A novel strategy for 1,3-propanediol recovery from fermentation broth and control of product colority using scraped thin-film evaporation for desalination
Yuanman Zhang,
Ji'an Luo,
Xuebing Zhao* and
Dehua Liu*
Institute of Applied Chemistry, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China. E-mail: zhaoxb@mail.tsinghua.edu.cn; dhliu@tsinghua.edu.cn; Fax: +86-10-62772130; Tel: +86-10-62772130
First published on 15th May 2015
Abstract
The biological production of 1,3-propanediol (1,3-PD) by microbial fermentation is promising because by-product glycerol produced in biodiesel production can be used as a carbon source. However, the salts present in the fermentation broth are negative to the downstream processing, particularly for the product colority. In the present work, we first studied the effects of several salts on the increase of colority and analyzed the possible mechanism. Ammonium salt ((NH4)2SO4) showed the most negative effect, which was probably due to the decrease of pH caused by the hydrolysis of ammonium salt thus facilitating the chromophoric reaction. A novel strategy was thus made by adjusting the initial pH of the feeding liquid for distillation. It was found that high pH (alkali condition) indeed reduced the distillate colority but showed no negative effects on the recovery yields of the main product 1,3-PD and major by-product 2,3-butanediol (2,3-BD). Scraped thin-film evaporation was greatly effective for desalination and recovering 1,3-PD and 2,3-BD with high recovery yields. High pH was also found to be beneficial to reduce the concentration of the impurity, acetic acid, in the distillate, which was of great importance for producing qualified 1,3-PD for polymerization. The novel strategy is thus very promising for recovering 1,3-PD from fermentation broth, particularly for the downstream processing at an industrial scale.
Introduction
1,3-Propanediol (1,3-PD) is an important bulk chemical. It can be used as emulsifiers, cosmetics, lubricants, and for medicine production with annual demand growing very fast.1,2 However, 1,3-PD is more famous as an important monomer for producing polytrimethylene terephthalate (PTT),1 which has been known for many years because of its excellent physical properties.3 PTT is reported to have several advantages versus PET and PBT such as better elastic recovery and lower modulus.3Commercially, 1,3-PD generally can be produced by chemical or biological synthesis.4–6 However, the application of the chemical synthesis has been limited by the requirement of high temperature, high pressure and expensive catalyst, use of non-renewable materials made from petroleum and the relative low yield. Thus, a cleaner, safer, more sustainable and economic way to produce 1,3-PD is necessary.7 Nowadays, several microorganisms have been reported to have the ability to convert glycerol into 1,3-PD.7,8 Owing to the rapid development of the biodiesel industry, the price of glycerol has decreased sharply since the glycerol is a main by-product of biodiesel production in a weight ratio of about 10%. It is reported that the price of crude glycerol (80%) dropped down from 55 cents per kg in 2004 to 4.4 cents per kg in 2006.9 The cheap glycerol attracts more attention for its bioconversion to 1,3-PD. However, there are still some problems restricting the large-scale biological production of 1,3-PD, one of which is the high cost of the downstream processing. The recovery of 1,3-PD from fermentation broth can make up 50–70% of the total production cost when by-product glycerol is used as the raw material.9–11 Technically, 1,3-PD is relatively difficult to recover and purify from fermentation broth because of the complexity of the broth, which contains the main product 1,3-PD, major by-product 2,3-butanediol (2,3-BD), un-converted feedstock glycerol, minor by-products acetic acid, succinic acid and lactic acid. The low volatility and high hydrophilic characteristics of 1,3-PD in dilute aqueous solutions (1,3-PD concentration ranging from 30–130 g L−1) also make the downstream processing more complicated.8,12–15
Two of the major problems that restrict the process of 1,3-PD recovery from fermentation broth are the presence of salts in the broth and the increase of product colority during purification. To date, a variety of separation methods have been introduced to separate the salts from the polyhydric alcohols such as distillation/evaporation, electrodialysis, liquid–liquid extraction, reactive extraction, aqueous two-phase extraction, chromatography, and membrane separation technology such as nanofiltration.9,11,15–25 All of those separation methods have their advantages and disadvantages, but no single method is proved to be efficient and economically feasible.9 For example, recent works by Bastrzyk et al.24,25 have shown that nanofiltration was very effective for the separation of post-fermentation glycerol solution. However, in our previous work (data not shown), we found that nanofiltration using organic membranes (DL-1812C and DK-1812, GE Co., USA) or 0.9 nm ceramic membrane (GFT, Germany) indeed was effective to remove multivalent organic salts such as citrate, lactate and succinate, but not as effective for the removal of acetate. Furthermore, the losses of 1,3-PD and 2,3-BD were also significant when nanofiltration was used for desalination. The presence of salts in the fermentation broth not only increases the viscosity of the broth, but also have a negative effect on the colority of 1,3-PD product. Therefore, a more effective process has to be developed to effectively remove salts with high recovery yields of 1,3-PD, 2,3-BD and glycerol and obtain 1,3-PD product that meets the criteria for polymerization of PTT.
