Green-emitting Lu3Al5O12:Ce3+ phosphor as a visible light amplifier for dye-sensitized solar cells†
Gill Sang Han‡
a,
Young Hyun Song‡a,
Dong Hoe Kimb,
Min-Ji Leea,
Dong Geon Leea,
Se-Hoon Hanc,
Yongjoo Kimc,
Mong-Kwon Jungd,
Dae-Ho Yoon*ae and
Hyun Suk Jung*a
aSchool of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon 440-746, Republic of Korea. E-mail: hsjung1@skku.edu; dhyoon@skku.edu
bDepartment of Materials Science and Engineering, Seoul National University, Seoul 151-744, Republic of Korea
cDongjin Semichem Co., LTD, Hwasung 445-935, Republic of Korea
dHyosung Corporation, R&D Business Labs, Anyang 431-080, Republic of Korea
eSKKU Advanced Institute of Nanotechnology (SAINT), Sungkyunkwan University, Suwon 440-746, Republic of Korea
First published on 26th February 2015
Abstract
A down convertor Lu3Al5O12:Ce3+ (LuAG) phosphor was used as a visible light amplifier for dye-sensitized solar cells (DSSCs). LuAG particles emitting green light with a wavelength of 470 to 650 nm, which is capable of stimulating Ru-dye molecules, enhanced the light-harvesting properties of a DSSC. The average short-circuit current density (Jsc) of the DSSC containing LuAG down conversion layers was approximately 9.6% higher than that of the mesoporous (mp) TiO2-based DSSC. Therefore, the average power conversion efficiency (PCE) for the DSSC with LuAG was 7.5% under AM 1.5 conditions, which was 8.7% higher than that for the mp-TiO2 based DSSC. The superior photovoltaic properties of the DSSC containing LuAG down conversion layers under UV light illumination revealed the effective wavelength down-converting properties of the LuAG down conversion layers.
Introduction
Dye-sensitized solar cells (DSSCs) have received a great deal of attention and have been actively researched owing to their high power conversion efficiency and low production cost, which makes them potential alternative photovoltaic devices to conventional thin-film solar cells.1,2Typical DSSCs are composed of a Ru-dye loaded porous nanocrystalline TiO2 film as the photoelectrode, Pt-coated fluorine tin oxide (FTO) glass as the counter electrode, and an electrolyte solution containing redox iodide/triiodide (I−/I3−) couples.3–6 In particular, photoelectrodes are key components for achieving high efficiencies because light absorption, charge generation, and charge collection take place at the photoelectrode. Significant effort has been made to boost the power conversion efficiency (PCE) of DSSCs by improving the light-harvesting and charge-collecting properties of photoelectrodes.7–10
Since the light penetration depth in nanocrystalline TiO2 film is longer than the electron diffusion length, a light-scattering layer that acts as a mirror has been employed to improve the light-harvesting properties of photoelectrodes. The light-scattering layers are composed of spherical TiO2 particles (>300 nm), which confine the incident light via the Mie scattering mechanism.11 Another way of improving the light-harvesting properties is to adopt a luminescent layer composed of up- or down-conversion phosphor nanoparticles to efficiently utilize the incident photons. Phosphors have the ability to convert the high or low energy of incident photons into energy, which is fairly suitable for the light absorption of the dye molecules.12,13 Recently, a Y3Al5O12:Ce3+ (YAG) phosphor was used as the down conversion layer in DSSCs.12 However, the down-converting effect of the YAG phosphor was not remarkably effective.13 The green emitting Lu3Al5O12:Ce3+ phosphor can be a good candidate as a down conversion layer in the way that it matches the wavelength in the absorption line of N719 dye as well as emits the high green emission.14
In the present study, we fabricated an incident light wavelength-tunable multi layered photoelectrode using a Lu3Al5O12:Ce3+ (LuAG) down conversion layer. The LuAG phosphor particles synthesized via the solid–state reaction method absorbed the 350 and 450 nm and emitted visible green light with a peak wavelength of 530 nm. That conversion resulted in excess charge generation from the N719 dye molecules. The DSSC with a LuAG/mesoporous-TiO2 multi layered electrode showed a PCE of 7.5% as compared to the cell without a down conversion layer (6.9%). The effect of the down-conversion characteristics of LuAG was confirmed by the superior photovoltaic properties for the DSSC containing a LuAG down conversion layer under UV light illumination.
