Total synthesis of Sparstolonin B, a potent anti-inflammatory agent†
Yongqiang Wangab,
Chao Wang*a,
Yuanxun Wanga,
Lijin Donga and
Jian Sun*a
aChengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China. E-mail: sunjian@cib.ac.cn; wangchao@cib.ac.cn; Tel: +86-28-8289-0805
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 15th January 2015
Abstract
Two concise routes for the first total synthesis of Sparstolonin B (SsnB) have been described. The synthesis of SsnB could be accomplished in 5 and 6 steps with 40% and 38% overall yield, respectively. The rhodium-catalysed oxidative coupling of benzoic acid with internal alkyne was used in both strategies to construct the core structure of SsnB.
Sparstolonin B (SsnB) is the first novel bioactive natural small molecule, isolated from a Chinese herb, Sparganium stoloniferum, by Liang and coworkers, which selectively blocks toll-like receptors 2 (TLR2) and TLR4 mediated inflammatory signalling by inhibiting the recruitment of MyD88 to the Toll/Il-1 receptor homologue domains of TLR2 and TLR4.1–3 TLRs play an essential part in the perception of microbes and shape the complex host responses that occur during infection.4 However, the signalling initiated by TLRs is a double-edged sword since a prolonged and exaggerated response can cause tissue and organ damage.5 Thus, blockade of excessive TLR signalling is a therapeutic approach being actively pursued for many chronic inflammatory diseases such as diabetes, Alzheimer's disease, autoimmune colitis and heart and brain ischemia/reperfusion injury.6–13 SsnB, therefore, is a promising lead for the development of selective TLR antagonist for inflammation control. But the low content of SsnB in plants hampered further studies, which attracted us to develop efficient and practical synthetic methods to prepare SsnB.
Architecturally, the structure of SsnB contains the core structure of both xanthone and isocoumarin. Xanthones14–18 and isocoumarins19–22 are key structural motifs in numerous important natural compounds with various biological activities and great attentions have been focused on the synthesis of their derivatives, but, to the best of our knowledge, the total synthesis of SsnB has not yet been achieved. Herein, we wish to report two routes for the first total synthesis of SsnB. The synthesis of SsnB could be accomplished in 5 steps with 40% overall yield in the first route, which use the intermolecular rhodium-catalysed oxidative coupling of benzoic acid with internal alkyne as the key step to form the isocoumarin structure of SsnB. In the second route, the B and C rings of SsnB were constructed in a single step via an intramolecular rhodium-catalysed oxidative coupling of benzoic acid with internal alkyne, and the synthesis of SsnB could be achieved in 6 steps with 38% overall yields.
On the basis of conventional retrosynthetic logic, we envisaged SsnB could be efficiently synthesized by the two strategies shown in Scheme 1. In the first strategy, we planned to disconnect the ether bond of the ring B, which can be constructed through various oxidation–cyclization reactions such as ceric ammonium nitrate mediated oxidation–cyclization.14,23–28 Intermediate 3 was in turn envisioned to be readily accessible from rhodium-catalysed oxidative coupling of 2,5-dimethoxybenzoic acid with internal alkyne 2.29–34 Therefore, the rhodium-catalysed oxidative coupling would be used to construct ring C and the oxidation–cyclization reaction would be used to construct ring B, which would complement the core structure of SsnB.
In the second strategy, the ring B and C could be constructed in a single step by a intramolecular rhodium-catalysed oxidative coupling of benzoic acid with alkyne, and the acid-mediated oxidation cyclization step could be avoid in the synthesis. The alkyne substituent in intermediate 10 would be installed through palladium catalysed Sonogashira coupling and the diaryl ether core would be constructed through a wide range of synthetic methods under both metal mediated and metal free conditions.
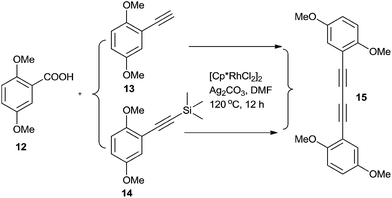
Initially, the commercially available 1-bromo-2,5-dimethoxybenzene 1 was converted to 2 through palladium and copper catalysed Sonogashira coupling in a 90% yield (Scheme 2). Intermediate 2 was converted to isocoumarin derivative 3 by ruthenium-catalysed oxidative alkenylation of 2,5-dimethoxybenzoic acid via twofold C–H bond cleavages in a 86% yield. The 2-hydroxyporp-2-yl substituent in 2 was found to be crucial for the chemo- and regioselectivity in the reaction. Replacing the 2-hydroxyporp-2-yl substituent in 2 with trimethylsilane and hydrogen atom, alkyne dimerization took place prior to coupling with 2,5-dimethoxybenzoic acid (Scheme 3). The 2-hydroxyporp-2-yl substituent in 3 could be removed upon treatment with a palladium catalyst system, which was developed by Miura and co-workers,30 and all the methyl protecting groups of phenols in 3 could be removed upon treatment with boron tribromide to get 5 in 69% yield for the two steps.
