Highly active thiol-functionalized SBA-15 supported palladium catalyst for Sonogashira and Suzuki–Miyaura cross-coupling reactions†
Shaheen M. Sarkar*,
Md. Lutfor Rahman and
Mashitah Mohd Yusoff
Faculty of Industrial Sciences & Technology, University Malaysia Pahang, 26300 Gambang, Kuantan, Pahang, Malaysia. E-mail: sha_inha@yahoo.com; Fax: +60 9 5492766; Tel: +60 9 5492399
First published on 27th November 2014
Abstract
Highly ordered mesoporous silica SBA-15 with pendent 3-mercaptopropyl groups has been prepared by condensation of surface silanols and (3-mercaptopropyl)trimethoxysilane. Treatment of the mercaptopropylated SBA-15 with (CH3CN)2PdCl2 gave a heterogeneous Pd-catalyst. The immobilized Pd-catalyst served as an efficient heterogeneous catalyst for Sonogashira and Suzuki–Miyaura cross coupling reactions of aryl halides. Furthermore, the SBA-15 supported Pd-catalyst was recovered by a simple filtration from the reaction mixture and reused five times without significant loss of its catalytic activity.
1. Introduction
Palladium-catalyzed C–C bond-forming reactions have played an important role in synthetic organic chemistry.1 The palladium-catalyzed coupling of an aryl halide with aryl boronic acid (Suzuki coupling)2,3 or terminal alkyne (Sonogashira coupling)4–6 are recognized as the most successful methods for forming C(sp2)–C(sp2) and C(sp)–C(sp2) C–C bonds, respectively. These reactions have shown widespread applications in the synthesis of natural products, biologically active molecules, and materials science.7–12 Many catalytic systems have been developed for the Suzuki–Miyaura and Sonogashira–Hagihara cross-coupling reactions using different palladium catalysts such as Pd(PPh3)4, but it is sensitive to air oxidation and thus require air-free conditions which pose significant inconvenience on synthetic applications.13–15 In addition, Sonogashira coupling reactions, Glaser-type oxidative dimerization of the terminal alkynes cannot be avoided in copper mediated reactions in which side products (diynes) are generally difficult to separate from the desired products, while copper acetylide is a potentially explosive reagent.16–21 Many palladium catalysts used in the Suzuki–Miyaura and Sonogashira–Hagihara reactions are homogeneous22–25 catalysts, in spite of many advantages, are impossible to be recovered and the residual palladium left in the product confines their use in bioactive molecules and large-scale synthesis. In order to overcome these drawbacks, in recent years, great efforts in catalysis research have been devoted to the introduction and application of effective heterogeneous catalysts.26–33 In this context, ligand-free palladium nanoparticles,34–39 as well as different ligands like palladacycles,40–42 N-heterocyclic carbene,43 Schiff bases,44–46 and dendrimers47 have been explored to be grafted on various inorganic and organic supports such as mesoporous silica,48–52 ionic liquids53,54 and polymers55–61 for the preparation of heterogeneous catalysts.Immobilization of catalysts to such solid supports opens the possibility for extending the benefits of heterogeneous system to homogeneous catalysts. The benefits may include ease of work-up procedures and the potential for reuse of the supported catalysts.62 In particular, highly ordered nanostructured mesoporous silica SBA-15 has extensively used as promising supports because it contains high surface area, porosity, and lots of surface silanol groups.63–66 These features can offer environmental-friendly catalytic systems as well as improved processing steps. The search for an efficient, readily available and recyclable heterogeneous catalytic system is still a major challenge.67–70
Our current interest in this field led to us to explore the immobilization of Pd-catalyst onto SBA-15 silica having nanopores. Herein we report the synthesis and characterization of SBA-15 supported Pd-catalyst and illustrate its application for the Sonogashira and Suzuki–Miyaura cross-coupling reactions under mild reaction conditions. The catalyst shows high catalytic activity in the coupling reactions of various aryl halides. The ease preparation of the catalyst, its long shelf life and its stability toward air, and its compatibility with a wide variety of aryl halides make it an ideal system for coupling reactions in aqueous phase.
