at room temperature. The molecular structure of 1 is illustrated in Fig. 1 including principal bond lengths and angles. X-Ray analysis shows that 1 is dinuclear, the twisted W-shaped 1,3,5-triazapentadienyl ligand coordinates to the two metal centers by η2-coordination mode and terminal bonding mode, respectively. The Ti1 atom is located in a pyramidal environment (center of Cp ring as one vertex) from one η5-cyclopentadienyl group, two nitrogen atoms from the ligand and two chlorine atoms, thus forming a distorted square pyramidal geometry at the Ti1 center with the Cp group occupying the apical position. The 1,3,5-triazapentadienyl ligand coordinates with Ti2 atom in the terminal bonding mode, and a flattened three-legged piano stool (N4–Ti2–Cl3 103.82(15)°; N4–Ti2–Cl4 101.11(14)°; Cl3–Ti2–Cl4 101.48(7)°) around Ti2 was formed. The piano stool structures were also found in other titanocene and yttrium guanidinate compounds17 and in the amido and imido lanthanide compounds with constrained bis(oxazoline)cyclopentadienyl ligand.18 The distance of Ti2–N4 is 1.782(4) Å, comparable to that of titanium imido complex Cp[Ph2(CH2N)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N]TiCl2 [1.792(2) Å]19 and other Ti compound Cp(Ph3P
N]TiCl2 [1.792(2) Å]19 and other Ti compound Cp(Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)TiCl2 [Ti–N: 1.78 Å] with strong π-donating ligand.20 The reaction of PhN(Li)SiMe3 with two equivalents of Me2NCN and further with one equivalent of Cp*TiCl3 in the presence of THF afforded an unusual ansa-bridged cyclopentadienyl titanium complex [{η5-C5Me4CH2–C(NMe2)
N)TiCl2 [Ti–N: 1.78 Å] with strong π-donating ligand.20 The reaction of PhN(Li)SiMe3 with two equivalents of Me2NCN and further with one equivalent of Cp*TiCl3 in the presence of THF afforded an unusual ansa-bridged cyclopentadienyl titanium complex [{η5-C5Me4CH2–C(NMe2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N}TiCl2],21 where the cyclopentadienyl ring and the mean plane of the Ti–N
N}TiCl2],21 where the cyclopentadienyl ring and the mean plane of the Ti–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C–C fragment form a dihedral angle of 88.08 (11)°.
C–C–C fragment form a dihedral angle of 88.08 (11)°.
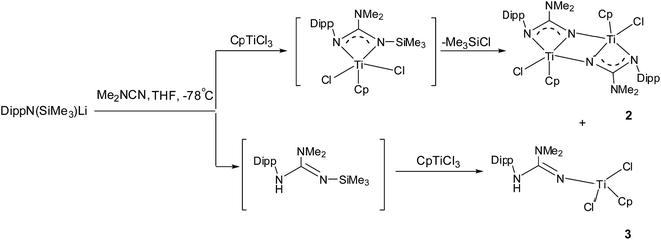
The titanocene guanidinates 2 and 3 were obtained from the same experiment, starting with equivalent portions of DippN(Li)SiMe3, (CH3)2NCN and CpTiCl3; and their separation was achieved by fractional crystallisation from dichloromethane. The formation of 2 underwent an attack of the N-centred nucleophile on Me2NCN, a salt metathesis and elimination of Me3SiCl during which an intermediate was thought to be formed and finally resulted in a thermodynamically product 2. The formation of 3 involved an unexpected hydrolysis of lithium guanidinate and elimination of Me3SiCl as well. The similar elimination reaction was found in other titanium(IV) complexes.22,23
The poor quality of crystals of 2 did not allow accurate information to be obtained on bond distances and angles but the data were adequate to establish its structure as shown in Scheme 1. 2 was characterised by multinuclear NMR spectra, while 3 was identified solely by its X-ray structure.