The key acid-mediated oxidation cyclization of 5 was achieved after extensive experimentation. We first found 5 could be converted to SsnB in the presence of pyridine hydrochloride in mesitylene at 160 °C for 2.5 h in 5% yield. Thus, various Bronsted and Lewis acids such as Yb(OTf)3, Zn(OTf)3 and ZnCl2 were screened in order to improve the yield. In the presence of 1.0 equivalent of ZnCl2, we found the yield of SsnB could be improved to 60% when 30 mg of 5 was used. Unfortunately, it dropped to 10% when the reaction was used to gram scale synthesis of SsnB. We envisioned the oxidation of one phenol ring in the intermediate 5 was crucial for the Lewis acid catalysed cyclization reaction since similar procedures have been reported.35 Therefore, several types of oxidant were added in this transformation, which were shown in Table 1. We glad to found 60% and 65% yield of SsnB could be obtained on gram scale in the presence of oxygen and copper(II) oxide, respectively (Table 1, entries 1 and 4). Finally, the yield could be improved to 75% when the reaction was carried out in the presence of 1.0 equivalent pyridinium p-toluenesulfonate in mesitylene under oxygen at 160 °C for 2.5 h (Table 1, entry 7), and the total synthesis of SsnB could be completed in 5 steps in a 40% overall yield.
| Entry | Conditions | Yieldb (%) |
|---|---|---|
| a 1.0 mmol of 5 was used.b Isolated yield of SsnB. | ||
| 1 | ZnCl2 (1.0 eq.), O2, mesitylene, 160 °C | 60 |
| 2 | ZnCl2 (1.0 eq.), KBrO3 (1.0 eq.), mesitylene, 160 °C | 30 |
| 3 | ZnCl2 (1.0 eq.), CuO (1.0 eq.), mesitylene, 160 °C | 45 |
| 4 | ZnCl2 (1.0 eq.), CuO (1.0 eq.), THF, 140 °C | 65 |
| 5 | FeCl3 (1.0 eq.), mesitylene, 160 °C | 61 |
| 6 | p-TsOH (1.0 eq.), O2, mesitylene, 160 °C | 61 |
| 7 | PPTs (1.0 eq.), O2, mesitylene, 160 °C | 75 |
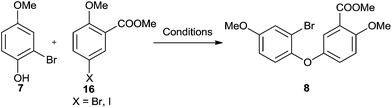
In the second route, we first planned to use Ullman reaction to construct the diaryl ether structure in 8. But we met unexpectedly difficult to get the desired product by these metal-catalysed protocols. For most of the tested reaction conditions shown in Table 2,36–41 no designed product 8 could be detected no matter bromo- or iodobenzene derivatives 16 were used to couple with 2-bromo-4-methoxyphenol 7. The highest yield we could get was 27% (Table 2, entry 7), which clearly shown the strategy to construct the diaryl ether 8 by Ullman reaction was not a proper choice in our total synthesis of SsnB (Scheme 4).
| Entry | Conditions | Yieldb (%) |
|---|---|---|
| a 1.0 mmol of 7 was used.b 1H NMR yield of 8. Ligand 1 = N,N-dimethylglycine; TMHD = 2,2,6,6-tetramethyl-3,5-heptanedione; ligand 2 = 2-[bis(2-methyl-2-propanyl)phosphino]-1-phenyl-1H-indole. | ||
| 1 | CuI, Fe(acac)3, K2CO3, DMF, 120 °C, 12 h | <5 |
| 2 | CuI, Cs2CO3, ligand 1, 1,2-dioxane, 90 °C, 12 h | <5 |
| 3 | CuO, DMF, 140 °C, 12 h | <5 |
| 4 | CuCl, Cs2CO3, TMHD, DMF/CH3CN, 120 °C, 24 h | <5 |
| 5 | CuO, K2CO3, pyridine, 120 °C, 16 h | <5 |
| 6 | Pd(OAc)2, K3PO4, ligand 2, toluene, 120 °C, 24 h | 21 |
| 7 | Cu, K2CO3, KI, DMF, 110 °C, 24 h | 27 |
| 8 | CuI, K3PO4, picolinic acid, 80 °C, 24 h | <5 |
We thought both the steric hindrance of the 2-bromide group of the phenol 7 and the electron donating property of the methoxyl group of the halobenzene 16 could be the reason for the low yields. Thus, 4-methoxyphenol 17 was used in the coupling reaction. As expected, the designed diaryl ether product 19 was obtained in an 87% yield. But we finally found the bromine atom could not be installed into the ortho-position of ether bond, and the meta-bromo substituted product 20 could be obtained in a good yield when the diaryl ether was treated with bromine in dichloromethane (Scheme 5).