2. Experimental
2.1. General information
All manipulations were performed under atmospheric conditions otherwise noted. Reagents and solvents were obtained from commercial suppliers and used without further purification. Water was deionized with a Millipore system as a Milli-Q grade. (CH3CN)2PdCl2 was purchased from Aldrich chemical industries, Ltd. Proton nuclear magnetic resonance (1H NMR, 500/400 MHz) and carbon nuclear magnetic resonance (13C NMR, 125 MHz) spectra were measured with a JEOL JNM ECA-500/400 spectrometer. The 1H NMR chemical shifts were reported relative to tetramethylsilane (TMS, 0.00 ppm). The 13C NMR chemical shifts were reported relative to deuterated chloroform (CDCl3 77.0 ppm). Elemental analyses were performed on a Yanaco CHN Corder MT-6 elemental analyzer by the chemical analysis team in Rikagaku Kenkyūjo (RIKEN), Wako, Japan. Inductively coupled plasma atomic emission spectrometry (ICP-AES) was performed on a Shimadzu ICPS-8100 equipment by the chemical analysis team in RIKEN Wako, Japan. N2 adsorption–desorption isotherms were measured with a BEL SORP mini II analyzer at liquid N2 temperature. Surface area (SBET) was calculated by the BET method, the pore volume (Vp) was determined by nitrogen adsorption at a relative pressure of 0.98, and average pore size (D) was determined from the desorption isotherms using the Barrett–Joyner–Halenda (BJH) method. The gas chromatography-mass spectrometry (GC-MS) was measured by an Agilent 7860A/JEOL JMS-T100GC equipped with a capillary column (DB-Wax, 0.25 mm i.d. × 30 m or HP-1, 0.32 mm i.d. × 30 m). Thin layer chromatography (TLC) analysis was performed on Merck silica gel 60 F254. Column chromatography was carried out on silica gel (Wakogel C-300).2.2. Preparation of the mesoporous SBA-15 silica
Mesoporous silica SBA-15 was synthesized as reported by Zhao.71 In a typical preparation procedure, 4 g of Pluronic P123 was dissolved with stirring in 30 g of water and 120 g of 2 M HCI solution at 35 °C. Then 8.5 g of TEOS was added into that solution under stirring. The mixture was maintained at 35 °C for 10–12 h and then at 60 °C for 48 h and then aged at higher temperature between 100 °C and 120 °C for 48 h under static conditions in a Teflon-lined autoclave to generate materials with uniform pore diameter from 4 to 10 nm. The solid product was recovered and washed with DI water.2.3. Preparation of the mercaptopropylated SBA-15 silica 1
SBA-15 silica (1.0 g) was added to a solution of (3-mercaptopropyl)trimethoxysilane (94 mg, 0.48 mmol) in toluene (12 mL) and the mixture was stirred at 105 °C for 12 h. The resultant mesoporous powder was collected by filtration, washed repeatedly with CH2Cl2 and dried under vacuo to afford (3-mercaptopropyl)trimethoxysilanized SBA-15 silica 1. Weight gain showed that 0.4 mmol of (3-mercaptopropyl)trimethoxysilane was immobilized on 1.0 g of the SBA-15.2.4. Preparation of the SBA-15 supported Pd-catalyst 2
SBA-15 silica 1 (1 g, 0.4 mmol) was added into a stirred solution of bis(acetonitrile)dichloropalladium (51.9 mg, 0.2 mmol) in CHCl3 (8 mL) and stirred for 12 h at 65 °C. The reaction mixture was filtered on a glass frit. The powder was washed with methylene chloride and dried under vacuum at 65 °C to afford SBA-15 supported Pd-catalyst 2. Weight gain and ICP analyses were showed that 0.16 mmol of Pd-complex anchored onto 1.0 g of SBA-15 silica 2.2.5. General procedure for the Sonogashira reaction
Aryl halide (1 mmol), phenylacetylene (1.1 mol equiv.), base (2 mol equiv.) and the Pd-catalyst 2 (7 mg, 0.1 mol%) was stirred at 75 °C for 1 h. The reaction mixture was cold at room temperature and diluted with EtOAc and the immobilized Pd-catalyst was separated by filtration. The organic layer was washed by H2O, dried over MgSO4 and evaporated under reduced pressure. The residue was purified by short column chromatography on silica gel eluted with n-hexane/EtOAc to afford the corresponding coupling products in up to 98% yield.2.6. General procedure for the Suzuki reaction
A mixture of aryl halide (1 mmol), boronic acid (1.1 mol equiv.), K2CO3 (2 mol equiv.), and the Pd-catalyst 2 (0.1 mol%) in 50% aqueous ethanol (1.5 mL) was stirred at 90 °C for 6 h. The reaction mixture was diluted with EtOAc and the Pd-catalyst 2 was separated by filtration. Organic layer was washed with water, dried over MgSO4 and evaporated under reduced pressure. The residue was purified by short column chromatography on silica gel to give the corresponding coupling products in up to 96% yield.3. Results and discussion