As shown in Fig. 2, complex 3 is a monomer, in which the titanium atom coordinated by one η5-cyclopentadienyl group, one guanidinato ligand and two chlorine atoms and forms a three-legged piano stool. The guanidinato ligand contacted with the centre metal in the η1-bonding mode. The distance of Ti–N(3) is 1.771(6) Å, significantly shorter than those of bis(imido)complex (C5H5)2Ti(–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)2[Ti–N(1): 1.944(3) Å, Ti–N(2): 1.956(2) Å],24 imido titanium compounds (C5H5)2Ti(Cl)N
CPh2)2[Ti–N(1): 1.944(3) Å, Ti–N(2): 1.956(2) Å],24 imido titanium compounds (C5H5)2Ti(Cl)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2 [Ti–N(1): 1.848(3) Å] and (C5H5)Ti(Cl)(μ-η1: η2-N
C(NMe2)2 [Ti–N(1): 1.848(3) Å] and (C5H5)Ti(Cl)(μ-η1: η2-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2)2 [Ti–N(1): 1.809(3) Å, Ti–N(4): 1.817(3) Å]17 and slightly shorter than that in 8-quinolyl-linked guanidinate titanium salt [{8-(2-CH3-C9H5N)N(H)C(NMe2)N}Ti(MeC5H4)-Cl2] [Ti1–N(3): 1.797(5) Å],25 suggesting stronger Ti–N(gua) bond of compound 3. The bond angle of Ti–N(3)–C(13) [172.2(5)°] in 3 is larger than the value in (C5H5)2Ti(Cl)N
C(NMe2)2)2 [Ti–N(1): 1.809(3) Å, Ti–N(4): 1.817(3) Å]17 and slightly shorter than that in 8-quinolyl-linked guanidinate titanium salt [{8-(2-CH3-C9H5N)N(H)C(NMe2)N}Ti(MeC5H4)-Cl2] [Ti1–N(3): 1.797(5) Å],25 suggesting stronger Ti–N(gua) bond of compound 3. The bond angle of Ti–N(3)–C(13) [172.2(5)°] in 3 is larger than the value in (C5H5)2Ti(Cl)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2 [Ti–N(1)–C(11) [170.2(3)°]],17 much larger than those in (C5H5)2Ti(–N
C(NMe2)2 [Ti–N(1)–C(11) [170.2(3)°]],17 much larger than those in (C5H5)2Ti(–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)2 [Ti–N(1)–C(11) [160.8(2)°]; Ti–N(2)–C(24) [161.0(2)°]]24 and in [{8-(2-CH3-C9H5N)N(H)C(NMe2)N}Ti(MeC5H4)–Cl2] [Ti(1)–N(3)–C(10) [157.5(5)°],26 also indicative of titanium imido feature of 3.
CPh2)2 [Ti–N(1)–C(11) [160.8(2)°]; Ti–N(2)–C(24) [161.0(2)°]]24 and in [{8-(2-CH3-C9H5N)N(H)C(NMe2)N}Ti(MeC5H4)–Cl2] [Ti(1)–N(3)–C(10) [157.5(5)°],26 also indicative of titanium imido feature of 3.
Compound 4 was conveniently prepared in 40% yield according to Scheme 2. Thus, treatment of PhN(SiMe3)Li with two equivalents of (CH3)2NCN in diethyl ether at −78 °C and further with one third equivalents of ZrCl4 resulted in the colorless crystalline [{PhNC(NMe2)NC(NMe2)N(SiMe3)}3ZrCl] (4). Complex 4 was air-sensitive and changed into white powder in 15 min when it exposed to air.
The molecular structure of 4 is illustrated in Fig. 3 including principal bond lengths and angles. The three chelated C1-symmetric 1,3,5-triazapentadienyl ligands are disposed around the zirconium atom in a propeller-like fashion via η2-coordination mode. The zirconium is the center of a chloride-capped distorted octahedron with three pairs of nitrogen atoms from three 1,3,5-triazapentadienyl ligands. Three C(NMe2)(NSiMe3) groups are located close to the chlorine atom and the three phenyl groups are arranged on the trans-position to the chlorine. The bond distance of Zr–Cl [2.437(1) Å] in 4 is slightly shorter than those of tris(benzamidinato)zirconium chloride complexes [(Siam)3ZrCl][Siam = N,N′ bis(trimethylsilyl)benzamidinat]][Zr–Cl 2.464(2) Å]26 and chiral zirconium benzamidinate complex [{C6H5C(N-TMS)(N-myrtanyl)}3ZrCl][Zr–Cl 2.475(3) Å,27 and tris(guanidinato)zirconium chloride compound [{PhNC(NMe2)NSiMe3}ZrCl[Zr–Cl 2.515(2) Å ].9a
The key bond lengths and angles of the ZrNCN moiety of 4 and its analogues zirconium guanidinate [PhNC(NMe2)N(SiMe3)] I9a and zirconium benzamidinate analogue [PhNC(C6H4Me-4)N(SiMe3)] II28 are shown in Scheme 3. In 4, the slightly longer Zr–N(C(NMe2)SiMe3) bond length than that of Zr–N(Ph) indicated its less steric impact in 4 than in its analogues I and II. This is also supported by the narrower dihedral angles between the planes NCN and NZrN in 4 (0.8, 0.8 and 1.1°) than in I (5.4, 6.1 and 6.5°) and II (8.2, 8.8 and 8.3°).