Experimental
Raw materials
Lu2O3 (High Purity Chemicals, 99.99%), Al2O3 (High Purity Chemicals, 99.99%), CeO2 (High Purity Chemicals, 99.9%), and H3BO3 (High Purity Chemicals, 99.9%) were used for the synthesis of green-emitting Lu3Al5O12 phosphors using a CeO2@B2O3 core–shell structure.Synthesis of Lu3Al5O12:Ce3+ phosphors using CeO2@B2O3 core–shell structure
The Lu3Al5O12:Ce3+(LuAG) phosphors were mixed with Lu2O3, Al2O3, and CeO2@B2O3 with a core–shell structure. First, CeO2@B2O3 particles were prepared by mixing CeO2 and H3BO3 powder in a flask with de-ionized water. The solution was magnetically stirred at 80 °C for 5 h. This mixture was dried at 100 °C for 24 h and then annealed at 800 °C for 7 h. The prepared powder was ground using an agate mortar for 30 min and then horizontally loaded into a tubular alumina furnace with an inner diameter of 50 mm. The temperature was increased to 1550 °C and maintained for 7 h in a reducing nitrogen atmosphere containing 5% H2 gas.Fabrication of DSSC
Nanocrystalline TiO2 particles (Dongjin Semichem Co. Ltd.) for the photoelectrode were doctor-bladed onto fluorine-doped tin oxide (FTO, Pilkington, TEC 8) and annealed at 450 °C in air for 1 h. For the LuAG/TiO2 based DSSCs, LuAG particles were doctor-bladed onto an mesoporous (mp) TiO2/FTO photoelectrode and annealed at 450 °C in air for 1 h, followed by N719 dye loading in a 5 × 10−4 M solution of ethanol overnight. Finally, a sandwich-type DSSC was fabricated with a Pt-coated FTO and an electrolyte (Iodolyte AN-50).Characterization
Cathodoluminescence (CL, Hitachi, S-4300SE) was employed to analyze the uniform luminescence area within the Lu3Al5O12:Ce3+ phosphor. The optical properties were analyzed using a room-temperature photoluminescence spectrometer (PL, PSI Co., Ltd./Korea) equipped with a 500 W Xenon discharge lamp as an excitation source. The thicknesses of the photoelectrodes were observed using a field-emission scanning electron microscope (FE-SEM, JSM 7600F, JEOL). Photovoltaic properties were measured by using a potentiostat (CHI660, CHI instrument), a solar simulator (Oriel Sol 3A class AAA, Newport), and a UV lamp at 365 nm. Optical reflectance and transmittance spectra were recorded using a UV-vis spectrometer (Lamda 35, Perkin-Elmer). The amount of dye molecules was determined by the measuring the desorbed dye in an alkaline alcoholic solution.Results and discussion
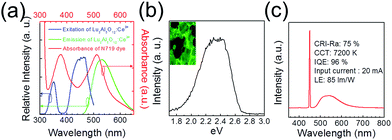
The synthesized phosphor materials showed the typical excitation and emission photoluminescence (PL) spectra of the LuAG phosphor. As shown in Fig. 1(a), two excitation spectra, i.e., a weak band with a maximum at 345 nm and a strong broad band from 400 to 500 nm, with a maximum peak at 460 nm, are associated with the electronic transition of Ce3+ ions from the 4f ground states 2F5/2 and 2F7/2 to the different crystal field splitting of the excited 5d state (ESI1†).15,16 The broad emission spectrum from 470 to 650 nm, with a maximum peak at 530 nm, is in good agreement with the spectra of typical LuAG phosphors. The emission spectrum is ascribed to the electron transition from the lowest crystal field-splitting component of the 5d level to the 4f ground state of Ce3+.17 Fig. 1(b) presents the cathode luminescence (CL) spectra, which is in a range similar to that of the PL spectra. The CL image shown in the inset of Fig. 1(b) exhibits uniform luminescence. The performance of the LuAG phosphor, the electroluminescence (EL) spectrum was analyzed in detail, as shown in Fig. 1(c). The EL spectrum consists of a blue LED chip of 450 nm and a LuAG phosphor whose quantum yield was 96%.Fig. 2(a) shows the cross-sectional image of the mp-TiO2 active layer with a thickness of approximately 11.5 μm. The total thickness of the LuAG particle-coated photoelectrode is approximately 20 μm (Fig. 2(b)). The SEM image demonstrates that the LuAG particles were uniformly coated on the mp-TiO2 active layer.
