Understanding the evolution of stratified extracellular polymeric substances in full-scale activated sludges in relation to dewaterability†
Weijun Zhanga,
Siwei Penga,
Ping Xiaoa,
Jie Hea,
Peng Yangab,
Shiwei Xucd and
Dongsheng Wang*a
aState Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, 18, Shuangqing Road, Beijing 100085, China. E-mail: wgds@rcees.ac.cn; Fax: +86-10-62849138; Tel: +86-10-62849138
bSchool of Civil and Architecture Engineer, Northeast Dianli University, Jilin 132012, Jilin Province, China
cSchool of Water Resources and Environment, China University of Geosciences, Beijing 100083, China
dBeijing Drainage Group Company Limited, Beijing 100024, China
First published on 24th November 2014
Abstract
Activated sludge is a highly changeable colloidal system. In this study, the dynamic variation in physicochemical characteristics, especially distribution and abundance of extracellular polymeric substances (EPS) of activated sludges from different WWTPs was investigated in order to establish the relationships between floc properties and the sludge dewatering property. Firstly, it was observed that the total EPS content of the activated sludge was significantly decreased with the rise in temperature. Three-dimensional fluorescence excitation–emission matrix (3D-EEM) spectroscopy analysis indicated that each sludge fraction (soluble EPS, loosely-bound EPS (LB-EPS), tightly bound EPS (TB-EPS) and pellet) from different WWTPs had a similar fluorescence fingerprint in the same time period. In addition, protein-like substances were found to be the dominant components in TB-EPS and pellets regardless of operating time for each WWTP sludge. At low temperatures, soluble EPS and LB-EPS also mainly contained protein-like compounds, while the amount of humic acids of them was increased significantly in the summer. According to Pearson's correlation analysis, normalized CST correlated well with the composition and content of soluble EPS, indicating that the change in soluble EPS properties caused fluctuation of sludge dewatering behavior. Finally, we proposed some operating strategies for improving the dewatering performance of activated sludge in full-scale WWTPs by regulating the soluble EPS properties.
1. Introduction
The management of wastewater sludge, now often referred to as biosolids, accounts for a major portion of the cost of the wastewater treatment process and represents significant technical challenges. Dewatering has been proven to be an efficient method to reduce sludge volume, cutting transportation and disposal cost.1,2Extracellular polymeric substances (EPSs) are the most crucial constituents of activated sludge and account for 60–80% of the total biomass.3 Consisting of high molecular-weight (MW) polysaccharides, proteins, glycoproteins, nucleic acids, phospholipids, and humic acids, EPS affects flocculating, settling and dewatering properties of activated sludge.3 Getting insight into the role of EPS on the sludge dewatering property became a very popular topic in last few decades. The history on this study has generally experienced three stages: (1) EPS content;4 (2) EPS composition and abundance;5,6 (3) fractionation of EPS7 and stratification of sludge floc.8,9 Initially, Knocke et al. suggested that biopolymer content had a major effect on dewatering performance of biological sludge, and sludge floc density determined the ultimate solid concentration after filtration dewatering.4 Houghton et al. also demonstrated that sludge dewaterability was mainly dependent on EPS content and there appeared to be an optimum level of EPS for each type of sludge (activated sludge or digested sludge) at which the sludge should show the best dewatering performance.10 Besides, EPS content also affected the surface charge, zeta potential, moisture content and floc stability.6 Afterward, it was found that EPS composition and abundance had more significant effects on sludge properties. Many studies suggested that mass ratio of protein and polysaccharide in EPS determined the sludge dewaterability.3 Especially, protein played a more important role that high content of bound protein