In a conventional recovery and purification of 1,3-PD from fermentation broth, the broth is usually filtrated by membrane to remove cell biomass and protein followed by a concentration/distillation process to remove a part of water. The concentrated broth is then treated for desalination before going to rectification column for 1,3-PD purification (Fig. 1). In the present work, the effects of several impurities including salts present in the fermentation broth on the colority of distillate and bottom liquid during distillation were first studied. Scraped thin-film evaporation (STFE) was further used to remove salts from the concentrated broth. The objective of this work is thus to study the possible mechanism for the increase of colority during distillation and further to make novel strategies to control the product colority.
 | ||
| Fig. 1 General flow scheme of fermentative production of 1,3-PD using biodiesel by-product crude glycerol as the carbon source. | ||
Materials and methods
The effect of salts on the colority
The fermentation broth was obtained according to our previous work.25 Before vacuum concentration to remove a part of water, the fermentation was treated by ultrafiltration using a ceramic membrane with pore size of 5 nm and cut-off molecular weight of 8000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (GFT, Germany). For investigating the effects of salts on the colority, the experiments were conducted with a simulated concentrated fermentation broth (SCFB), which consists of 240.0 g L−1 1,3-PD, 50.0 g L−1 2,3-butanediol (2,3-BD), 7.00 g L−1 glycerol, and the rest was deionized water. The natural pH of this solution was 6.08. The salts used in this experiment, including (NH4)2SO4, K2HPO4, KH2PO4, MgSO4, sodium succinate, sodium acetate and sodium lactate, were analytically pure and the same as those used or produced in the fermentative production of 1,3-PD.25 To investigate their effects on colority, single salt was added to 450.0 g SCFB placed in a round-bottom flask for vacuum distillation. The flask was then heated at 4.00 kPa of absolute pressure using an electric heating jacket until the temperature reached 180.0 °C and kept for 30 min. The pH of the bottom liquid was not controlled during heating. Samples of distillate and bottom liquid were then taken and the absorbance was measured at 460 nm for comparing the colority26 using a spectrophotometer (772E, Spectrum Shanghai, China).
000 (GFT, Germany). For investigating the effects of salts on the colority, the experiments were conducted with a simulated concentrated fermentation broth (SCFB), which consists of 240.0 g L−1 1,3-PD, 50.0 g L−1 2,3-butanediol (2,3-BD), 7.00 g L−1 glycerol, and the rest was deionized water. The natural pH of this solution was 6.08. The salts used in this experiment, including (NH4)2SO4, K2HPO4, KH2PO4, MgSO4, sodium succinate, sodium acetate and sodium lactate, were analytically pure and the same as those used or produced in the fermentative production of 1,3-PD.25 To investigate their effects on colority, single salt was added to 450.0 g SCFB placed in a round-bottom flask for vacuum distillation. The flask was then heated at 4.00 kPa of absolute pressure using an electric heating jacket until the temperature reached 180.0 °C and kept for 30 min. The pH of the bottom liquid was not controlled during heating. Samples of distillate and bottom liquid were then taken and the absorbance was measured at 460 nm for comparing the colority26 using a spectrophotometer (772E, Spectrum Shanghai, China).