 | ||
| Fig. 2 Cross-sectional SEM images of (a) mp-TiO2 on FTO glass and (b) LuAG down conversion layer/mp-TiO2/FTO photoelectrodes. | ||
Fig. 3(a) shows the photocurrent–voltage (J–V) curves for the pristine mp-TiO2 and LuAG/mp-TiO2 based DSSCs under an irradiance of AM 1.5. The average values of short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and power conversion efficiency (PCE) with standard deviations are listed in Table 1. The PCE of the LuAG/mp-TiO2 based DSSC (7.5%) is approximately 8.7% higher than that of the mp-TiO2 based DSSC (6.9%) under an irradiance of AM 1.5. The significantly increased Jsc from 13.6 to 14.9 mA cm−2 (an approximately 9.6% increase) is responsible for the improved PCE. Also, the incident photon-to-current efficiency (IPCE) for the LuAG/mp-TiO2-based DSSC is higher than that of the mp-TiO2 based DSSC in terms of the overall wavelength (Fig. 3(b)).
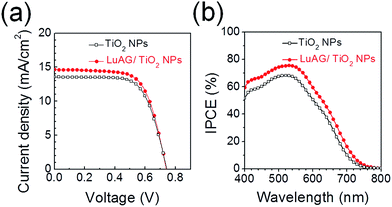 | ||
| Fig. 3 (a) The photovoltaic J–V curves and (b) IPCE data of the mp-TiO2-and LuAG/mp-TiO2-based DSSCs. | ||
| Jsc (mA cm−2) | Voc (V) | FF (%) | η (%) | |
|---|---|---|---|---|
| TiO2 NPs | 13.6 ± 0.5 | 0.75 ± 0.01 | 67.9 ± 1.1 | 6.9 ± 0.3 |
| LuAG/TiO2 NPs | 14.9 ± 0.7 | 0.75 ± 0.01 | 67.1 ± 1.4 | 7.5 ± 0.5 |
To understand the effect of the LuAG layer on the photovoltaic properties of the DSSC, the electrochemical impedance spectra (EIS), which provides information on the interfaces in DSSCs, were measured and the results are plotted in Fig. 4(a). Each semicircle and the total series resistance of both cells are almost identical, which indicates that the charge transport and recombination characteristics for both DSSCs are almost identical. Moreover, the Bode plots for the two DSSCs exhibit the same frequencies at maximum peaks, confirming the identical electron lifetimes (Fig. 4(b)).18,19 The similar EIS data for the DSSCs demonstrate that the LuAG layer does not affect the charge transport and recombination properties of the DSSCs, which is consistent with the fact that the Voc and FF values for the DSSCs are identical (Table 1).
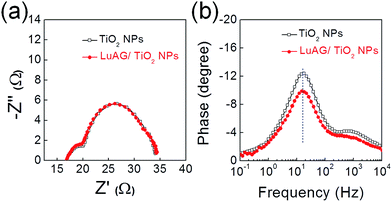 | ||
| Fig. 4 (a) Nyquist plots and (b) plots of imaginary parts of the impedance as a function of frequency of mp-TiO2 and LuAG/mp-TiO2- based DSSCs. | ||
The short-circuit current density is in proportion to the product of the light-harvesting efficiency (ηlh), injection efficiency (ηinj), and charge-collection efficiency (ηcc), which is expressed as follows:20
| Jsc ∝ ηlhηinjηcc |
The electron-injection efficiency and charge-collection efficiency can be ignored because the same dye molecules (N719 dye) and TiO2 nanoparticles, respectively, were employed. Therefore, the difference in the photocurrent densities of the LuAG/mp-TiO2 and mp-TiO2 based DSSCs originates from the difference in light-harvesting efficiency. In this study, three factors may have had an impact on the light-harvesting efficiency: (1) the amount of dye loading, (2) the light-scattering capability of LuAG particles, and (3) the down-conversion effect.