3.1. Characterizations of the SBA-15 supported Pd-catalyst 2
The immobilization of Pd-catalyst 2 onto nanostructured mesoporous SBA-15 silica was performed in two steps (Scheme 1). Treatment of SBA-15 silica with (3-mercaptopropyl)triethoxysilane in refluxing toluene for 12 h gave mercaptopropylated SBA-15. The incorporation of the 3-mercaptopropyl precursor was confirmed by elemental analysis and weight gain provided SBA-15 supported mercapto precursor 1 with loading ratio of 0.40 mmol g−1. The SBA-15 supported mercapto precursor 1 was then treated with (CH3CN)2PdCl2 (0.2 mmol) in CHCl3 at 65 °C for 12 h. The resultant brown color SBA-15 supported Pd-catalyst 2 was filtrated on a glass filter, washed by CH2Cl2 and dried under vacuum at 65 °C to give SBA-15 supported Pd-catalyst 2. Elemental analysis and weight gain showed that 0.16 mmol of palladium complex was anchored onto 1.0 g of 2. The loading amount (0.16 mmol g−1) of Pd-catalyst 2 was further determined by ICP-AES analysis. Recently, L. Dai et al.72 prepared sulfur-modified SBA-15 supported amorphous palladium catalyst which efficiently promoted alcohol oxidation reaction. From their report it is confirmed that the palladium was strongly coordinate with the sulfur atoms.The TEM images were obtained before and after modification of the SBA-15 silica (Fig. 1 and 2, ESI†). Both images showed that the hexagonal symmetry of the pore arrays is conserved after immobilization of the Pd-complex onto the SBA-15 silica. The small-angle X-ray diffraction patterns of SBA-15 shows a strong diffraction peak at 100 and two small diffraction peaks at 110 and 200 planes (Fig. 3, ESI†). The presence of guest moieties on the mesoporous framework of SBA-15, resulting in the decrease of intensity of the peaks. The narrow pore size distribution demonstrating a uniform mesoporosity of the SBA-15 supported Pd-catalyst 2. The structural information about the Pd-catalyst 2, the N2 sorption isotherm plot show a type-IV isotherm with a H2 hysteresis loop, indication that the Pd-catalyst 2 still maintains a good mesoporous cage-link structure (Fig. 4, ESI†). The BET surface area and pore volume of SBA-15 is 750 m2 g−1 and 0.83 cm3 g−1, whereas in Pd-catalyst 2, this volume decrease to 465 m2 g−1 and 0.38 cm3 g−1, respectively (Table 1, ESI†). The surface area, pore volume and pore size in Pd-catalyst 2 are reduced during the functionalization of organic moiety onto the parent SBA-15.
3.2. Sonogashira cross-coupling reaction
We then investigated the catalytic efficiency of Pd-catalyst 2 in the Sonogashira cross-coupling reaction without presence of copper and solvent. The Sonogashira cross-coupling of aryl halide and terminal alkyne is a useful tool for the synthesis of aryl-substituted acetylene. This method has been widely used for the synthesis of natural products, biologically active molecules73 nonlinear optical materials and molecular electronics74 dendrimeric and polymeric materials75 macrocycles with acetylene links76 and polyalkynylated molecules.77 With the heterogeneous Pd-catalyst 2 in hand, the Sonogashira coupling reaction was carried out using iodobenzene with phenylacetylene in the presence of Et3N. The supported Pd-catalyst 2 (0.1 mol%) efficiently promoted the Sonogashira cross-coupling reaction under solvent and copper free reaction conditions to give the corresponding coupling product in 92% yield (Table 1, entry 1). Then we performed the reaction using (i-pr)2EtN and piperidine and found piperidine is the best effective base in this cross-coupling reaction.Due to the high catalytic activity of the SBA-15 supported Pd-catalyst 2 we then extended the scope of the Sonogashira coupling reaction using different aryl halides. Excellent catalytic activity was observed in the couplings of iodobenzene, deactivated 4-iodoanisole and 4-iodotoluene (Table 2, 3a–c, X = I, isolated yield) as well as activated 4-iodoacetophenone, 4-iodo-nitrobenzene, 4-cyanoiodobenzene and 4-iodobenzotrifluoride (3d–g, X = I). The deactivated aryl iodides possessing electron-donating group showed a slight drop in reactivity compared to the activated aryl iodides possessing electron-withdrawing group. It is worthy to note that the immobilized Pd-catalyst 2 showed remarkably catalytic activities in the coupling of bromobenzene and substituted bromobenzenes to give the corresponding coupling products (3a–g, X = Br) in up to 96% yield with 100% selectivity.