was beneficial to sludge dewatering.11 On the contrary, Murthy and Novak reported that sludge dewaterability deteriorated with increasing protein concentration of EPS.12 EPS of activated sludge particles are not uniform and are likely to have a dynamic double-layered structure of loosely bound EPS (LB-EPS) diffused from the tightly bound EPS (TB-EPS).13 According to the strength of their binding to floc, Li et al. used a two step heat extraction method to separate the LB-EPS and TB-EPS from sludge floc. The sludge dewatering property was found to be much more strongly correlated with the concentration of LB-EPS than with the concentration of TB-EPS, and excessive LB-EPS could weaken the floc structure resulting in poor bioflocculation and sludge–water separation performance.7 Additionally, they found that the sludge flocculating behavior, settleability, compressibility and dewaterability correlated well with LB-EPS content rather than TB-EPS content.14 Yu et al. fractionated sludge floc into five layers (supernatant, slime, LB-EPS, TB EPS and pellet) with combined ultrasound and centrifugation treatment. A strong correlation was found between the parameters for sludge dewatering characteristics and protein concentration in the supernatant, slime, and LB-EPS, while no correlation showed between organic composition in the pellet and the sludge flocs as a whole.9 Furthermore, three-dimensional excitation emission matrix combined with parallel factor analysis was proven to be an effective tool to characterize the biopolymers. Yu et al. also found that sludge dewaterability was mainly determined by protein, humic acid and fulvic acid in slime and LB-EPS layers.8 In addition, it was noted that divalent and multivalent cations could bind the EPS together with electrostatic force, thus affected EPS distribution and composition and dewatering behavior of activated sludge.2,15
As mentioned above, great efforts have been made to understand the effect of physicochemical properties of sludge floc on sludge–liquid separation in biological system. However, the dynamic variation in physicochemical properties, especially EPS distribution and composition of activated sludge from different full-scale WWTPs with operating time and its influence on sludge dewatering property is still not fully understood. Therefore, the aims of this study were to: (1) understand the physicochemical properties of activated sludge floc collected from five different WWTPs in Beijing; (2) investigate the dynamic variation of distribution, abundance and composition of EPS with operating time (from April to July, 2013) by using 3D-EEM spectroscopy; (3) get an insight into relationships between EPS properties and other physicochemical properties of floc and sludge dewatering behavior.
2. Materials and methods
2.1. Sludge samples
24 wastewater sludge samples were collected from sludge recycle pumping lines of the 5 full-scale WWTPs in Beijing from April to July, 2013, each mainly treating domestic wastewater (Table 1). The locations of WWTPs investigated in this study were shown in Fig. S1 of ESI.† The operating status and sampling date of activated sludges from different wastewater treatment plants (WWTPs) can be found in Table 2. The collected samples were transported to a laboratory within 6 h after sampling and then stored at 4 °C prior to analysis. The characteristics of activated sludge obtained from different WWTPs were given in Table 3.| Treatment plant | Source of wastewater | Treatment capacity (ton per day) | Biological process | Chemical dosage | SRTa (days) | SVIb (mL g−1) |
|---|---|---|---|---|---|---|
| a Solids retention time (SRT).b Sludge volume index (SVI); data obtained from the treatment plants. | ||||||
| Qinghe MBR (QHM) | Mainly domestic | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Membrane bioreactor (MBR) | — | 10–12 | 96–197 |
| Qinghe A/A/O (QHA) | Mainly domestic | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Anaerobic/Anoxic/Oxic (A/A/O) | — | 20–30 | 45–101 |
| Jiuxianqiao (JXQ) | Mainly domestic | 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Oxidation ditch | Aluminum sulfate | 10–12 | 140–308 |