Scraped thin-film evaporation (STFE)
The STFE experiments were performed using real fermentation broth obtained according to our previous work.25 Before STFE, the fermentation broth was first treated by membrane filtration to remove protein and bacteria cells followed by vacuum evaporation to remove a part of the water. The concentrated fermentation broth consisted of 242.1 g L−1 1,3-PD, 48.6 g L−1 2,3-BD, 67.3 g L−1 glycerol, 30.0 g L−1 sodium acetate, 36.2 g L−1 sodium succinate, 28.1 g L−1 sodium lactate, and the rest was water. A lab-scale scraped thin-film evaporator was used in the experiments. Its structure parameters and operation conditions are shown in Table 1. About 5000 g concentrated broth was continuously pumped into the evaporator at a speed of 16.7 mL min−1 (≈25.0 g min−1). The condensate at the top of evaporator was analyzed for colority and compositions.| Experimental conditions | Value |
|---|---|
| Evaporator area (m2) | 0.2 |
| Feeding flow (mL min−1) | 16.7 |
| Evaporator temperature (°C) | 220.0 |
| Condenser temperature (°C) | 3.0 |
| Pressure (kPa) | 0.30 |
| Scraper speed (rpm) | 390 |
Analytical methods
The concentration of 1,3-PD, 2,3-BD and glycerol were determined by a high performance liquid chromatography (HPLC) system (Shimadzu, Japan) equipped with a refractive index detector and an AMINEX HPX-87H column (Bio-Rad, USA). 5 mM H2SO4 was used as a mobile phase at a flow rate of 0.8 mL min−1 at column temperature of 65 °C. The recovery yield of 1,3-PD, 2,3-BD and glycerol during STFE is defined as follows:
 | (1) |
The colority of distillate and bottom liquid was characterized by the absorbance at 460 nm.25 However, the colority of the final product 1,3-PD after decoloration by active charcoal was determined by a colorimetric method with platinum–cobaltic standard solution26 because the absorbance at 460 nm is too low.
Results and discussion
Effect of several salts on the colority
Several inorganic and organic salts were singly added into the SCFB to study their effects on the colority of distillate and bottom liquid obtained in distillation. The concentrations of the added salts were selected based on the experimental results of distillation of real fermentation broth. The determined absorbances at 460 nm are shown in Table 2. Corresponding photos of the bottom liquids are shown in Fig. 2.| Salts | Concentration (g L−1)a | A460 (distillate) | A460 (bottom liquid) |
|---|---|---|---|
| a The initial pH was the natural pH of SCFB. No pH adjustment was performed after salt was added. | |||
| No salts before heating | 0 | 0.006 | 0.006 |
| No salts after heating | 0 | 0.033 | 0.121 |
| (NH4)2SO4 | 20.00 | 0.047 | 0.993 |
| K2HPO4 | 3.45 | 0.030 | 0.204 |
| KH2PO4 | 1.25 | 0.027 | 0.163 |
| MgSO4 | 1.00 | 0.034 | 0.104 |
| Sodium succinate | 36.17 | 0.041 | 0.133 |
| Sodium acetate | 28.11 | 0.042 | 0.177 |
| Sodium lactate | 30.00 | 0.032 | 0.151 |
It is clear that (NH4)2SO4 showed significant negative effect on the colority of distillate and bottom liquid. The color of the bottom liquid after distillation became dark yellow compared with light yellow for other samples. It indicated that (NH4)2SO4 might play as a catalyst or reactant in the colorization reaction.