| a Molar ratio: aryl halide (1 mmol), Pd-catalyst 2 (0.1 mol%), phenylacetylene (1.1 mol equiv.) and piperidene (2 mol equiv.). |
|---|
 |
Recently, B. Movassagh et al.46 reported Pd(II) Schiff base complex supported on multi-walled carbon nanotubes for Sonogashira cross-coupling reaction of aryl iodide using 1.2 mol% of Pd under copper free reaction conditions with prominent decreased of the catalytic activity (1st cycle 99, 4th cycle 82%). However our catalyst efficiently (0.1 mol%) promoted the Sonogashira reaction (twelve times faster) without significance loss of its catalytic activity (1st cycle 100, 4th 96%).
The reusability of immobilized Pd-catalyst is one of the important factors for practical application of heterogeneous catalyst. We then turn attention to recover and reuse of the SBA-15 supported Pd-catalyst 2. In order to test the reusability, we carried out the coupling reaction of iodobenzene in the presence of 0.1 mol% of Pd-catalyst 2.
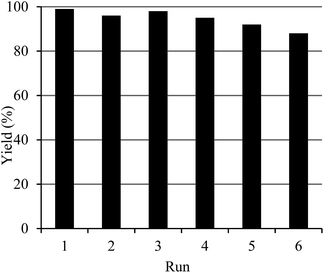
After completion of the reaction, it was diluted with EtOAc and the SBA-15 supported Pd-catalyst 2 was recovered from the reaction mixture by simple filtration, washed with EtOAc and used it for the next cycle without changing the reaction conditions. It is noteworthy that the catalyst could be used six times without significance loss of its activity (Fig. 1).
3.3. Suzuki–Miyaura cross-coupling reaction
The palladium-catalyzed Suzuki cross-coupling of aryl halides with arylboronic acids has become a general and convenient synthetic route in organic chemistry toward biaryls, which has been employed in many areas of organic synthesis, including natural product synthesis. By observing the high catalytic activity of SBA-15 supported Pd-catalyst 2 in the Sonogashira reaction hence, we explore the catalytic activity of Suzuki–Miyaura cross-coupling reaction of aryl halides under mild reaction conditions. With the heterogeneous Pd-catalyst 2 (0.1 mol%), we then tested Suzuki–Miyaura cross coupling reaction of 4-iodoanisole with phenylboronic acid in aqueous ethanol at 90 °C to give the corresponding biaryl product in 96% isolated yield (Table 3, 4a). High catalytic activity was observed in the coupling of deactivated aryl iodides such as 4-iodotoluene, 4-iodophenol and 4-iodoaniline (4b–e) as well as activated 4-iodoacetophenone and 4-cyanoiodobenzene (4f, 4g). In order to investigate the scope of aryl halides with phenylboronic acid, various aryl bromides were used. The coupling of activated or deactivated aryl bromides cleanly proceeded with 0.1 mol% of Pd-catalyst 2 (4a–g) to give the corresponding coupling products in up to 94% isolated yield. It is notable that, aryl chloride was also coupled with phenylboronic acid to give the biaryl products in up to 54% yield (4f, 4g). Recently, A. C. Sullivan et al.78 reported palladium ethylthioglycolate modified silica as a heterogeneous catalyst for Suzuki–Miyaura cross coupling reaction of aryl bromide in xylene using 5 mol% of palladium where the catalytic activity is dramatically decreased (1st cycle 99, 3rd cycle 90%). Here we used only 0.1 mol% of catalyst which showed better catalytic activity in aqueous ethanol as well as reusability of the catalyst (1st cycle 99, 3rd cycle 98%).| a Molar ratio: aryl halide (1 mmol), Pd-catalyst 2 (0.1 mol%), boronic acid (1.1 mol equiv.), K2CO3 (2 mol equiv.), EtOH/H2O (1.5 mL). |
|---|
 |
In general, Suzuki coupling reactions catalyzed by solid-supported Pd follow the usual reaction mechanism (similar to homogenous catalysis, Scheme 2), but the full understanding of mechanism in heterogeneous conditions still remains an open question. In order to determine whether the catalysis was due to the SBA-15 supported Pd-complex 2 or to a homogeneous palladium complex that comes off the support during the reaction and then returns to the support at the end (release and recapture mechanism), we performed the Pd leaching test. To determine the Pd content in the solution, a part of the filtrate (4a, X = I, after solvent removal) was placed in a 20 mL crucible, heated to 600 °C at a heating rate of 10 °C min−1, and then calcined at 600 °C for 3.5 h. The residue was dissolved in 15 mL aqua-regia and diluted to 20 mL. The Pd leaching in solution was determined by inductively coupled plasma mass spectrometry (ICP-MS) analysis. It revealed that the leaching of Pd from the catalyst into the solution was lower than 0.21 ppm after six consecutive run, which suggests the heterogeneous nature of the prepared SBA-15 supported Pd-catalyst 2 (Fig. 2).
4. Conclusion
In conclusion, we have developed nanostructured SBA-15-supported Pd-catalyst 2 for Sonogashira and Suzuki–Miyaura cross-coupling reactions. The heterogenized Pd-catalyst was found to be highly active catalyst for these coupling reactions of aryl iodide, bromide as well as chloride system. The Sonogashira cross-coupling reaction was performed without presence of copper and solvent-free reaction conditions. Furthermore, the catalyst could be simply recovered and reused without significant loss of its activity.Acknowledgements
We gratefully acknowledge the finical support of the University Malaysia Pahang, Fund no. RDU140124.Notes and references
- X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef.
- K. Sonogashira, T. Yatake, Y. Tohda, S. Takahashi and N. Hagihara, J. Chem. Soc., Chem. Commun., 1977, 291 RSC.
- S. Takahashi, Y. Kuroyama, K. Sonogashira and N. Hagihara, Synthesis, 1980, 627 CrossRef CAS.
- Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1998 Search PubMed.
- I. Paterson, R. D. M. Davies and R. Marquez, Angew. Chem., Int. Ed., 2001, 40, 603 CrossRef CAS.
- H. Nakamura, M. Aizawa, D. Takeuchi, A. Murai and O. Shimoura, Tetrahedron Lett., 2000, 41, 2185 CrossRef CAS.
- D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed.
- K. C. Nicolaou and E. J. Sorensen, Classics in Total Synthesis, Wiley-VCH, Weinheim, 1996, p. 582 Search PubMed.
- E. I. Negishi and A. de Meijere, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- T. Suzuka, Y. Okada, K. Ooshiro and Y. Uozumi, Tetrahedron, 2010, 66, 1064 CrossRef CAS PubMed.
- M. Cai, J. Sha and Q. Xu, Tetrahedron, 2007, 63, 4642 CrossRef CAS PubMed.
- J. C. Hierso, A. Fihri, R. Amardeil, P. Meunier, H. Doucet and M. Santelli, Tetrahedron, 2005, 61, 9759 CrossRef CAS PubMed.
- K. Sonogashira, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, New York, 1991, vol. 3 Search PubMed.
- R. Chinchilla and C. Nájera, Chem. Soc. Rev., 2011, 40, 5084 RSC.
- D. Sengupta, J. Saha, G. De and B. Basu, J. Mater. Chem. A, 2014, 2, 3986 CAS.
- S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- W. S. Zhang, W. J. Xu, F. Zhang and G. R. Qu, Chin. Chem. Lett., 2013, 24, 407 CrossRef CAS PubMed.
- F. Nador, M. A. Volpe, F. Alonso, A. Feldhoff, A. Kirschning and G. Radivoy, Appl. Catal., A, 2013, 455, 39 CrossRef CAS PubMed.
- T. Fujihara, S. Yoshida, H. Ohta and Y. Tsuji, Angew. Chem., Int. Ed., 2008, 47, 8310 CrossRef CAS PubMed.
- T. Fujihara, S. Yoshida, J. Terao and Y. Tsuji, Org. Lett., 2009, 11, 2121 CrossRef CAS PubMed.
- H. Doucet and J. C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834 CrossRef CAS PubMed.
- H. Plenio, Angew. Chem., Int. Ed., 2008, 47, 6954 CrossRef CAS PubMed.