As shown in Fig. 5(a), the UV-vis absorption spectra of the desorbed dye solutions obtained by immersing both photoelectrodes in an alkaline solution clearly show that the amounts of dye loading on both the LuAG/mp-TiO2 and mp-TiO2 based photoelectrodes are identical. We also measured the reflectance of the photoelectrodes, which is related to the light-scattering capability (Fig. 5(b)). Given that an efficient light-scattering layer exhibits more than 55% optical reflectance,9 the optical reflectance for the LuAG/mp-TiO2 based DSSC is slightly higher than that for the mp-TiO2 based one, which is below 30% across the whole range of wavelength. The light-scattering capability of LuAG layers can also enhance the photocurrent density of the LuAG/mp-TiO2 based DSSC. The IPCE (Fig. 3(b)) supports the enhanced quantum efficiency at longer wavelengths. These results demonstrate that the light scattering effect of LuAG layers cannot be ignored.
In order to confirm the down-conversion effect of the LuAG down conversion layer, we calculated the integrated Jsc values based on IPCE data from 300 to 600 nm, as shown in Fig. 5(c). The mp-TiO2 and LuAG/mp-TiO2 based DSSC show the integrated Jsc of 7.99 mA cm−2 and 8.96 mA cm−2, respectively. We also measured J–V curves under UV irradiation alone at maximum peak wavelength of approximately 365 nm (0.283 Sun), as presented in Fig. 5(d). The mp-TiO2 based DSSC had values of 5.25 mA cm−2, 0.64 V, 54.5%, and 1.83% for Jsc, Voc, FF, and PCE, respectively. On the other hand, the LuAG/mp-TiO2 based DSSC showed respective values of 8.53 mA cm−2, 0.67 V, 52.4%, and 3.01% (Table 2). Although the amount of dye loading for each DSSC was identical, the PCE of the LuAG/mp-TiO2 based DSSCs was 64% higher than that of the mp-TiO2 based DSSC owing to the 62% increase in the short-circuit current density under UV illumination. These results indicate that the LuAG phosphors absorbed UV light and converted the UV light into visible light (532 nm). This amplified visible light is much more suitable for stimulating Ru-dye molecules. ESI2† shows the spectral energy distribution for the 365 nm UV lamp. The transmittances of FTO glass and mp-TiO2 (11.5 μm) on FTO glass are approximately 82.7% and 50.2%, respectively, at 365 nm. These results show that the FTO glass cannot cut off the 365 nm UV light and that approximately 50% of the incident UV light that passed through the mp-TiO2 on FTO glass is capable of stimulating the LuAG layer, contributing to the improved light-harvesting properties of DSSCs.
| Jsc (mA cm−2) | Voc (mV) | FF (%) | η (%) | |
|---|---|---|---|---|
| TiO2 NPs | 5.25 | 0.64 | 54.5 | 1.83 |
| LuAG/TiO2 NPs | 8.53 | 0.67 | 52.4 | 3.01 |
Conclusions
In summary, we prepared a green-light-emitting Lu3Al5O12:Ce3+ (LuAG) phosphor with a size of 2–8 μm. This phosphor absorbed 345 and 460 nm UV light and emitted 532 nm visible light, which is suitable for stimulating N719 dye molecules. These LuAG particles were applied to DSSCs as down-conversion optical layers. The employment of a LuAG overlayer led to a significant increase of short-circuit current density (Jsc) under AM 1.5 conditions, consequently improving the power conversion efficiency (PCE) by 8.7% in comparison with the mp-TiO2 based DSSC. The incident photon-to-current efficiency (IPCE) data showed that the LuAG overlayer improved the light-harvesting properties of the DSSC. The combination of J–V curve measurements of the DSSCs under 365 nm UV illumination and transmittance measurements of the photoelectrodes clearly demonstrated that the enhancement of the light-harvesting property of the LuAG/TiO2 based DSSC is ascribed to the down-conversion effect of the LuAG phosphor amplifying 532 nm visible light, which is much more suitable for stimulating Ru-dye molecules. These results demonstrate that the LuAG phosphor is another alternative down-conversion material that is capable of improving the light-harvesting efficiency of DSSCs.Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea under contract no. NRF-2014R1A2A2A01007722. This research was also supported by the Nano.Material Technology Development Program through National Research Foundation of Korea (NRF) grants funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea (2012M3A7B4049967) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2059280). This work was supported by the industrial strategic technology development program (10041041, Development of non-vacuum and non-lithography based 5 mm width Cu interconnect technology for TFT backplane) funded by the Ministry of Knowledge Economy (MKE, Korea).Notes and references
- B. O'regan and M. Grätzel, Nature, 1991, 353, 797–740 CrossRef.