| Gaobeidian (GBD) | Mainly domestic | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
A/O (primary settling tank) | Aluminum sulfate | 10–12 | 87–116 |
| Xiaohongmen (XHM) | Mainly domestic | 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
A/A/O (primary settling tank) | — | 10–12 | 103–152 |
| Sample | Treatment plant | Operating conditions | Sampling date | ||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | COD (mg L−1) | NH4+–N (mg L−1) | TP (mg L−1) | COD/nitrogen ratio | |||
| 1 | QHAO | 15.0 | 249 | 48.9 | 5.5 | 5.1 | April 16, 2013 |
| 2 | QHM | 15.0 | 696 | 53.4 | 5.7 | 13.0 | April 16, 2013 |
| 3 | JXQ | 14.4 | 656 | 60.3 | 6.5 | 10.9 | April 16, 2013 |
| 4 | GBD | 14.9 | 390 | 41.2 | 4.3 | 9.5 | April 16, 2013 |
| 5 | XHM | 15.5 | 613 | 55.5 | 6.0 | 11.0 | April 16, 2013 |
| 6 | QHAO | 20.3 | 539 | 51.2 | 6.2 | 10.5 | May 06, 2013 |
| 7 | QHM | 19.6 | 850 | 54.9 | 6.1 | 15.5 | May 06, 2013 |
| 8 | JXQ | 19.9 | 698 | 59.8 | 5.8 | 11.7 | May 06, 2013 |
| 9 | GBD | 19.6 | 440 | 39.7 | 4.3 | 11.1 | May 06, 2013 |
| 10 | XHM | 19.5 | 619 | 57.3 | 7.61 | 10.8 | May 06, 2013 |
| 11 | QHAO | 22.3 | 508 | 50.2 | 6.0 | 10.1 | June 03, 2013 |
| 12 | QHM | 22.8 | 837 | 50.9 | 6.8 | 16.4 | June 03, 2013 |
| 13 | JXQ | 22.8 | 700 | 53.8 | 6.5 | 13.0 | June 03, 2013 |
| 14 | GBD | 22.5 | 548 | 41.3 | 5.2 | 13.3 | June 03, 2013 |
| 15 | XHM | 23.0 | 616 | 55.2 | 5.9 | 11.2 | June 03, 2013 |
| 16 | QHAO | 24.1 | 441 | 45.1 | 5.5 | 9.8 | June 25, 2013 |
| 17 | QHM | 24.0 | 744 | 41.6 | 6.6 | 17.9 | June 25, 2013 |
| 18 | JXQ | 23.8 | 578 | 48.6 | 5.7 | 11.9 | June 25, 2013 |
| 19 | GBD | 23.7 | 430 | 36.9 | 4.6 | 11.7 | June 25, 2013 |
| 20 | QHAO | 25.1 | 441 | 40.2 | 6.1 | 11.0 | July 10, 2013 |
| 21 | QHM | 24.5 | 744 | 42.5 | 4.7 | 17.5 | July 10, 2013 |
| 22 | JXQ | 25.1 | 578 | 42.4 | 5.0 | 13.6 | July 10, 2013 |
| 23 | GBD | 25.0 | 430 | 32.5 | 3.9 | 13.2 | July 10, 2013 |
| 24 | XHM | 25.3 | 441 | 42.2 | 5.0 | 10.5 | July 10, 2013 |
| Sample | CST (s g−1 L−1) | d0.1 (μm) | d0.5 (μm) | d0.9 (μm) | Zeta (mV) | pH | VSS/TSS (%) |
|---|---|---|---|---|---|---|---|
| 1 | 17.36 | 44.08 | 124.66 | 263.70 | −17.90 | 6.81 | 72.56 |
| 2 | 13.79 | 23.47 | 68.36 | 151.85 | −12.10 | 7.04 | 63.85 |
| 3 | 15.78 | 26.08 | 84.85 | 234.12 | −14.30 | 7.15 | 69.63 |
| 4 | 4.74 | 40.52 | 106.93 | 220.16 | −17.40 | 7.08 | 67.34 |
| 5 | 30.93 | 11.93 | 42.33 | 114.86 | −16.30 | 7.08 | 64.63 |
| 6 | 6.26 | 36.77 | 105.25 | 215.94 | −10.50 | 6.53 | 74.00 |
| 7 | 4.05 | 22.34 | 65.07 | 144.61 | −11.10 | 6.76 | 63.91 |
| 8 | 11.75 | 22.48 | 72.23 | 195.00 | −13.00 | 6.71 | 68.81 |
| 9 | 3.57 | 41.19 | 108.78 | 222.91 | −18.20 | 6.69 | 66.56 |
| 10 | 7.18 | 17.77 | 56.43 | 125.16 | −25.80 | 6.81 | 67.43 |
| 11 | 4.36 | 31.94 | 91.06 | 190.85 | −8.70 | 6.64 | 70.35 |
| 12 | 3.83 | 30.23 | 79.41 | 166.94 | −12.40 | 6.92 | 62.83 |
| 13 | 8.78 | 17.14 | 54.80 | 133.97 | −15.80 | 7.04 | 63.20 |
| 14 | 4.65 | 47.93 | 117.80 | 231.23 | −17.00 | 6.84 | 59.58 |
| 15 | 4.14 | 17.90 | 54.80 | 112.82 | −8.50 | 6.98 | 60.26 |
| 16 | 4.14 | 26.37 | 78.61 | 163.37 | −12.00 | 6.78 | 72.80 |
| 17 | 3.55 | 33.82 | 89.38 | 189.68 | −12.10 | 6.90 | 59.92 |
| 18 | 5.39 | 17.62 | 51.87 | 129.87 | −14.00 | 7.17 | 65.47 |
| 19 | 3.59 | 41.61 | 120.73 | 244.04 | −15.20 | 6.82 | 62.17 |
| 20 | 3.72 | 23.44 | 70.93 | 150.26 | −10.30 | 6.81 | 65.67 |
| 21 | 5.30 | 34.59 | 85.79 | 170.09 | −12.80 | 7.06 | 56.45 |
| 22 | 4.11 | 19.02 | 53.04 | 123.33 | −12.10 | 7.07 | 64.22 |
| 23 | 6.76 | 32.36 | 106.56 | 231.14 | −12.30 | 6.91 | 58.31 |
| 24 | 7.66 | 15.63 | 44.14 | 93.73 | −10.40 | 6.96 | 61.36 |
2.2. Analytical methods
2.3. Extraction and analysis of EPS
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 20 min and separated as TB-EPS. Finally, the solid residues were again re-suspended with the buffer to the original volume. This fraction was the pellet. The particulates present in the supernatant, soluble EPS, LB-EPS, and TB-EPS solutions were removed with polytetrafluoroethylene membranes with a pore size of 0.45 μm prior to fluorescence EEMs and dissolved organic carbon (DOC) analysis.