To further investigate the possible mechanisms for the increase of colority, FTIR spectra of concentrated broth, distillate and control (solution prepared using pure 1,3-PD, 2,3-BD, glycerol and water) were recorded as shown in Fig. 3. It is clear that two new peaks appeared for the concentrated broth at 1711 cm−1 and 1572 cm−1. The peak at 1711 cm−1 is attributed to the carbonyl group in carboxyl or ketone, whereas the absorption peak at 1572 cm−1 might be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N or C
N or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. It indicated that oxidation reaction might take place during distillation, and the ammonium salt might take part in or facilitate the reaction. However, no significant difference between the spectra of distillate and control were found even if the initial pH of the liquid increased from 7 to 12. It was probably because the concentration of chromophores in the distillate was too low to be detected by FTIR. To further study the new compounds formed in the distillation, GC-MS (gas chromatography-mass spectrometry) was used to analyze the concentrated broth. It was found that 3-hydroxy-2-butanone and 2,3-butanedione were detected, which was in accordance with the FTIR results. However, no amide compound was detected probably due to the very low concentration.
C. It indicated that oxidation reaction might take place during distillation, and the ammonium salt might take part in or facilitate the reaction. However, no significant difference between the spectra of distillate and control were found even if the initial pH of the liquid increased from 7 to 12. It was probably because the concentration of chromophores in the distillate was too low to be detected by FTIR. To further study the new compounds formed in the distillation, GC-MS (gas chromatography-mass spectrometry) was used to analyze the concentrated broth. It was found that 3-hydroxy-2-butanone and 2,3-butanedione were detected, which was in accordance with the FTIR results. However, no amide compound was detected probably due to the very low concentration.
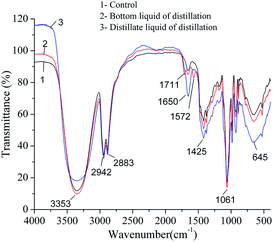 | ||
| Fig. 3 FTIR spectra of concentrated broth, distillate and control sample (prepared by mixing pure 1,3-PD, 2,3-BD, glycerol and water). | ||
3-Hydroxy-2-butanone is one of the main co-products during the biological synthesis of 2,3-BD by Klebsiella pneumoniae. It is the precursor of 2,3-BD and can be transformed into 2,3-BD by acetoin reductase.27 Because 2,3-BD is produced as a co-product during the biological synthesis of 1,3-PD, the presence of 3-hydroxy-2-butanone is also a great possibility. 3-Hydroxy-2-butanone is easy to convert into 2,3-butanedione, which is a yellow liquid. Another possible mechanism for the increase of colority during distillation is the Maillard reaction. The Maillard reaction usually takes place between an amino group and an α-hydroxyl carbonyl moiety of a reducing sugar.28 The amino acid and ammonium salt in the fermentation broth could provide amino groups, while the reducing sugar and other carbonyl compound in the broth such as 3-hydroxy-2-butanone could provide α-hydroxyl carbonyl moiety. However, according to the GC-MS results, Maillard reaction probably is not a main reason for the increase of colority. Moreover, it is also possible that the presence of ammonium salt increases the acidity of the solution, and thus the formation of 2,3-butanedione can be facilitated from 3-hydroxy-2-butanone or even from 2,3-BD. It also has been reported that the acids in the broth can promote the conversion of chromophore precursors to chromophores.29 It thus can be concluded that the increase of the colority of the concentrated broth was mainly due to the presence of ammonium salt, which increases the acidity of solution by hydrolysis (eqn. (2)) and facilitates the formation of chromophores.
| NH4+ + H2O ⇄ NH3·H2O + H+ | (2) |
Therefore, a novel strategy can be made by adjusting the pH of the solution to weaken or eliminate the chromophoric reaction during distillation. The formed salts can be simultaneously removed using a scraped thin-film evaporator. The effects of several factors on simple distillation and STFE were thus further investigated as follows.