- H. S. Jung and J. K. Lee, J. Phys. Chem. Lett., 2013, 4, 1682 CrossRef CAS.
- N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338 CrossRef CAS PubMed.
- A. J. Frank, N. Kopidakis and J. van de Lagemaat, Coord. Chem. Rev., 2004, 248, 1165–1179 CrossRef CAS PubMed.
- M. Quintana, T. Edvinsson, A. Hagfeldt and G. Boschloo, J. Phys. Chem. C, 2007, 111, 1035 CAS.
- X. Fang, T. Ma, G. Guan, M. Akiyama, T. Kida and E. Abe, J. Electroanal. Chem., 2004, 570, 257–263 CrossRef CAS PubMed.
- M. Gratzel, Inorg. Chem., 2005, 44, 178–180 CrossRef PubMed.
- J. K. Lee and M. J. Yang, Mater. Sci. Eng., B, 2011, 176, 1142–1160 CrossRef CAS PubMed.
- S. H. Han, S. Lee, H. Shin and H. S. Jung, Adv. Energy Mater., 2011, 1, 546–550 CrossRef CAS.
- G. S. Han, S. Lee, J. H. Noh, H. S. Chung, J. H. Park, B. S. Swain, J.-H. Im, N.-G. Park and H. S. Jung, Nanoscale, 2014, 6, 6127 RSC.
- Q. Fu and W. Sun, Appl. Opt., 2001, 4, 1354 CrossRef.
- G. Zhu, X. Wang, H. Li, L. Pan, H. Sun, X. Liu, T. Lv and Z. Sun, Chem. Commun., 2012, 48, 958–960 RSC.
- W. Z. He, T. S. Atabaev, H. K. Kim and Y. H. Hwang, J. Phys. Chem. C, 2013, 117, 17894–17900 CAS.
- K. Blazek, A. Krasnikov, K. Nejezchleb, M. Nikl, T. Savikhina and S. Zazubovich, Phys. Status Solidi B, 2004, 241, 1134–1140 CrossRef CAS.
- W. Z. He, T. S. Atabaev, H. K. Kim and Y. H. Hwang, J. Phys. Chem. C, 2013, 117, 17894–17900 CAS.
- A. Birkel, K. A. Denault, N. C. George, C. E. Doll, B. Hery, A. A. Mikhailovsky, C. S. Birkel, B.-C. Hong and R. Seshadri, Chem. Mater., 2012, 24, 1198–1204 CrossRef CAS.
- J. Xu, Y. Shi, J. Xie and F. Lei, J. Am. Ceram. Soc., 2013, 96, 1930–1936 CrossRef CAS PubMed.
- T. Hoshikawa, M. Yamada, T. Kikuchi and K. Eguchi, J. Electrochem. Soc., 2005, 152, E68 CrossRef CAS PubMed.
- J. Van de Lagemaat, N.-G. Park and A. J. Frank, J. Phys. Chem. B, 2000, 104, 2044 CrossRef CAS.
- M. Gratzel, Inorg. Chem., 2005, 44, 6841 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The crystal field splitting diagram of Ce3+ ions, and UV-vis transmittance of the FTO glass, mp-TiO2 layer (11.5 μm) on FTO glass and spectral energy distribution of UV lamp. See DOI: 10.1039/c5ra00378d |
| ‡ G. S. Han and Y. H. Song contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |