New insight into rare-earth doped gadolinium molybdate nanophosphor assisted broad spectral converters from UV to NIR for silicon solar cells†
Pawan Kumarab and
Bipin Kumar Gupta*a
aCSIR – National Physical Laboratory, Dr K S Krishnan Road, New Delhi, 110012, India. E-mail: bipinbhu@yahoo.com; Fax: +91-11-45609310; Tel: +91-11-45609385
bAcademy of Scientific and Innovative Research (AcSIR), CSIR – National Physical Laboratory, New Delhi – 110012, India
First published on 19th February 2015
Abstract
We have successfully synthesized rare-earth doped gadolinium molybdate Gd2(MoO4)3:Re3+ (Re3+ = Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphors for solar cell application as a broad spectral converter from the ultraviolet (UV) to the near infrared (NIR) regions in a single host lattice using a facile solid state reaction method. The gross structure, surface morphology and microstructure of these nanophosphors have been investigated by X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and transmission/high-resolution transmission electron microscopy (TEM/HRTEM) techniques , respectively. Photoluminescence (PL) and time-resolved photoluminescence (TRPL) spectroscopic methods have been used to explore the striking luminescence properties of the synthesized nanophosphors. The Gd2(MoO4)3:Eu3+ nanophosphor exhibits a hypersensitive red emission (616 nm) at an excitation wavelength in the range of 250–475 nm corresponding to a 5D0–7F2 transition. The Gd2(MoO4)3:Tb3+ and Gd2(MoO4)3:Tm3+ nanophosphors demonstrate a strong green emission at 541 nm and a deep blue emission at 453 nm upon an excitation wavelength of 378 nm and 266 nm, respectively. Moreover, the upconversion characteristic of the Gd2(MoO4)3:Er3+/Yb3+ nanophosphor exhibits strong green emission at 545 nm and red emission at 657 nm corresponding to 4S3/2–4I15/2 and 4F9/2–4I15/2 transitions respectively. Furthermore, the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor emits in the NIR spectrum region at 994 nm upon a 980 nm excitation wavelength. Hence, the obtained PL emission results with a lifetime in milliseconds reveal that these nanophosphors could be futuristic promising broad spectral converter phosphors which may possibly integrate with the next-generation Si-solar cell to enhance the efficiency of the cell.
1. Introduction
Energy is directly related to the currency of any country. There is no doubt that it is always in huge demand, but unluckily, it is always in short supply which is insufficient to match the unparalleled population explosion and our changing comfort lifestyle. Undeniably, we are facing an energy crisis and it has become the most important commodity. Energy consumption will have almost doubled by 2050 compared to the energy consumption in 2001.1,2 Consequently, renewable energy sources that can provide sufficient energy to meet the worldwide energy demand have attained huge attention.1–5 Sunlight is the most important renewable source of energy which can be directly converted into heat and electricity. Moreover, sunlight is a plentiful source of energy; available almost everywhere in the world without any cost.5 The photovoltaic cells which convert sunlight directly into electricity are the most prominent source of renewable energy. However, photovoltaic cells are only contributing a limited portion of the energy demand. Therefore, over the past few decades, significant effort has been directed to the development of efficient photovoltaic cells. Still, the efficient and economic cost conversion of sunlight into electricity remains a challenging task.6–8 Commercial availability and the economic approach of crystalline and polycrystalline solar cells have been dominating the photovoltaic market to date.9,10 Therefore, an immense amount of research has been devoted to the development of efficient and low cost crystalline and polycrystalline solar cells in the past few decades.A major drawback of Si-solar cells is that they don’t utilize the full solar spectrum when sunlight falls on their surface which limits the energy conversion efficiency. It is well established that the solar spectrum has photons ranging from 250–2500 nm (ultraviolet to infrared) at air mass 1.5 global (AM 1.5G). But, photovoltaic cells only utilize a small portion of the solar spectrum which is attributed only to photons that match the band gap of the material.11 In general; there are two kinds of loss in solar cells which limit their efficiency. One is that the photons with an energy higher than the band gap (UV radiations) are not efficiently used; the excess energy of the photons is dissipated in the form of heat. Secondly, the photons with low energy (IR radiation) are not absorbed by solar cells and these are transmitted. It is observed that the 70% loss in solar cells is related to these losses, known as spectral mismatch. One of the promising ways to address this issue is by using downshift (DS) or upconversion (UC) materials as a spectral converter to minimise these losses (thermalisation and non-absorbed losses). A downshift material can absorb the photons with high energy and emit photons that have a lower energy in the visible spectrum region which are subsequently absorbed by the solar cell without any heat dissipation. Upconversion materials absorb two photons that have an energy less than that of the band gap of the solar cell and are converted into a utilized photon (having energy in the visible spectrum region) which is absorbed by the solar cell. This approach to minimising the thermalisation and non-absorbed losses by applying a luminescent layer on the solar cell is termed as third generation solar conversion.5 Although there are many materials that have been proposed as luminescence concentrators for the enhancement of solar cell efficiency, lanthanide doped materials are the most suitable materials for solar spectral converters due to their electron rich energy level structure which offers a superficial photon management. Therefore, significant effort has been dedicated to the enhancement of the efficiency of solar cells via modification of the solar spectrum using trivalent lanthanide doped materials.12–23
Among the various trivalent lanthanide ions (Ln3+), materials doped with Eu3+/Er3+ ions (downshift/upconversion phosphors) have gained more inquisitiveness as spectral converters. These materials can emit photons in the visible spectrum which are useful in solar cell applications to create electron–hole pairs. Moreover, host lattices containing d-block transition elements (like tungstate and molybdate) have an advantage over other host lattices because the excitation band is broad due to the charge transfer (CT) in the O–Mo or O–W bond occurring in the UV region, which is sufficiently transferred to the trivalent lanthanide ion.24 Furthermore, the Gd2(MoO4)3 host matrix possesses beneficial properties such as a high refractive index, high thermal stability, low toxicity and high photochemical stability.
In the present investigation, we have synthesized Gd2(MoO4)3:Re3+ (Re3+ = Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphors for a proposed solar cell application where these nanophosphors act as a spectral converter nanophosphor. These nanophosphors have the capability to absorb light in a broad range from UV to IR and can emit red, green and blue emission in the visible and NIR regions. The Gd2(MoO4)3:Eu3+ downshift nanophosphor demonstrates a photoluminescence emission peak at 616 nm (hypersensitive red emission) upon broad excitation ranging from 250–475 nm. Moreover, other two downshift nanophosphors Gd2(MoO4)3:Tb3+ and Gd2(MoO4)3:Tm3+ exhibit the emission peaks at 541 nm (strong green) and 453 nm (deep blue) respectively. Furthermore, the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor shows strong green emission at 545 nm and red emission at 657 nm.
Additionally, the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor exhibits emission at 994 nm (NIR region) upon a 980 nm excitation wavelength, which is rarely reported in the literature.25,26 Thus, Gd2(MoO4)3:Re3+ (Re3+, Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphors could be efficiently used as spectral converters, which can absorb light in the 250–475 nm/NIR spectrum region and emit in the visible and NIR region which is highly desirable for the modification of the Si-solar cell spectrum. Hence, the obtained results provide a new podium of Gd2(MoO4)3:Re3+ (Re3+, Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphors to examine their interesting photoluminescence properties in detail for a proposed Si-solar cell application.
2. Experimental
2.1 Materials
The precursors; Y2O3 (99.99%), Eu2O3 (99.99%), Tm2O3 (99.99%), Tb4O7 (99.99%), Er(NO3)3·5H2O (99.99%), Yb2O3 (99.99%) and (NH4)6Mo7O24·4H2O (AR grade, 99%) were purchased from Sigma-Aldrich. All reagents were of analytical (AR) grade and used as received without further purification.2.2 Synthesis of Gd2(MoO4)3:Re3+ (Re3+, Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphors
A facile solid state reaction was used for the synthesis of Gd2(MoO4)3:Re3+ (Re3+, Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) which can be easily scaled-up for large quantities. In order to optimize the concentration and temperature, first we synthesized the red emitting Gd2−x(MoO4)3:Eux3+ (x = 0.1 to 0.5) nanophosphor using a solid state reaction method. According to the stoichiometric ratio, the starting materials Gd2O3, Eu2O3, and (NH4)6Mo7O24·4H2O were taken. After precise weighing of these materials, the materials were appropriately crushed in an agate mortar for homogeneous mixing. The homogeneous mixture was kept in an alumina crucible and heated in a box furnace at a temperature of 1000 °C for 3 h. The doping concentration of Eu3+ was varied (x = 0.1 to 0.5) in order to attain the optimum dopant concentration for the strongest red emission and it was observed that x = 0.2 is the optimum concentration. Furthermore, in order to compare the luminescence intensity with sintering temperature, the Gd1.8(MoO4)3:Eu0.23+ phosphor was heated at 800 °C, 900 °C, 1000 °C and 1100 °C. The green and blue nanophosphors were synthesized by doping with different rare-earth ions (Tb3+ and Tm3+). The nanophosphor was doped with Tb3+ ions were doped to achieve the green emission and Tm3+ ions for blue emission. Similarly, the Gd2(MoO4)3:Er3+/Yb3+ (Er3+ = 5 mol% and Yb3+ = 10 mol%) upconversion nanophosphor were also synthesized by a solid state reaction method keeping the same conditions as for the downshift nanophosphors. Using this method, the yield of the materials was more than 90% in all cases (downshift and upconversion nanophosphors) with a high degree of homogeneity throughout the mass. The versatility of this method is such that one could easily synthesize large quantities of homogeneous rare earth doped nanophosphors with a narrow size distribution.2.3 Characterization
The crystal structure was investigated using X-ray powder diffraction (XRD) with a Bruker AXS D8 Advance X-ray diffractometer, using Cu Kα1 radiation (λ = 1.5406 Å). The surface morphology was studied using Carl ZEISS EVOR-18 equipment at a 10 kV operating voltage. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) micrographs were recorded using a Tecnai G2 S-Twin transmission electron microscope with a field emission gun operating at 300 kV. An Edinburgh spectrometer was used for photoluminescence (PL) and time-resolved photoluminescence (TRPL) spectroscopy, where a xenon lamp acted as the source of excitation. To estimate the absolute luminescence quantum efficiency of the QC phosphors, an integrating sphere equipped with an Edinburgh spectrometer (model F900) instrument was used, and then the integrated fractions of luminous flux and radiant flux were measured using the standard method; quantum efficiency was evaluated. The NIR PL emission and PL mapping of the nanophosphors was performed using a WITech alpha 300R+ confocal PL microscope system, where 375 and 980 nm diode lasers act as source of excitations.3. Results and discussion
A facile solid state reaction method has been used to synthesize Gd2(MoO4)3:Re3+ (Re3+, Eu3+, Tb3+, Tm3+ and Er3+/Yb3+). The X-ray diffraction (XRD) technique has been carried out to investigate the gross structure and phase purity of the nanophosphors. The XRD pattern of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor is illustrated in Fig. 1(a). The XRD result reveals that the nanophosphor has an orthorhombic crystalline structure with space group Pba2. The estimated lattice parameters for the Gd1.8(MoO4)3:Eu0.23+ nanophosphor are a = (10.1631 ± 0.0023) Å, b = (10.1132 ± 0.0021) Å and c = (10.74 ± 0.0027) Å, which are comparable with the existing Gd2(MoO4)3 host (JCPDS card no. 20-0408). The estimated crystallite size of the Gd1.8(MoO4)3:Eu0.23+ nanophosphor is ∼28 nm using the standard Scherrer formula. The XRD patterns of Gd2−x(MoO4)3:Eux3+ downshift nanophosphors for different concentrations (x = 0.1 to 0.5) are demonstrated in Fig. S1 (see ESI†). The lattice parameters for all variants of the Gd2−x(MoO4)3:Eux3+ nanophosphors (x = 0.1 to 0.5) were calculated from the observed d-values through a least square fitting method using computer program based unit cell refinement software.27 The unit cell volume was estimated from these parameters and is exhibited in Table S1.† It was observed that the unit cell volume increased with Eu3+-doping concentration up to a value of 0.2 and then after this it started to decrease. The decrease in the unit cell volume decreases the inter-ionic distance between the Eu3+ ions which leads to an increase in non-radiative emission which decreases the luminescence intensity. Furthermore, the obtained results for the different concentrations (x = 0.1 to 0.5) of Gd2−x(MoO4)3:Eux3+ nanophosphors, with their unit cell parameters, are consistent with the obtained PL emission results. Therefore, the emission intensity is maximum for doping concentration x = 0.2. Fig. S2† exhibits the XRD patterns of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor at different sintering temperatures (800 °C, 900 °C, 1000 °C and 1100 °C).28 The obtained XRD patterns of the Gd1.8(MoO4)3:Eu0.23+ nanophosphor at different sintering temperatures reveals that the relative peak intensity of Gd1.8(MoO4)3:Eu0.23+ increases when the sintering temperature increases from 800 to 1000 °C. Above 1000 °C, the secondary phases start to appear, and as a result it affects the PL intensity of the Gd1.8(MoO4)3:Eu0.23+ nanophosphor, which is further discussed in detail in the PL section. Fig. 1(b) demonstrates the XRD pattern of the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor. The XRD patterns of the Gd1.8(MoO4)3:Tb0.23+ and Gd1.8(MoO4)3:Tm0.23+ downshift nanophosphors are illustrated in Fig. S3 (see ESI†). | ||
| Fig. 1 The XRD patterns of the (a) Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor and (b) Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor. | ||
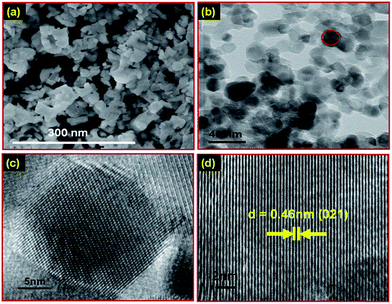
Scanning electron microscopy (SEM) has been used to explore the surface morphology of the synthesized nanophosphors. Fig. 2(a) exhibits the SEM image of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor. To gain more information about the microstructure of the Gd1.8(MoO4)3:Eu0.23+ nanophosphor, transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) was performed. The TEM image of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor is shown in Fig. 2(b). The TEM image shows that the average particle size of the nanophosphor is ∼24 nm. The size distribution histogram of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor is shown in Fig. S4 (see ESI†). The HRTEM image of a selected individual nanoparticle (marked in Fig. 2(b)) is shown in Fig. 2(c). Fig. 2(d) represents that the HRTEM image of the nanophosphor exhibits the clear lattice fringes without any distortion, which confirms that the nanophosphor has good crystal quality. The estimated d-spacing is measured to be ∼0.46 nm from Fig. 2(d), which is analogous to the d-spacing corresponding to the (021) plane (JCPDS card no. 20-0408). Moreover, the selected area electron diffraction (SAED) pattern of the Gd1.8(MoO4)3:Eu0.23+ nanophosphor was also obtained. Fig. S5 (see ESI†) demonstrates the SAED pattern of Gd1.8(MoO4)3:Eu0.23+ which clearly exhibits that the nanophosphor is highly crystalline. Furthermore, energy dispersive X-ray analysis (EDAX) was performed for elemental detection. Fig. S6 (see ESI†) shows the EDAX spectrum of the Gd2(MoO4)3:Eu0.23+ downshift nanophosphor which confirms the presence of Gd, Mo, O and Eu. Moreover, the elemental analysis for the upconversion nanophosphor was also performed. The EDAX spectrum of the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor is shown in Fig. S7 (see ESI†) which confirms the presence of Gd, Mo, O, Er and Yb.
Furthermore, PL and TRPL spectroscopy have been carried out to explore the spectroscopic characteristics of the synthesized nanophosphor to examine the feasibility of the nanophosphor for the proposed spectral converter application. The photoluminescence excitation (PLE) spectrum of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor is illustrated in Fig. 3(a). The excitation spectrum has a broad band around 290 nm and sharp peaks in the range of 350–475 nm. The broad band at ∼290 nm is due to the energy charge transfer between Mo–O. It is well known that a transition metal containing host matrix (like VO43−, NbO43−, WO42− and MoO42− group containing host matrices) has an additional advantage over the other host matrices as it can absorb a broad range in the UV spectrum region due to the charge transfer between the electron deficient transition metal ion and electron rich oxygen ion.24,29 Therefore, the energy transfer between the transition metal atom and the oxygen atom is a very important factor to enhance the luminescence properties of inorganic phosphors. The sharp peaks are characteristics of the f–f transition within the 4f6 electron shell of the Eu3+ ion in the host lattices. The two strong excitation peaks at 395 nm and 465 nm are attributed to the 7F0–5L6 and 7F0–5D2 transitions of the Eu3+ ion, respectively.30 Fig. 3(b) depicts the emission spectrum of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor at an excitation wavelength of 395 nm. The emission spectrum shows hyperfine red emission at 616 nm with a quantum efficiency of ∼84%. The emission peaks at 589 nm, 596 nm, 616 nm, 623 nm and 701 nm are attributed to 5D0–7F1, 5D0–7F1, 5D0–7F2, 5D0–7F2 and 5D0–7F4 transitions, respectively. The strong red emission of the nanophosphor has been obtained by optimising the doping concentration of Eu3+ ions. Fig. 3(c) reveals the variation in luminescence intensity with the doping concentration of Eu3+ ions. It has been observed that the luminescence intensity increases with an increase in the doping concentration of Eu3+ up to 10 mol%. However, with a further increase of Eu3+ concentration, the luminescence intensity starts to decrease and it decreases rapidly after 20 mol% concentration. The decrease in luminescence intensity with an increase in Eu3+ concentration is due to the decrease in the distance between two Eu3+ ions in the host matrix. This decrease in the distance between the Eu3+ ions reduces the probability of the radiative transitions of the Eu3+ ions due to mutual interaction at the shortened distances between two Eu3+ ions at high doping concentrations which leads to non-radiative emission which decreases luminescence intensity. Therefore, the obtained result suggests that 10 mol% is the optimised doping concentration. In addition, the Gd1.8(MoO4)3:Eu0.23+ nanophosphor has been sintered at different temperatures (800 to 1100 °C) for optimization of the sintering temperature for luminescence intensity. The variation in luminescence intensity with sintering temperature is shown in Fig. S8 (see ESI†). It has been observed that 1000 °C is the optimum temperature having the highest luminescence intensity. Furthermore, the PL emission intensity of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor decreases at 1100 °C and above. The decrease in PL emission intensity at 1100 °C is due to the formation of secondary phases at high temperatures which was earlier observed in the XRD pattern of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor at 1100 °C. Fig. 3(d) exhibits the emission spectra of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor at different excitation wavelengths. It shows that a 395 nm excitation wavelength has the maximum emission intensity which signifies that 395 nm is the prominent excitation wavelength for a maximum emission intensity. The proposed energy level diagram for the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor has been illustrated in Fig. S9 (see ESI†) which explicated the downshift mechanism for the luminescence process in the Gd1.8(MoO4)3:Eu0.23+ nanophosphor.
Moreover, the photoluminescence properties of the Gd1.8(MoO4)3:Tb0.23+ and Gd1.8(MoO4)3:Tm0.23+ downshift nanophosphors have also been carried out to explore the spectroscopic characteristics of the synthesized nanophosphors to examine the feasibility of these nanophosphors with different dopants for possible spectral converter application. Fig. S10 (see ESI†) exhibits the photoluminescence properties of the Gd1.8(MoO4)3:Tb0.23+ and Gd1.8(MoO4)3:Tm0.23+ downshift nanophosphors. Fig. S10(a)† shows that the emission spectrum of the Gd1.8(MoO4)3:Tb0.23+ downshift nanophosphor has strong green emission at 541 nm upon excitation at 378 nm. Fig. S10(b)† shows the excitation spectrum of the Gd1.8(MoO4)3:Tb0.23+ downshift nanophosphors at emission of 541 nm. The emission spectrum of the Gd1.8(MoO4)3:Tm0.23+ downshift nanophosphor is illustrated in Fig. S11(c).† The emission spectrum of the Gd1.8(MoO4)3:Tm0.23+ downshift nanophosphor shows hypersensitive blue emission at 453 nm upon excitation at 266 nm. Fig. S10(d)† shows the excitation spectrum of the Gd1.8(MoO4)3:Tm0.23+ downshift nanophosphor at emission of 453 nm. The TRPL technique is an imperative and non-destructive tool which has been used to determine the decay lifetime of the nanophosphor. The lifetime data of a nanophosphor is very important and it helps to decide a suitable application for the nanophosphor. Fig. S11(a) (see ESI†) shows the decay profile of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor at emission of 616 nm upon an excitation wavelength of 395 nm for 7F0–5D2 transitions of Eu3+ ions. The inset in Fig. S11(a)† exhibits the exponential fitting parameters of the decay profile of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor. The decay profile has been best fitted to the double exponential function as described in eqn (1):31
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (1) |
The obtained parameters after the double exponential fitting are listed in the inset of Fig. S11(a).† The decay lifetimes of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor are τ1 ∼ 0.56 ms and τ2 ∼ 0.81 ms. The average decay lifetime of the Gd1.8(MoO4)3:Eu0.23+ nanophosphor is τav ∼ 0.64 ms which is measured using eqn (2) as described below.
| τav = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (2) |
Therefore, the achieved PL and TRPL results suggest that the Gd1.8(MoO4)3:Re0.23+ (Re3+ = Eu3+, Tb3+ and Tm3+) downshift nanophosphors meets the stringent criteria of Si-solar cells and it is highly suitable for Si-solar cells as well as other display and biological applications. The CIE colour co-ordinates of Gd1.8(MoO4)3:Re0.23+ (Re3+ = Eu3+, Tb3+ and Tm3+) are exhibited in Fig. S11(b) (see ESI†). The symbolic marks A (x = 0.66, y = 0.33), B (x = 0.34, y = 0.60) and C (x = 0.14, y = 0.11) demonstrate the CIE colour co-ordinates for Gd1.8(MoO4)3:Eu0.23+, Gd1.8(MoO4)3:Tb0.23+ and Gd1.8(MoO4)3:Tm0.23+ emission, respectively.
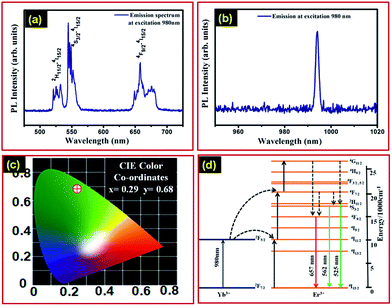
In order to explore the upconversion nature of the Gd2(MoO4)3:Er3+/Yb3+ nanophosphor, we have also performed PL spectroscopy of the synthesized upconversion nanophosphor. Conceptually, upconversion is a process in which the consecutive absorption of two photons of lower energy (IR spectrum region) and emission of a higher energy photon (visible spectrum region) occurs.32 Upconversion materials are of particular interest for solar cell application because these materials utilize the IR region of the solar spectrum which is transmitted by solar cells.15,33–38 The emission spectra of the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor upon excitation at a wavelength of 980 nm are shown in Fig. 4(a) and (b). Fig. 4(a) exhibits the strong green emission at 545 nm and red emission at 657 nm, which are attributed to 4S3/2–4I15/2 and 4F9/2–4I15/2 transitions, respectively. Additionally, the emission spectrum also demonstrates a weak emission centred at a wavelength of 525 nm which is attributed to the 2H11/2–4I15/2 transition. Moreover, the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor has a strong emission at 994 nm, as shown in Fig. 4(b), which is rarely reported in the literature to the best of our knowledge. This emission is also efficiently contributing to the enhancement of the solar cell efficiency because of the fact that the energy of these photons is very near to that of the band gap of the solar cell. The CIE colour co-ordinates of the Gd2(MoO4)3:Er3+/Yb3+ nanophosphor corresponding to upconversion emission at 980 nm excitation wavelength are exhibited in Fig. 4(c) with values x = 0.29 and y = 0.68.
Fig. 4(d) is a schematic diagram for the upconversion process in the Gd2(MoO4)3:Er3+/Yb3+ nanophosphor. In the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor, the Yb3+ ion acts as a sensitizer and Er3+ is used as the dopant, which provides the emission. Yb3+ is used for the sensitizer because it can absorb broadly in the NIR region as compared to the Er3+ ion. In the case of upconversion, both Yb3+ and Er3+ ions absorbed incident photons of 980 nm in wavelength.
However, absorption across the 2F7/2–2F5/2 transition in Yb3+ ions involves more photons compared to absorption across the 4I15/2–4I11/2 transition in the case of Er3+ ions. Therefore, Yb3+ ions absorb most of the incident photons. Furthermore, Yb3+ ions can also sufficiently transfer energy to Er3+ ions because the energy of the 2F5/2 level in Yb3+ ions is similar to the energy of the 4I11/2 level of the Er3+ ions.30 The excited Yb3+ ions relax to the ground state and transfer energy to neighbouring Er3+ ions. Therefore, the excited Er3+ ions absorb energy from Yb3+ ions and are promoted to a higher excitation state which increases the population in a higher energy state. Otherwise, Er3+ ions can absorb two low energy photons simultaneously which drives Er3+ ions to a higher energy states. The relaxation of the excited Er3+ ions to the ground state produces upconversion and emits a strong green coloured emission corresponding to a 4S3/2–4I15/2 transition whereas red emission is seen in the case of a 4F9/2–4I15/2 transition.
Furthermore, the PL emissions of the Gd2(MO4)3:Eu3+ and Gd2(MoO4)3:Er3+/Yb3+ nanophosphors are also comparable with other similar host systems with the same dopants (GdPO4:Eu3+, GdBO3:Eu3+, GdPO4:Er3+/Yb3+ and GdBO3:Er3+/Yb3+). Fig. S12(a) (see ESI†) shows the emission spectra of Gd2(MO4)3:Eu3+, GdPO4:Eu3+ and GdBO3:Eu3+ downshift nanophosphors under an excitation wavelength of 395 nm. Fig. S12(b) (see ESI†) exhibits the emission spectra of Gd2(MoO4)3:Er3+/Yb3+, GdPO4:Er3+/Yb3+ and GdBO3:Er3+/Yb3+ upconversion nanophosphors at an excitation wavelength of 980 nm. The obtained results shown in Fig. S12† reveal that a Gd2(MO4)3 host lattice based nanophosphor has high emission in both downshift as well as upconversion forms which may be due to an efficient energy transfer from the host lattice to the activator compared to other similar host lattice systems.
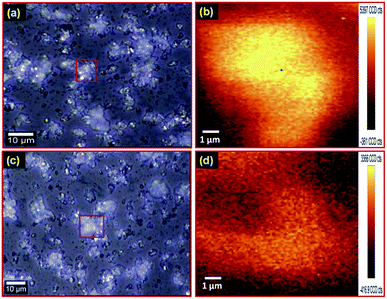
Moreover, PL mapping of downshift as well as upconversion nanophosphors was performed for confirmation of uniform emission from the nanophosphors. Fig. 5(a) exhibits the optical image of the Gd1.8(MoO4)3:Eu0.23+ downshift nanophosphor on a glass slide. The PL map of the red marked area in optical image Fig. 5(a) under an excitation wavelength of 375 nm is shown in Fig. 5(b). Fig. 5(b) clearly shows that the nanophosphor has uniform PL emission. The optical image of the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor is illustrated in Fig. 5(c). Fig. 5(d) reveals the PL mapping image of the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor of the marked region in Fig. 5(c) under an excitation wavelength of 980 nm. The obtained result reveals that the photoluminescence emission intensity is uniformly distributed in both Gd1.8(MoO4)3:Eu0.23+ downshift and Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphors.