Fortunately, we found intermediate 8 could be efficiently constructed by arylation of 2-bromo-4-methoxybenzenol with diaryliodonium salt 6 in a 72% yield. The diaryliodonium salt 6 could be easily prepared from methyl-2-methoxybenzoate upon treatment with iodine and 3-chloroperoxybenzoic acid.42 The intermediate 8 could be converted to 9 by palladium catalysed Sonogashira coupling, followed by treatment with lithium hydroxide to get 10 in 84% yield for the two steps. With the intermediate 10 in hand, we started to construct the ring B and C by rhodium-catalyzed oxidative coupling. We were glad to see ring B and C could be efficiently constructed in a single step under the same reaction conditions that were used before for the construction of intermediate 3, and the key intermediate 11 could be obtained in 85% yield. The designed product SsnB could be obtained by removing the 2-hydroxyporp-2-yl and methyl substituents of 11 upon treatment with palladium catalyst system and boron tribromide, respectively (Scheme 6).
Conclusions
In conclusion, two concise routes for the total synthesis of SsnB, a novel bioactive natural small molecule isolated from a Chinese herb Sparganium stoloniferum, have been described. The synthesis of SsnB could be accomplished in 5 steps with 40% overall yield in the first route, which use the intermolecular rhodium-catalysed oxidative coupling of benzoic acid with internal alkyne as the key step to form the isocoumarin structure of SsnB. In the second route, the B and C rings of SsnB were constructed in a single step via an intramolecular rhodium-catalysed oxidative coupling of benzoic acid with internal alkyne, and the synthesis of SsnB could be achieved in 6 steps with 38% overall yields. The current synthetic methods provide a promising future to get large quantity of SsnB for a variety of pathological and physiological studies.Acknowledgements
We are grateful for financial support from the Western Light Talents Training Program of Chinese Academy of Sciences and the National Natural Science Foundation of China (Project no. 21272227 and 21402185).Notes and references
- Q. Liang, Q. Wu, J. Jiang, J. Duan, C. Wang, M. D. Smith, H. Lu, Q. Wang, P. Nagarkatti and D. Fan, J. Biol. Chem., 2011, 286, 26470 CrossRef CAS PubMed.
- Q. Liang, F. Yu, X. Cui, J. Duan, Q. Wu, P. Nagarkatti and D. Fan, Arch. Pharmacal Res., 2013, 36, 890 CrossRef CAS PubMed.
- H. R. Bateman, Q. Liang, D. Fan, V. Rodriguez and S. M. Lessner, PLoS One, 2013, 8, e70500 CAS.
- X. Z. West, N. L. Malinin, A. A. Merkulova, M. Tischenko, B. A. Kerr, E. C. Borden, E. A. Podrez, R. G. Salomon and T. V. Byzova, Nature, 2010, 467, 972 CrossRef CAS PubMed.
- S. Basith, B. Manavalan, T. H. Yoo, S. G. Kim and S. Choi, Arch. Pharmacal Res., 2012, 35, 1297 CrossRef CAS PubMed.
- K. Zhang, B. Zhou, Y. Wang, L. Rao and L. Zhang, Eur. J. Cancer, 2013, 49, 946 CrossRef CAS PubMed.
- X. Wang, C. Smith and H. Yin, Chem. Soc. Rev., 2013, 42, 4859 RSC.
- E. Vacchelli, L. Galluzzi, A. Eggermont, W. H. Fridman, J. Galon, C. Sautes-Fridman, E. Tartour, L. Zitvogel and G. Kroemer, Oncoimmunology, 2012, 1, 894 CrossRef PubMed.
- S. J. Liao, Y. H. Zhou, Y. Yuan, D. Li, F. H. Wu, Q. Wang, J. H. Zhu, B. Yan, J. J. Wei, G. M. Zhang and Z. H. Feng, Breast Cancer Res. Treat., 2012, 133, 853 CrossRef CAS PubMed.
- D. J. Connolly and L. A. J. O'Neill, Curr. Opin. Pharmacol., 2012, 12, 510 CrossRef CAS PubMed.
- X. Liu, J. Zheng and H. Zhou, Int. Immunopharmacol., 2011, 11, 1451 CrossRef CAS PubMed.
- L. A. O'Neill, C. E. Bryant and S. L. Doyle, Pharmacol. Rev., 2009, 61, 177 CrossRef PubMed.