Beside the synthesis of organoamido group 4 complexes, our interest is focused on their applications. Therefore, complexes 2 and 4 were tested as procatalysts for ethylene polymerization with methylaluminoxane (MAO) as the activator under varied conditions. Table 2 summarized ethylene polymerization results.
| Entry | Cat. | P/atm | T/°C | Al/M | Yield (mg) | Activityb | Tmd/°C | Mwe (× 10−4) | Mw/Mne |
|---|---|---|---|---|---|---|---|---|---|
| a Polymerization conditions: 5 μmol catalyst, cocat: MAO, 100 mL toluene, 0.5 hour.b g mol−1 h−1.c 0.25 hour.d Determined by DSC.e Determined by GPC. | |||||||||
| 1 | 4 | 1 | 30 | 500 | Trace | — | — | — | — |
| 2 | 4 | 10 | 30 | 500 | 5.80 | 2.32 × 103 | — | — | — |
| 3 | 4 | 10 | 50 | 500 | 7.60 | 3.04 × 103 | — | — | — |
| 4 | 4 | 10 | 70 | 500 | 8.90 | 3.56 × 103 | — | — | — |
| 5 | 4 | 10 | 70 | 1500 | 18.1 | 7.24 × 103 | — | — | — |
| 6 | 4 | 10 | 70 | 2500 | 25.4 | 1.02 × 104 | — | — | — |
| 7 | 2 | 10 | 30 | 500 | 394 | 1.57 × 105 | 133.1 | 67.4 | 1.86 |
| 8 | 2 | 10 | 30 | 1500 | 659 | 2.63 × 105 | 133.1 | 82.6 | 2.01 |
| 9 | 2 | 10 | 30 | 2000 | 1623 | 6.49 × 105 | 131.6 | 87.8 | 1.94 |
| 10 | 2 | 10 | 30 | 2500 | 1134 | 4.54 × 105 | 130.5 | 71.6 | 1.95 |
| 11 | 2 | 10 | 50 | 2000 | 3792 | 1.52 × 106 | 132.8 | 23.5 | 3.76 |
| 12 | 2 | 10 | 70 | 2000 | 2646 | 1.06 × 106 | 133.4 | 42.8 | 3.17 |
| 13c | 2 | 10 | 50 | 2000 | 1584 | 1.27 × 106 | 131.2 | 55.6 | 2.04 |
When activated with MAO under 1 atm of ethylene, complex 4 showed absent polymerization activity compared to that under 10 atm of ethylene (Entry 1 and 2), therefore, 10 atm pressure of ethylene was employed in the remaining experiments, in which other reaction parameters were changed. At a ratio of Al/Zr 500, with the temperature from 30 to 70 °C, the activity of 4 increased from 2.32 × 103 to 3.56 × 103 g PE mol−1 h−1 (Entry 2–4). At 70 °C, with the higher Al/Zr molar ratio of 2500 (Entry 6), complex 4 showed relatively higher activity (1.02 × 104 g PE mol−1 h−1). Comparing with other L3ZrCl type analogues, the catalytic activity of 4 is slightly higher than those of tris(benzamidinato)zirconium chloride complexes such as [N(SiMe3)C(C6H4Me-4)NPh]3ZrCl] (8.6 × 103 g PE mol−1 h−1 bar−1),28 [{PhC(NCy)(NSiMe3)}3ZrCl] (5.44 × 103 g PE mol−1 h−1).29 The catalytic activity of 4 is comparable to that of sufficiently similar complex [PhNC(NMe2)NSiMe3]3ZrCl (1.05 × 104 g PE mol−1 h−1)9a that we reported, although the angle between the propeller flaps in complex 4 [mean value 92.3°] is larger by 1.9° than that in [PhNC(NMe2)NSiMe3]3ZrCl [mean value 90.4°], suggesting that the change of ligands in 4 didn't affect the catalytic activity of the compound significantly. The moderate activities were also found in other amide-based polymerization catalysts such as [PhC(NR)2]2ZrCl2 (R = iPr, Cy), and [PhC(NiPr)2]2ZrCl2.30