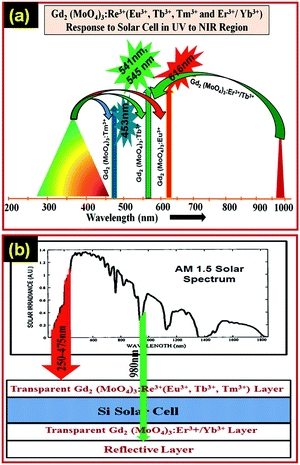
The Gd2(MoO4)3:Eu3+ downshift nanophosphor can absorb photons of high energy in the range of 250–475 nm and give a strong red emission. The Si-solar cell cannot utilise photons falling in this range of energy because of thermal losses. The Gd2(MoO4)3:Eu3+ downshift nanophosphor can easily absorb the photons falling in this region and emits in the visible region which can be easily absorbed by the Si-solar cell for electron–hole pair generation. The NIR region is also not absorbed by solar cells because for the NIR region solar cell is transparent. The Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor absorbs in the NIR region and emits in the visible region which can be easily absorbed by Si-solar cells. Therefore, the efficiency of the Si-solar cell could be enhanced by coating a thin layer of the downshift nanophosphor on the front side and an upconversion nanophosphor layer on the back side of the Si-solar cell. Fig. 6(a) and (b) illustrate a proposed spectral converter for Gd2(MoO4)3:Re3+ (Re3+ = Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphors to enhance the efficiency of Si-solar cells. Thus, the obtained spectroscopy results reveal that the Gd2(MoO4)3:Re3+ (Re3+ = Eu3+, Tb3+, Tm3+ and Er3+/Yb3+) nanophosphor could be a good choice as a spectral converter for upcoming next generation Si-solar cells.
4. Conclusions
We have successfully synthesized Gd2(MoO4)3:Re3+ (Re3+ = Eu3+, Tb3+ and Tm3+) (downshift) and Gd2(MoO4)3:Er3+/Yb3+ (upconversion) nanophosphors by a customized solid state reaction method which can be easily scaled-up for large quantities. The structural and microstructure studies show that the nanophosphors have an orthorhombic crystal structure with an average size in the range of ∼24–28 nm. Gd2(MoO4)3 has an advantage over other host matrices as the excitation band is broad due to the charge transfer in O–Mo in the UV region, which sufficiently transfers energy to trivalent lanthanide ions. The Gd2(MoO4)3:Eu3+ downshift nanophosphor demonstrates hypersensitive red emission at 616 nm corresponding to excitation in the range of 250–475 nm. The Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor shows strong green emission at 545 nm upon an excitation of 980 nm. The co-doping of Yb3+ ions assists the broad absorption in the NIR region as Yb3+ ions can absorb a broader range in the NIR region compared to Er3+ ions. Furthermore, the Gd2(MoO4)3:Er3+/Yb3+ upconversion nanophosphor demonstrates emission at 994 nm, which is rarely reported. Moreover, TRPL spectroscopy demonstrates a PL lifetime of τav ∼ 0.64 ms. Thus, the obtained PL and TRPL spectroscopy results of the synthesized nanophosphor legitimates its potential use as a broad spectral converter for next generation Si-solar cells.Acknowledgements
The authors wish to thank Director, N.P.L., New Delhi, for his keen interest in the work. The authors are thankful to Prof. O. N. Srivastava (Banaras Hindu University, Varanasi) for his encouragement. Mr Pawan Kumar gratefully acknowledges the University Grant Commission (UGC), Govt of India, for financial assistance under RGNF Research Fellowship, Award no. F1-17.1/2011-12/RGNF-SC-PUN-12604/(SA-III/Website). The authors are grateful to the CSIR TAPSUN program providing PL mapping instrument facility.References
- G. D. Scholes, G. R. Fleming, A. Olaya-Castro and R. Grondelle, Nat. Chem., 2011, 3, 763–774 CrossRef CAS PubMed.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- O. Morton, Nature, 2006, 443, 19–22 CrossRef CAS PubMed.
- B. M. van der Ende, L. Aartsa and A. Meijerink, Phys. Chem. Chem. Phys., 2009, 11, 11081–11095 RSC.
- X. Huang, S. Han, W. Huang and X. Liu, Chem. Soc. Rev., 2013, 42, 173–201 RSC.
- A. J. Nozik and J. Miller, Chem. Rev., 2010, 110, 6443–6445 CrossRef CAS PubMed.
- H. A. Atwater and A. Polman, Nat. Mater., 2010, 9, 205–213 CrossRef CAS PubMed.
- P. K. Nayak, G. G. Belmonte, A. Kahn, J. Bisquert and D. Cahen, Energy Environ. Sci., 2012, 5, 6022–6039 CAS.
- A. Goetzberger, C. Hebling and H. W. Schock, Mater. Sci. Eng., 2003, 40, 1–46 CrossRef.
- B. van der Zwaan and A. Rabl, Sol. Energ., 2003, 74, 19–31 CrossRef.
- B. S. Richards, Sol. Energy Mater. Sol. Cells, 2006, 90, 2329–2337 CrossRef CAS PubMed.
- O. M. ten Kate, M. de Jong, H. T. Hintzen and E. van der Kolk, J. Appl. Phys., 2013, 114, 084502 CrossRef PubMed.
- C. Strumpela, M. McCann, G. Beaucarne, V. Arkhipov, A. Slaoui, V. Svrcek, C. del Canizo and I. Tobias, Sol. Energy Mater. Sol. Cells, 2007, 91, 238–249 CrossRef PubMed.