Effects of initial pH and pressure on simple distillation
Because 1,3-PD, 2,3-BD and glycerol have high boiling points of 210 °C, 183 °C, and 290 °C, respectively, it is better to recover them by distillation under low pressure to reduce the chromophoric reactions. Therefore, after adjusting the pH of solution, vacuum distillation was carried out at three pressure levels, namely, 0.14 kPa, 2.00 kPa and 4.00 kPa, respectively. Six pH levels in the range of 7–12 were considered. The results on the yields of main products (1,3-PD, 2,3-BD) and glycerol are shown in Fig. 4. Corresponding effects of pH and pressure on distillate colorities (A460) are shown in Fig. 5. It can be known that the recovery yields of 1,3-PD and 2,3-BD were generally higher than 95%, whereas the yield of glycerol was 15–55% depending on distillation pressure. Decreasing pressure was beneficial to the recovery of 1,3-PD and 2,3-BD. pH showed no significant effects on the yields of the main products. However, the distillate colority indeed decreased with an increase of pH. It indicated that alkali pH could really reduce the chromophoric reaction to form the chromophores. This result further corroborated that the formation of chromophores during distillation were acid-catalyzed reactions. | ||
| Fig. 4 The recovery yields of 1,3-PD, 2,3-BD and glycerol at different initial pH and pressure in a simple distillation. (A) At 0.14 kPa, (B) at 2.00 kPa, and (C) at 4.00 kPa. | ||
 | ||
| Fig. 5 The absorbance of distillate at 460 nm (A460) at different initial pH obtained by a simple distillation. | ||
Therefore, it can be known that a relatively high initial pH and low pressure should be employed in distillation to decrease the colority. However, there are still several issues limiting the application of simple distillation in the downstream processing of 1,3-PD recovery, one of which refers to the high salinity of the fermentation broth. Moreover, NaOH was consumed and new salt was formed by adjusting the initial pH of the broth. The salts not only have a negative effect on the colority of distillate but also limit the continuous operation for 1,3-PDO recovery. Therefore, desalination is important for the subsequent rectification for 1,3-PDO purification, and thus a scraped thin-film evaporator was employed to recover 1,3-PDO and 2,3-BD and remove salts continuously.
Effects of pH on scraped thin-film evaporation (STFE)
During STFE, concentrated fermentation broth was continuously pumped into the evaporator. The effects of pH on the recovery yields of 1,3-PD, 2,3-BD and glycerol at a pressure of 0.30 kPa are shown in Fig. 6. The yields of 1,3-PD and 2,3-BD were higher than 97%, and pH showed no significant effects on the yields. This result was similar to that of simple distillation. However, glycerol yield was much considerably than that of simple distillation. This was mainly because that scraped thin-film evaporator could provide more surface to evaporate the liquid, and thus higher efficiency for evaporation was achieved. | ||
| Fig. 6 The recovery yields of 1,3-PD, 2,3-BD and glycerol in the scraped thin-film evaporation at pressure of 0.30 kPa. | ||
The effects of pH on the colority (A460) of distillate obtained by STFE are shown in Fig. 7. The A460 decreased significantly with an increase of pH from 7.0 to 12.0, and the lowest colority was obtained at pH 12. It was also found that the impurity concentrations in distillate were influenced by the pH of feeding liquid. For example, acetic acid, which was a by-product in the fermentation broth produced by the metabolism of the bacteria used for 1,3-PD production, was detected as an impurity in the distillate. When the pHs of feeding liquid were 7, 9 and 10, the acetic acid concentrations were 15.24, 8.06 and 5.16 g L−1, respectively. No acetic acid was detected when pH was higher than 11. This is because the acetic acid concentration in the concentrated broth was controlled by the following equilibrium reaction:
| CH3COOH ⇄ CH3COO− + H+ | (3) |
The pKa of acetic acid is 4.76 at 25 °C, corresponding to a dissociation constant (Ka) of 1.76 × 10−5. In the experiments, the total concentration of acetic acid and acetate in the feeding liquid was 0.747 mol L−1. It thus can be calculated that the concentrations of acetic acid ([HAc]) at pHs of 7, 8, 9, 10, 11, 12, and 12.5 are about 0.255, 0.0255, 0.00255, 0.000255, 2.55 × 10−5, 2.55 × 10−6 and 8.05 × 10−7 g L−1, respectively. [HAc] decreased by 10 times when pH increased by 1 unit. Therefore, an alkali condition is very good for reducing the hydrolysis of acetate to form acetic acid. [HAc] actually can be neglected when pH is 11. Therefore, under acid condition (low pH), most of acetic acid exists as the form of molecule, and thus it was easy to evaporate into the distillate. Increasing pH moves the equilibrium of eqn (3) to the right and thus less acetic acid was detected in the distillate. Removing acetic acid is very important to increase the quality of 1,3-PD product, because acetic acid has a negative effect on co-polymerization of 1,3-PD and terephthalic acid to produce PTT. The existence of acetic acid may terminate the polymerization reaction. According to experimental results, when the initial pH of the feeding liquid was higher than 11, no acetic acid or acetate was detected in the distillate. Therefore, adjusting the initial pH before STFE is also of great importance on this aspect.