000g for 20 min and separated as TB-EPS. Finally, the solid residues were again re-suspended with the buffer to the original volume. This fraction was the pellet. The particulates present in the supernatant, soluble EPS, LB-EPS, and TB-EPS solutions were removed with polytetrafluoroethylene membranes with a pore size of 0.45 μm prior to fluorescence EEMs and dissolved organic carbon (DOC) analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm min−1, using excitation and emission slit bandwidths of 10 nm. Each scan had 37 emission and 27 excitation wavelengths.
000 nm min−1, using excitation and emission slit bandwidths of 10 nm. Each scan had 37 emission and 27 excitation wavelengths.2.4. Statistical analysis
Correlation analysis was carried out using the software SPSS version 16.0 for Windows (SPSS). Pearson's correlation coefficient (R) was used to evaluate the linear correlation between physicochemical properties and normalized CST. According to Dancey and Reidy's suggestions, this R from −1 to +1 A correlation of 1, whether it's positive or negative, is a perfect correlation. A correlation of 0 denotes no relationship between two variables. A correlation of 0.7 to 0.9 (positive or negative) is regarded as a strong correlation. R of 0.4 to 0.6 means moderate correlation. R of 0.1 to 0.3 regarded as weak correlations. The correlations were considered statistically significant at a 95% confidence interval (p < 0.05).183. Results and discussion
3.1. Physicochemical properties of sludge floc
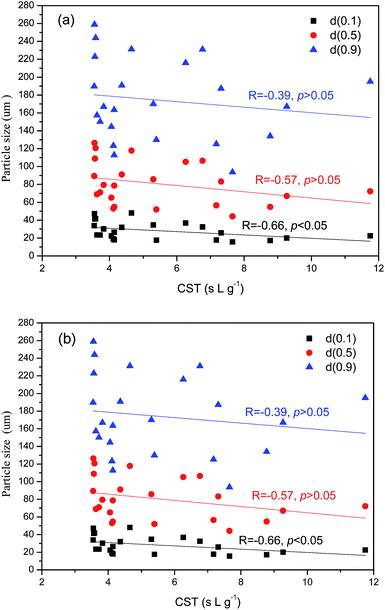
As depicted in Table 3, CST for activated sludge from various WWTPs showed different changing trends with operating time. Sludge from JXQ WWTP exhibited the poorest dewatering property while no significant variation in sludge dewaterability for other sludges. It is worthy to note that the CST for most of sludges reached minimum in the summer. The average floc size (d0.5) of all sludge samples were in the range from 54.80 μm to 126.33 μm, no obvious variation was observable for each WWTP with operating time. The average floc size of GBD, QHA, JXQ and QHM were 95 μm, 83 μm, 62 μm and 71 μm respectively. In addition, the all sludge particles carried negative charge with a zeta potential of −3.8 to −19 mV. This is due to the ionization of the anionic functional groups, such as carboxylic and phosphate. It should be pointed out that the aluminum sulfate was dosed in influent of secondary settling tank for enhancing phosphorus removal in GBD WWTP. The trivalent cations (Al3+, Fe3+) can serve to bind the negatively charged EPS and improve bioflocculation through electrical neutralization and bridging.19 Therefore, the activated floc size in GBD WWTP was larger than that in other WWTPs.The results of Pearson correlation analysis was given in Table S1.