- H. Firouzabadi, N. Iranpoor and A. Ghaderi, Org. Biomol. Chem., 2011, 9, 865 CAS.
- J. Zhang, M. Đaković, Z. Popović, H. Wu and Y. Liu, Catal. Commun., 2012, 17, 160 CrossRef CAS PubMed.
- B. Bahramian, M. Bakherad, A. Keivanloo, Z. Bakherad and B. Karrabi, Appl. Organomet. Chem., 2011, 25, 420 CrossRef CAS.
- Y. He and C. Cai, Catal. Commun., 2011, 12, 678 CrossRef CAS PubMed.
- M. Islam, P. Mondal, K. Tuhina, A. S. Roy, S. Mondal and D. Hossain, J. Organomet. Chem., 2010, 695, 2284 CrossRef CAS PubMed.
- D. H. Lee, M. Choi, B. W. Yu, R. Ryoo, A. Taher, S. Hossain and M. J. Jin, Adv. Synth. Catal., 2009, 351, 2912 CrossRef CAS.
- D. A. Alonso and C. Nájera, Cross-coupling and Heck reactions, in Science of Synthesis: Water in Organic Synthesis, ed. S. Kobayashi, George Thieme Verlag KG, Stuttgart, 2012, p. 535 Search PubMed.
- K. H. Shaughnessy, Metal-catalyzed cross-couplings of aryl halides to form C–C bonds in aqueous media, in Metal-Catalyzed Reactions in Water, ed. P. H. Dixneuf and V. Cadierno, Wiley, Weinheim, 2013, pp. 1–46 Search PubMed.
- P. Li, L. Wang and H. Li, Tetrahedron, 2005, 61, 8633 CrossRef CAS PubMed.
- K. Qu, L. Wu, J. Ren and X. Qu, ACS Appl. Mater. Interfaces, 2012, 4, 5001 CAS.
- M. P. Lorenzo, J. Phys. Chem. Lett., 2012, 3, 167 CrossRef.
- A. B. Patil, D. S. Patil and B. M. Bhanage, J. Mol. Catal. A: Chem., 2012, 365, 146 CrossRef CAS PubMed.
- M. Nasrollahzadeh, M. Maham and M. M. Tohidi, J. Mol. Catal. A: Chem., 2014, 391, 83 CrossRef CAS PubMed.
- Y. S. Feng, X. Y. Lin, J. Hao and H. J. Xu, Tetrahedron, 2014, 70, 5249 CrossRef CAS PubMed.
- B. Izabela, A. Gniewek and A. M. Trzeciak, J. Organomet. Chem., 2011, 696, 3601 CrossRef PubMed.
- K. Karami, N. Rahimi and M. B. Shehni, Tetrahedron Lett., 2012, 53, 2428 CrossRef CAS PubMed.
- J. Dupont and M. Pfeffer, Palladacycles: Synthesis, Characterization and Applications, Wiley-VCH, Weinheim, 2008 Search PubMed.
- A. Taher, J. B. Kim, J. Y. Jung, W. S. Ahn and M. J. Jin, Synlett, 2009, 2477 CAS.
- Y. He and C. Cai, J. Organomet. Chem., 2011, 696, 2689 CrossRef CAS PubMed.
- Y. Li, X. Fu, B. Gong, X. Zou, X. Tu and J. Chen, J. Mol. Catal. A: Chem., 2010, 322, 55 CrossRef CAS PubMed.
- M. Navidi, N. Rezaei and B. Movassagh, J. Organomet. Chem., 2013, 743, 63 CrossRef CAS PubMed.
- J. C. G. Martinez, R. W. J. Scott and R. M. Crooks, J. Am. Chem. Soc., 2003, 125, 11190 CrossRef PubMed.
- H. Qiu, S. M. Sarkar, D. H. Lee and M. J. Jin, Green Chem., 2008, 10, 37 RSC.
- V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599 CrossRef CAS PubMed.
- W. Chang, J. Shin, G. Chae, S. R. Jang and B. J. Ahn, J. Ind. Eng. Chem., 2013, 19, 739 CrossRef CAS PubMed.
- W. Chen, P. Li and L. Wang, Tetrahedron, 2011, 67, 318 CrossRef CAS PubMed.
- J. Huang, F. Zhu, W. He, F. Zhang, W. Wang and H. Li, J. Am. Chem. Soc., 2010, 132, 1494 CrossRef PubMed.