It should be noted that decolorization by activated charcoal adsorption is necessary for the post-treatment of rectification product, as shown in Fig. 1, in order to obtain qualified 1,3-PD for producing PTT. It seems that colority control is not necessary for STFE if a post-decolorization was used. However, we have found that the initial pH of concentrated broth showed significant effects on the amount of activated charcoal used for post-treatment (Fig. 8) to produce qualified 1,3-PD (colority < 10 Hazen units). The consumption of activated charcoal was greatly reduced when pH was adjusted from 7 (natural pH of the broth at the end of fermentation) to 12.5. For example, the loading of activated charcoal can be reduced by 60% when initial pH was increased from 7 to 12.5. Nevertheless, pH showed no significant effect on the purity of 1,3-PD. One reason for this was that the rectification column used in the experiments was efficient enough to purify 1,3-PD with high purity (99.8%). The other reason was that the amount of chromophores present in the crude 1,3-PD product was very low even if a dark yellow color was observed. Therefore, the production cost would be reduced greatly by adjusting the initial pH of concentrated broth before desalination using STFE.
 | ||
| Fig. 8 Effect of pH on the weight of activated charcoal used for the decoloration of crude 1,3-PD product. | ||
Conclusions
The salts present in the fermentation broth of 1,3-PD production by microbial fermentation showed negative effects on downstream processing. One of the problems is the increase of product colority after distillation. It was found that (NH4)2SO4 showed the most negative influence on the increase of colority, which was probably due to the decrease of pH (increase of acidity) caused by the hydrolysis of (NH4)2SO4. A novel strategy was thus made by adjusting the initial pH of concentrated broth before distillation and desalination. The experimental results demonstrated that the colority of distillate indeed was decreased at high pH. The optimal pH was found to be 12. Scraped thin-film evaporation was further used for desalination and recovering the main product 1,3-PD and by-product 2,3-BD. Increasing pH from 7 to 12.5 showed no negative effects on the recovery yields of 1,3-PD and 2,3-BD, but greatly decreased the colority of the distillate. It was also found that the acetic acid concentration in the distillate was greatly reduced by increasing the pH. No acetic acid was detected at initial pH higher than 11. The reduction of colority greatly reduced the amount of activated charcoal used in post-treatment to obtain qualified 1,3-PD. The novel strategy is thus very promising for recovering and purifying 1,3-PD from fermentation broth, especially for industrial processing.Acknowledgements
The authors express their gratitude to the support from Tsinghua University Initiative Support Program (2011Z02140, 2012Z98148 and 2012Z02295).Notes and references
- Z. Wang, Z. Wu and T. Tan, Biotechnol. Bioprocess Eng., 2013, 18(4), 697–702 CAS
.
- Z. Song, Y. Sun, B. Wei and X. Z. Eng, Life Sci., 2013, 13(5), 487–495 CrossRef CAS PubMed
.
- J. V. Kurian, J. Polym. Environ., 2005, 13(2), 159–167 CrossRef CAS PubMed
.
- D. Arntg and N. Wiegand, EurPat Appl, EP 412337, 1991
.