- E. J. Hennessy, A. E. Parker and L. A. J. O'Neill, Nat. Rev. Drug Discovery, 2010, 9, 293 CrossRef CAS PubMed.
- Y. Xu, J. Zhou, C. Zhang, K. Chen, T. Zhang and Z. Du, Tetrahedron Lett., 2014, 55, 6432 CrossRef CAS PubMed.
- D. K. Winter, D. L. Sloman and J. A. Porco Jr, Nat. Prod. Rep., 2013, 30, 382 RSC.
- K.-S. Masters and S. Braese, Chem. Rev., 2012, 112, 3717 CrossRef CAS PubMed.
- O. Chantarasriwong, A. Batova, W. Chavasiri and E. A. Theodorakis, Chem.–Eur. J., 2010, 16, 9944 CrossRef CAS PubMed.
- H. T. Nguyen, M.-C. Lallemand, S. Boutefnouchet, S. Michel and F. Tillequin, J. Nat. Prod., 2009, 72, 527 CrossRef CAS PubMed.
- S. Pal, V. Chatare and M. Pal, Curr. Org. Chem., 2011, 15, 782 CrossRef CAS.
- M. Wilsdorf and H.-U. Reissig, Angew. Chem., Int. Ed., 2014, 53, 4332 CrossRef CAS PubMed.
- A. Saeed and P. A. Mahesar, Tetrahedron, 2014, 70, 1401 CrossRef CAS PubMed.
- G.-H. Ma, B. Jiang, X.-J. Tu, Y. Ning, S.-J. Tu and G. Li, Org. Lett., 2014, 16, 4504 CrossRef CAS PubMed.
- J. Hu, E. A. Adogla, Y. Ju, D. Fan and Q. Wang, Chem. Commun., 2012, 48, 11256 RSC.
- N. Barbero, R. SanMartin and E. Domínguez, Tetrahedron, 2009, 65, 5729 CrossRef CAS PubMed.
- L. Hintermann, R. Masuo and K. Suzuki, Org. Lett., 2008, 10, 4859 CrossRef CAS PubMed.
- K. C. Nicolaou, H. Xu and M. Wartmann, Angew. Chem., Int. Ed., 2005, 44, 756 CrossRef CAS PubMed.
- K. C. Nicolaou and J. Li, Angew. Chem., 2001, 113, 4394 CrossRef.
- M. M. Johnson, J. M. Naidoo, M. A. Fernandes, E. M. Mmutlane, W. A. van Otterlo and C. B. de Koning, J. Org. Chem., 2010, 75, 8701 CrossRef CAS PubMed.
- X.-X. Guo, J. Org. Chem., 2013, 78, 1660 CrossRef CAS PubMed.
- Y. Unoh, K. Hirano, T. Satoh and M. Miura, Tetrahedron, 2013, 69, 4454 CrossRef CAS PubMed.
- D. Nandi, D. Ghosh, S.-J. Chen, B.-C. Kuo, N. M. Wang and H. M. Lee, J. Org. Chem., 2013, 78, 3445 CrossRef CAS PubMed.
- R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2012, 48, 2030 RSC.
- L. Ackermann, J. Pospech, K. Graczyk and K. Rauch, Org. Lett., 2012, 14, 930 CrossRef CAS PubMed.
- T. Satoh and M. Miura, Chem.–Eur. J., 2010, 16, 11212 CrossRef CAS PubMed.
- J. W. Benbow, B. L. Martinez and W. R. Anderson, J. Org. Chem., 1997, 62, 9345 CrossRef CAS.
- S. Zhang and X. Liu, Synlett, 2010, 2011, 268 CrossRef PubMed.
- Q. Cai, G. He and D. Ma, J. Org. Chem., 2006, 71, 5268 CrossRef CAS PubMed.
- C. V. Ramana, M. A. Mondal, V. G. Puranik and M. K. Gurjar, Tetrahedron Lett., 2007, 48, 7524 CrossRef CAS PubMed.
- N. Jung and S. Brase, Eur. J. Org. Chem., 2009, 2009, 4494 CrossRef.
- J. L. Carr, D. A. Offermann, M. D. Holdom, P. Dusart, A. J. White, A. J. Beavil, R. J. Leatherbarrow, S. D. Lindell, B. J. Sutton and A. C. Spivey, Chem. Commun., 2010, 46, 1824 RSC.
- S. Harkal, K. Kumar, D. Michalik, A. Zapf, R. Jackstell, F. Rataboul, T. Riermeier, A. Monsees and M. Beller, Tetrahedron Lett., 2005, 46, 3237 CrossRef CAS PubMed.
- N. Jalalian, T. B. Petersen and B. Olofsson, Chem.–Eur. J., 2012, 18, 14140 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15948a |
| This journal is © The Royal Society of Chemistry 2015 |