† It can be seen from Fig. 1(a) that moderate correlation was found between sludge dewaterability (measured with CST) and d0.1 (R = −0.66, p < 0.05) rather than d0.5 and d0.9, indicating that the large amounts of fine colloidal particles were detrimental to sludge dewatering process. Interestingly, it was found that pH negatively correlated with d0.5 (R = −0.83, p < 0.05) and d0.9 (R = −0.82, p < 0.05) (Fig. 1(b)). This result revealed that sludge particle size distribution was affected by solution pH. Liao et al. suggested that the protons (H+) present within the bulk liquid tend to neutralize the floc negative charges, more especially the negative functional groups located on the EPS surface, improving the particle flocculating property.20 Meanwhile, Zhu et al. demonstrated that the activated sludge growing in weak acid conditions were larger than that growing in mild alkali medium.21 Karr and Keinath observed that the supracolloidal solids of particle size in the range from 1–100 μm were reduced in the presence of acids.22 It was generally accepted that the filter medium and cake layer were more likely to be blocked by fine bio-colloidal particles. Furthermore, it was interesting to note that VSS/TSS was negatively correlated to the solution pH level (R = −0.75, p < 0.01). The sludge with higher organic content was more likely to undergo hydrolysis acidification process. Overall, no strong correlation was found between normalized CST and properties of sludge floc.
3.2. EPS distribution and composition in sludge flocs
 | ||
| Fig. 3 Variation in protein (a) and polysaccharide (b) concentration of four EPS fractions in activated sludge from different WWTPs. | ||
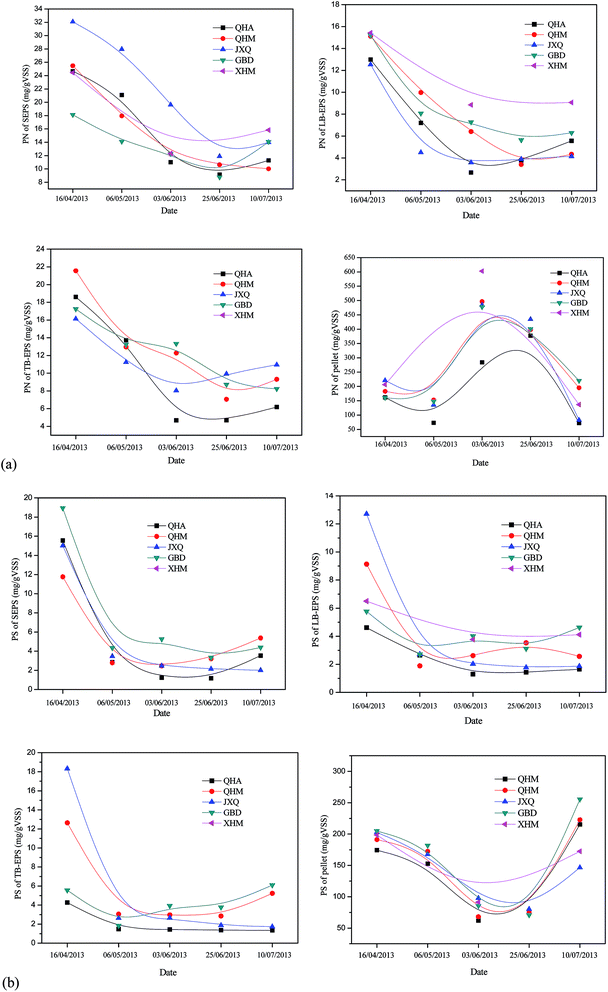
Most microbial exopolysaccharides are highly soluble in water, and capsule-forming polysaccharides are attached to the cells surface through covalent bonds to other surface polymers.24 Fig. 3(b) showed that a similar changing trend was observed for polysaccharide in EPS fractions from different WWTPs. The extractable PS in all sludge layers showed very similar pattern, it reached the minimum in summer (22 May to 10 July 2004). More than 80% of PS was located in pellet. Since the polysaccharides are highly biodegradable polymers, they are very likely to be more easily degraded in the presence of high content of exoenzymes in summer.
 | ||
| Fig. 4 Change in EEM profiles of sludge from QHM WWTP with time (a) 2013-04-16 (b) 2013-05-06 (c) 2013-05-23 (d) 2013-06-03 and (e) 2013-07-10. | ||
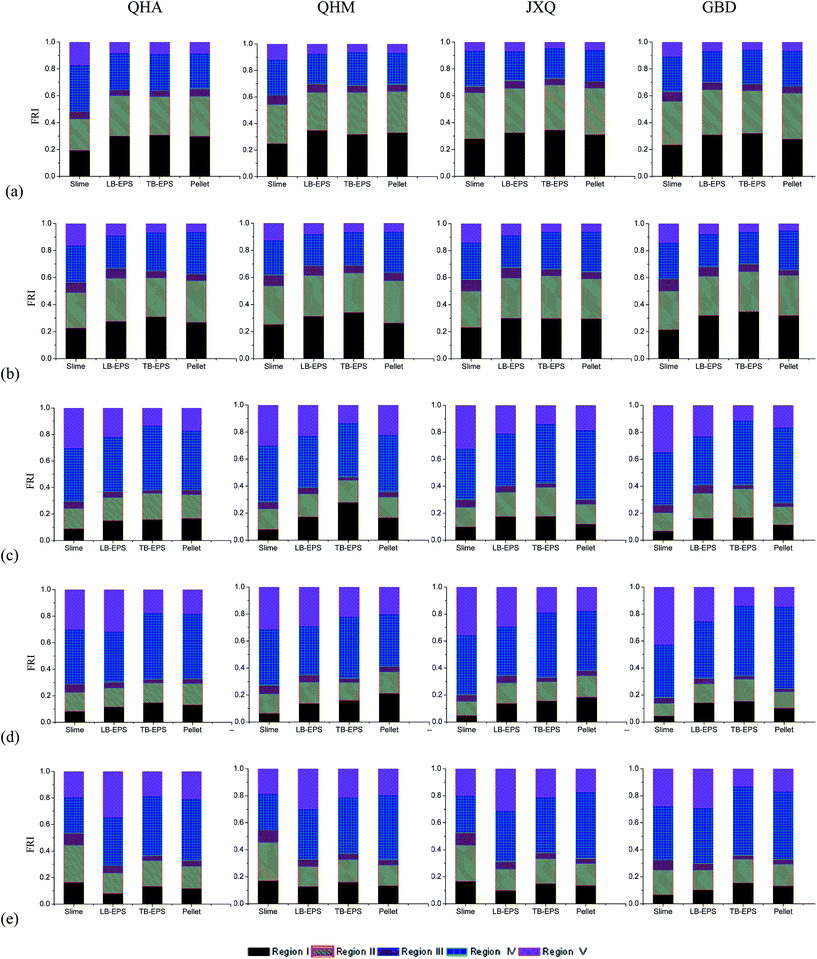
In order to better understand the influence of chemical composition of DOMs in four floc layers on sludge dewaterability, the fluorescence regional integration (FRI) method was employed to analyze the five excitation–emission regions as described by Chen.26 As shown in Fig. S6,† peaks at shorter wavelengths (<250 nm) and shorter emission wavelengths (<350 nm) are associated with simple aromatic proteins such as tyrosine and tryptophan (Regions I and II). Peaks at intermediate excitation wavelengths (250–280 nm) and shorter emission wavelengths (<380 nm) also are related to protein-like substances (region IV) while peaks located at the excitation wavelengths (200–250 nm) and the emission wavelengths (>380 nm) represent fulvic acid-like substances (region III). Peaks at longer excitation wavelengths (>280 nm) and longer emission wavelengths (>380 nm) are related to humic substances (region V). It can be seen from Fig. 5 that relative proportion of humic-like substances in soluble EPS and LB-EPS was increased by around 20–30% while that of aromatic protein-like substances reduced by about 20% from April to July, 2013. Furthermore, it is interesting to note that tryptophan-like substances increased significantly in the summer, because they were related to microbial activity which could be enhanced with increase in temperature.