For 2/MAO system, the optimal Al/Ti ratio was 2000 (Entry 9) and the highest activity of 1.52 × 106 g PE mol−1 h−1 (Entry 11) was observed at 50 °C. The titanocene guanidinate 2 showed relatively higher activity for the polymerization of ethylene than that of complex 4. This is probably due to the introducing of the cyclopentadienyl group into the structure of 2, the formation and stabilization of the catalytically active species can be achieved by more electron donation through the cyclopentadienyl fragment especially for the electron-deficient zirconium complex.31 With the increase of the Al/Ti molar ratio from 500 to 2500 (Entries 7–10), the molecular weight of the polymers initially increased and then decreased. Meanwhile, the Tm of the resultant polyethylene decreased from 133.1 to 130.5 °C. Increasing the reaction temperature from 30 to 70 °C (Entries 9, 11 and 12), the activity initially increased and then decreased and the Tm of the resultant polymer increased from 131.6 to 133.4 °C. In addition, NMR data showed the formation of linear polyethylenes.32
Conclusions
Several organoamido group 4 complexes have been synthesized. In titanocene guanidinate complexes 1 and 3, the guanidinato ligand coordinated the metal center in an unusual terminal bonding mode. Both in titanocene guanidinate 2 and 1,3,5-triazapentadienyl zirconium complex 4, the ligand coordinated to the metal via η2-coordination mode, a bridge and side-on chelating coordination mode was found in 2 and a propeller-like fashion was found in 4. Complexes 1 and 3 showed efficient ligand π-donation to the metal. Compounds 1 and 4 are the first examples of group 4 complexes bearing the 1,3,5-triazapentadienyl ligands. Complex 4 showed moderate and 2 showed high activity in ethylene polymerization. At present we are designing other chloroguanidinate zirconocene and hafnocene complexes and L2MCl2 type group 4 complexes containing 1,3,5-triazapentadienyl ligands such that their catalytic activities will be higher in olefin polymerization.Acknowledgements
We thank the Natural Science Foundation of China (No. 21371111), Shanxi Scholarship Council of China (No. 2013-025) and Shanxi Functional Organometallic Compound Information Net Project (No. 2013091022).References
- F. T. Edelmann, Angew. Chem., Int. Ed., 1995, 34, 2466 CrossRef CAS.
- R. Kempe, Eur. J. Inorg. Chem., 2003, 791 CrossRef CAS.
- C. Jones, P. C. Junk, S. G. Leary and N. A. Smithies, Inorg. Chem. Commun., 2003, 6, 1126 CrossRef CAS.
- H. Fuhrmann, S. Brenner, P. Arndt and R. Kempe, Inorg. Chem., 1996, 35, 6742 CrossRef CAS.
- (a) P. J. Bailey and S. Pace, Coord. Chem. Rev., 2001, 214, 91 CrossRef CAS; (b) F. T. Edelmann, Coord. Chem. Rev., 1994, 137, 403 CrossRef.
- L. Bourget-Merle, M. F. Lappert and J. R. Severn, Chem. Rev., 2002, 102, 3031 CrossRef CAS PubMed.
- A. R. Siedle, R. J. Webb, R. A. Newmark, M. Brostrom, D. A. Weil, K. Erickson, F. E. Behr and V. G. J. Young, Fluorine Chem., 2003, 122, 175 CrossRef CAS.
- H. V. R. Dias and S. Singh, Inorg. Chem., 2004, 43, 7396 CrossRef CAS PubMed.
- (a) M.-S. Zhou, H.-B. Tong, X.-H. Wei and D.-S. Liu, J. Organomet. Chem., 2007, 692, 5195 CrossRef CAS; (b) M.-S. Zhou, S. Zhang, H.-B. Tong, W.-H. Sun and D.-S. Liu, Inorg. Chem. Commun., 2007, 1262 CrossRef CAS; (c) M. Fan, Q. K. Yang, H. B. Tong, S. F. Yuan, B. Jia, D. L. Guo, M. S. Zhou and D. S. Liu, RSC Adv., 2012, 2, 6599 RSC; (d) M.-S. Zhou, S.-P. Huang, L.-H. Weng, W.-H. Sun and D.-S. Liu, J. Organomet. Chem., 2003, 665, 237 CrossRef CAS.