- T. Miao, L. Wang, P. H. Li and J. C. Yan, Synthesis, 2008, 3828 CAS.
- M. S. Sarkar, H. Qiu and M. J. Jin, J. Nanosci. Nanotechnol., 2007, 7, 3880 CrossRef CAS PubMed.
- S. P. Schweizer, J. M. Becht and C. L. Drian, Tetrahedron, 2010, 66, 765 CrossRef CAS PubMed.
- M. Bakherad and S. Jajarmi, J. Mol. Catal. A: Chem., 2013, 370, 152 CrossRef CAS PubMed.
- A. Alonso, A. Shafir, J. Macanás, A. Vallribera, M. Muñoz and D. N. Muraviev, Catal. Today, 2012, 193, 200 CrossRef CAS PubMed.
- M. Bakherad, A. Keivanloo and S. Samangooei, Chin. J. Catal., 2014, 35, 324 CrossRef CAS.
- M. Nasrollahzadeh, M. Khalaj and A. Ehsani, Tetrahedron Lett., 2014, 55, 5298 CrossRef CAS PubMed.
- S. M. Islam, P. Mondal, A. S. Roy, S. Mondal and D. Hossain, Tetrahedron Lett., 2010, 51, 2067 CrossRef CAS PubMed.
- M. M. Heravi, E. Hashemi, Y. S. Beheshtiha, S. Ahmadi and T. Hosseinneja, J. Mol. Catal. A: Chem., 2014, 394, 74 CrossRef CAS PubMed.
- N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217 CrossRef CAS PubMed.
- C. Nozaki, C. G. Lugmair, A. T. Bell and T. D Tilley, J. Am. Chem. Soc., 2002, 124, 13194 CrossRef CAS PubMed.
- D. Zhao, J. Sun, Q. Li and G. D. Stucky, Chem. Mater., 2000, 12, 275 CrossRef CAS.
- J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56 CrossRef CAS.
- M. N. Alam and S. M. Sarkar, React. Kinet., Mech. Catal., 2011, 103, 493 CrossRef CAS PubMed.
- S. M. Sarkar, M. N. Alam and M. R. Miah, React. Kinet. Catal. Lett., 2009, 96, 175 CrossRef CAS.
- M. J. Jin and M. S. Sarkar, Stud. Surf. Sci. Catal., 2007, 165, 709 CrossRef CAS.
- M. J. Jin and M. S. Sarkar, Stud. Surf. Sci. Catal., 2007, 165, 697 CrossRef CAS.
- A. Fodor, Z. Hell and P. R. Laurence, Appl. Catal., A, 2014, 484, 39 CrossRef CAS PubMed.
- D. Zhao, J. Feng and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- K. Liu, Z. Chen, Z. Hou, Y. Wang and L. Dai, Catal. Lett., 2014, 144, 935 CrossRef CAS PubMed.
- N. D. P. Cosford, L. Tehrani, J. Roppe, E. Schweiger, N. D. Smith, J. Anderson, L. Bristow, J. Brodkin, X. Jiang, I. McDonald, S. Rao, M. Washburn and M. A. Varney, J. Med. Chem., 2003, 46, 204 CrossRef CAS PubMed.
- L. Brunsveld, E. W. Meijer, R. B. Prince and J. S. Moore, J. Am. Chem. Soc., 2001, 123, 7978 CrossRef CAS PubMed.
- J. Li, A. Ambroise, S. I. Yang, J. R. Diers, J. Seth, C. R. Wack, D. F. Bocian, D. Holten and J. S. Lindsey, J. Am. Chem. Soc., 1999, 121, 8927 CrossRef CAS.
- S. Höger, S. Rosselli, A. D. Ramminger and V. Enkelmann, Org. Lett., 2002, 4, 4269 CrossRef PubMed.
- K. Onitsuka, M. Fujimoto, N. Ohshiro and S. Takahashi, Angew. Chem., Int. Ed., 1999, 38, 689 CrossRef CAS.
- M. A. Hashimi, A. C. Sullivan and J. R. H. Wilson, J. Mol. Catal. A: Chem., 2007, 273, 298 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of SBA-15 and Pd-catalyst 2, XRD, pore size distribution, loading amount of organic moieties onto the mesoporous silica SBA-15 and NMR data of the coupling products. See DOI: 10.1039/c4ra13322f |
| This journal is © The Royal Society of Chemistry 2015 |