- T. Trupke, M. A. Green and P. Würfel, J. Appl. Phys., 2002, 92, 4117 CrossRef CAS PubMed.
- H. Q. Wang, M. Batentschuk, A. Osvet, L. Pinna and C. J. Brabec, Adv. Mater., 2011, 23, 2675–2680 CrossRef CAS.
- S. F. H. Correia, V. de Z. Bermudez, S. J. L. Ribeiro, P. S. Andre, R. A. S. Ferreira and L. D. Carlos, J. Mater. Chem. A, 2014, 2, 5580–5596 CAS.
- W. Guo, K. Zheng, W. Xie, L. Sun, L. Shen, C. Liu, Y. He and Z. Zhang, Sol. Energy Mater. Sol. Cells, 2014, 124, 126–132 CrossRef CAS PubMed.
- S. K. W. MacDougall, A. Ivaturi, J. Marques-Hueso, K. W. Krämer and B. S. Richards, Sol. Energy Mater. Sol. Cells, 2014, 128, 18–26 CrossRef CAS PubMed.
- M. G. Debije and P. P. C. Verbunt, Adv. Energy Mater., 2012, 2, 12–35 CrossRef CAS.
- J. de Wild, A. Meijerink, J. K. Rath, W. G. J. H. M. van Sark and R. E. I. Schropp, Energy Environ. Sci., 2011, 4, 4835–4848 CAS.
- E. Klampaftis, D. Ross, K. R. McIntosh and B. S. Richards, Sol. Energy Mater. Sol. Cells, 2009, 93, 1182–1194 CrossRef CAS PubMed.
- W. G. J. H. M. van Sark, Renewable Energy, 2013, 49, 207–210 CrossRef CAS PubMed.
- P. F. Scudo, L. Abbondanza, R. Fusco and L. Caccianotti, Sol. Energy Mater. Sol. Cells, 2010, 94, 1241–1246 CrossRef CAS PubMed.
- X. Liu, L. Li, H. M. Noh, B. K. Moon, B. C. Choib and J. H. Jeong, Dalton Trans., 2014, 43, 8814–8825 RSC.
- X. F. Liang, X. Y. Huang and Q. Y. Zhang, J. Fluoresc., 2009, 19, 285–289 CrossRef CAS PubMed.
- P. Kumar, J. Dwivedi and B. K. Gupta, J. Mater. Chem. C, 2014, 2, 10468 RSC.
- B. K. Gupta, D. Haranath, S. Saini, V. N. Singh and V. Shanker, Nanotechnology, 2010, 21, 055607 CrossRef PubMed.
- S. Abtmeyer, R. Pązik, R. J. Wiglusz, M. Małecka, G. A. Seisenbaeva and V. G. Kessler, Inorg. Chem., 2014, 53, 943–951 CrossRef CAS PubMed.
- A. Dwivedi, A. K. Singh and S. B. Rai, Dalton Trans., 2014, 43, 15906 RSC.
- T. V. Gavrilovic, D. J. Jovanovic, V. Lojpur and M. D. Dramicanin, Sci. Rep., 2014, 4, 4209 Search PubMed.
- B. K. Gupta, N. N. Tharangattu, S. A. Vithayathil, Y. Lee, S. Koshy, A. L. M. Reddy, A. Saha, V. Shanker, V. N. Singh, B. A. Kaipparettu, A. A. Martí and P. M. Ajayan, Small, 2012, 8, 3028–3034 CrossRef CAS PubMed.
- M. Haase and H. Schafer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS PubMed.
- A. Shalav, B. S. Richards, T. Trupke, K. W. Krämer and H. U. Güdel, Appl. Phys. Lett., 2005, 86, 013505 CrossRef PubMed.
- D. Chen, L. Lei, A. Yang, Z. Wang and Y. Wang, Chem. Commun., 2012, 48, 5898–5900 RSC.
- A. C. Pana, C. del Canizo, E. N. M. Santos, J. P. Leitao and A. Luque, Sol. Energy Mater. Sol. Cells, 2010, 94, 1923–1926 CrossRef PubMed.
- K. Mishra, S. K. Singh, A. K. Singh, M. Rai, B. K. Gupta and S. B. Rai, Inorg. Chem., 2014, 53, 9561–9569 CrossRef CAS PubMed.
- F. Lahoz, C. P. Rodrıguez, S. E. Hernandez, I. R. Martın, V. Lavın and U. R. R. Mendoza, Sol. Energy Mater. Sol. Cells, 2011, 95, 1671–1677 CrossRef CAS PubMed.
- Y. Y. Cheng, B. Fuckel, R. W. MacQueen, T. Khoury, R. G. C. R. Clady, T. F. Schulze, N. J. E. Daukes, M. J. Crossley, B. Stannowski, K. Lips and T. W. Schmidt, Energy Environ. Sci., 2012, 5, 6953–6959 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15383a |
| This journal is © The Royal Society of Chemistry 2015 |