- T.-G. Ong, G. P. A. Yap and D. S. Richeson, Organometallics, 2002, 21, 2839 CrossRef CAS.
- A. Devi, R. Bhakta, A. Milanov, M. Hellwig, D. Barreca, E. Tondello, R. Thomas, P. Ehrhart, M. Winter and R. Fischer, Dalton Trans., 2007, 1671 RSC.
- M.-S. Zhou, P. Li, H.-B. Tong, Y.-P. Song, T. Gong, J.-P. Guo, L.-H. Weng and D.-S. Liu, Inorg. Chem., 2008, 47, 1886 CrossRef CAS.
- (a) M.-S. Zhou, Y.-P. Song, T. Gong, H.-B. Tong, J.-P. Guo, L.-H. Weng and D.-S. Liu, Inorg. Chem., 2008, 47, 6692 CrossRef CAS PubMed; (b) M.-S. Zhou, T. Gong, X.-L. Qiao, H.-B. Tong, J.-P. Guo and D.-S. Liu, Inorg. Chem., 2011, 50, 1926 CrossRef CAS PubMed; (c) D. Tian, Q.-W. Xie, L. Yan, H.-B. Tong and M.-S. Zhou, Inorg. Chem. Commun., 2015, 58, 35 CrossRef CAS.
- G. M. Sheldrick, Correction Software, University of Göttinggen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, Program for the Solution of Crystal Structures, University of Göttinggen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELXTL version 6.14, Bruker Analytical X-ray Instruments, Inc., Madison, WI, USA, 2003 Search PubMed.
- J. Zhang, F.-Y. Han, Y.-N. Han, Z.-X. Chen and X.-G. Zhou, Dalton Trans., 2009, 1806 RSC.
- N. L. Lampland, J. Zhu, M. Hovey, B. Jana, A. Ellern and A. D. Sadow, Inorg. Chem., 2015, 54, 6938 CrossRef CAS.
- W. P. Kretschmer, C. Dijkhuis, A. Meetsma, B. Hessen and J. H. Teuben, Chem. Commun., 2002, 608 RSC.
- I. A. Latham, G. J. Leigh, G. Huttner and I. Jibril, J. Chem. Soc., Dalton Trans., 1986, 377 RSC.
- D.-L. Guo, H.-B. Tong and M.-S. Zhou, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, m611 CAS.
- W. J. Meerendonk, K. Schröder, E. A. C. Brussee, A. Meetsma, B. Hessen and J. H. Teuben, Eur. J. Inorg. Chem., 2003, 427 CrossRef.
- M. P. Coles and P. B. Hitchcock, Organometallics, 2003, 22, 3255 CrossRef.
- T. Zippel, P. Arndt, A. Ohff, A. Spannenberg, R. Kempe and U. Rosenthal, Organometallics, 1998, 17, 4429 CrossRef CAS.
- P. Wang, H.-F. Li, X.-Y. Xue and X. Chen, Dalton Trans., 2015, 44, 4718 RSC.
- D. Walther, R. Fischer, H. Görls, J. Koch and B. Schweder, J. Organomet. Chem., 1996, 508, 13 CrossRef CAS.
- C. Averbuj, E. Tish and M. S. Eisen, J. Am. Chem. Soc., 1998, 120, 8640 CrossRef CAS.
- P. B. Hitchcock, M. F. Lappert and P. G. Merle, Dalton Trans., 2007, 585 RSC.
- Q.-K. Yang, M.-S. Zhou, H.-B. Tong, D.-L. Guo and D.-S. Liu, Inorg. Chem. Commun., 2013, 29, 1 CrossRef CAS.
- J. Richter, F. T. Edelmann, M. Noltemeyer, H.-G. Schmidt, M. Schmulinson and M. S. Eisen, J. Mol. Catal. A: Chem., 1998, 130, 149 CrossRef CAS.
- K. Nomura, J.-Y. Liu, S. Padmanabhan and B. Kitiyanan, J. Mol. Catal. A: Chem., 2007, 267, 1 CrossRef CAS.
- W. Zuo, W.-H. Sun, S. Zhang, P. Hao and A. Shiga, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3415 CrossRef CAS.
Footnote |
| † CCDC 1424470–1424472. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra20868h |
| This journal is © The Royal Society of Chemistry 2015 |