- L. H. Slaugh and P. R. Weider, U.S. Pat., US 5256827, 1993
.
- K. D. Allen, J. P. Arhancet and D. C. Eubanks, et al., U.S. Pat., US 5777182, 1998
.
- G. Kaur, A. K. Srivastava and S. Chand, Biochem. Eng. J., 2012, 64, 106–118 CrossRef CAS PubMed
.
- H. S. Wu and Y. J. Wang, Ind. Eng. Chem. Res., 2012, 51(33), 10930–10935 CrossRef CAS
.
- Z. L. Xiu and A. P. Zeng, Appl. Microbiol. Biotechnol., 2008, 78(6), 917–926 CrossRef CAS PubMed
.
- S. Wang, H. Dai, Z. Yan, C. Zhu, L. Huang and B. Fang, Eng. Life Sci., 2014, 14(5), 485–492 CrossRef CAS PubMed
.
- Z. Li, H. Teng and Z. Xiu, Process Biochem., 2011, 46(2), 586–591 CrossRef CAS PubMed
.
- T. Boonsongsawat, A. Shotipruk, V. Tantayakom, P. Prasitchoke, C. Chandavasu and P. Boonnoun, Sep. Sci. Technol., 2010, 45(4), 541–547 CrossRef CAS PubMed
.
- J. Hao, H. Liu and D. Liu, Ind. Eng. Chem. Res., 2005, 44(12), 4380–4385 CrossRef CAS
.
- J. Hao, F. Xu, H. Liu and D. Liu, J. Chem. Technol. Biotechnol., 2006, 81(1), 102–108 CrossRef CAS PubMed
.
- R. C. Wu, Y. Z. Xu, Y. Q. Song, J. A. Luo and D. H. Liu, Sep. Purif. Technol., 2011, 83, 9–14 CrossRef PubMed
.
- D. R. Kelsey, U.S. Pat., US 5527973, 1996
.
- T. T. Ames, U.S. Pat., US 6361983, 2002
.
- Y. Gong, Y. Tang, X. Wang, L. Yu and D. Liu, Desalination, 2004, 161(2), 169–178 CrossRef CAS
.
- J. Malinowski, J. Biotechnol. Tech., 1999, 13(2), 127–130 CrossRef CAS
.
- Y. J. Fang and P. Zhou, Sep. Sci. Technol., 2006, 41(2), 329–340 CrossRef CAS PubMed
.
- J. J. Malinowski, Biotechnol. Progr., 2000, 16(1), 76–79 CrossRef CAS PubMed
.
- M. H. Cho, S. I. Joen, S. H. Pyo, S. Mun and J. H. Kim, Process Biochem., 2006, 41(3), 739–744 CrossRef CAS PubMed
.
- D. Szymanowska-Powałowska, J. Piątkowska and K. Leja, BioMed Res Int., 2013, 2013, 949107, DOI:10.1155/2013/949107
.
- J. Bastrzyk and M. Gryta, Desalin. Water Treat., 2015, 53(2), 319–329 CrossRef CAS PubMed
.
- J. Bastrzyk, M. Gryta and K. Karakulski, Chem. Pap., 2014, 68(6), 757–765 Search PubMed
; L. He, X. Zhao, K. Cheng, Y. Sun and D. Liu, Appl. Biochem. Biotechnol., 2013, 169(1), 312–326 CrossRef CAS PubMed
.
- M. Seapan, G. F. Diffendall, R. E. Trotter, T. T. Ames and F. G. Gallagher, U.S. Pat., US 7211699, 2007
.
- E. Celińska and W. Grajek, Biotechnol. Adv., 2009, 27(6), 715–725 CrossRef PubMed
.
- V. A. Yaylayan, Trends Food Sci. Technol., 1997, 8(1), 13–18 CrossRef CAS
.
- H. B. Sunkara and J. U. I. I. Robert, U.S. Pat., US 6235948. 2001
.
| This journal is © The Royal Society of Chemistry 2015 |