3.3. Correlations of influent quality and operating condition and EPS properties of AS
Table 4 showed that the temperature was negatively correlated with content of SEPS (R = −0.86, p < 0.01), LB-EPS (R = −0.89, p < 0.01), TB-EPS (R = −0.77, p < 0.01) and pellet (R = −0.89, p < 0.01). PS content in SEPS, LB-EPS, TB-EPS and PN in SEPS were also negatively correlated to water temperature. There was no correlation between C/N ratios of the wastewater and EPS properties in the sludge. These results indicated that temperature had significant effect on EPS production of activated sludges. Table 2 showed that the operating temperature of biochemical tank rose from 15 to 25 °C from April to July while the influent COD concentration decreased. This result agreed with the finding of Wang et al., who observed that content of bound EPS reached maximum in the winter while minimum in the summer.29 The high EPS production at low temperature might be attributed to a shift in microbial populations due to the stress on microorganisms at low temperatures and/or a reduced degradation of these substances due to kinetic considerations.29| Operating conditions | Sludge fractions | PS | PN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEPS | LB | TB | Pellet | SEPS | LB | TB | Pellet | SEPS | LB | TB | Pellet | ||
| a Correlation is significant at the 0.01 level (2-tailed).b Correlation is significant at the 0.05 level (2-tailed); SEPS-soluble EPS; LB-loosely bound EPS; TB-Tightly bound EPS; OC-organic content; ZP-zeta potential; PS-polysaccharide; PN-protein. | |||||||||||||
| Organic loading | Pearson correlation | −0.49 | −0.66 | −0.51 | −0.33 | −0.48 | −0.26 | −0.22 | −0.47 | −0.16 | −0.60 | −0.44 | 0.58 |
| Significance | 0.32 | 0.05 | 0.27 | 0.65 | 0.29 | 0.73 | 0.82 | 0.31 | 0.90 | 0.08 | 0.36 | 0.11 | |
| Carbon/nitrogen (C/N) | Pearson correlation | −0.57 | −0.64 | −0.52 | −0.49 | −0.55 | −0.40 | −0.26 | −0.36 | −0.27 | −0.58 | −0.49 | 0.47 |
| Significance | 0.13 | 0.05 | 0.21 | 0.27 | 0.12 | 0.42 | 0.72 | 0.50 | 0.71 | 0.08 | 0.21 | 0.26 | |
| Temperature (°C) | Pearson correlation | −0.86a | −0.89a | −0.77a | −0.89a | −0.68b | −0.71b | −0.69b | 0.30 | −0.72b | −0.51 | −0.41 | 0.20 |
| Significance | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.01 | 0.66 | 0.19 | 0.01 | 0.37 | 0.83 | |
3.4. Relationship between characteristics of different sludge fractions and dewaterability
Table 5 presented the relationships between CST and distribution, abundance, composition of four sludge fractions. A strong positive correlation was found between CST and total content of soluble EPS (R = 0.79, p < 0.01) rather than organic content of other fractions (Fig. 6(a)). It is generally believed that the increase in content of total EPS are positive correlated to dynamic viscosity of mixed liquor, resulting in a higher degree of accumulation of polymers and sludge particles on membrane surface and furthermore deterioration of sludge filterability and dewaterability.30 However, there was no significant correlation between LB-EPS content and CST. This was contradictory to the finding of Li and Yang, who found that sludge properties (settleability, filterability and dewaterability) were mainly influenced by the characteristics of LB-EPS.7| SEPS | LB | TB | Pellet | S1 | S2 | S3 | S4 | S5 | LB1 | LB2 | LB3 | LB4 | LB5 | TB1 | TB2 | TB3 | TB4 | TB5 | P1 | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Correlation is significant at the 0.05 level (2-tailed).b Correlation is significant at the 0.01 level (2-tailed).c SEPS-soluble EPS, LB-LB-EPS, TB-TB-EPS, S1-region I of soluble EPS, S2-region II of soluble EPS, S3-region III of soluble EPS, S4-region IV of soluble EPS,… | ||||||||||||||||||||||||
| CST | 0.79b | 0.61 | 0.36 | 0.64 | 0.74a | 0.76a | 0.62 | 0.75a | 0.41 | 0.57 | 0.60 | 0.60 | 0.60 | 0.48 | 0.42 | 0.43 | 0.41 | 0.22 | −0.12 | 0.63 | 0.55 | 0.63 | 0.59 | 0.61 |
| SEPS | 1 | 0.89b | 0.84b | 0.85b | 0.94b | 0.95b | 0.93b | 0.95b | 0.51 | 0.88b | 0.85b | 0.90b | 0.89b | 0.65b | 0.88b | 0.88b | 0.87b | 0.64 | 0.56 | 0.86b | 0.84b | 0.86b | 0.81b | 0.78b |
| LB | — | 1 | 0.72a | 0.93b | 0.91b | 0.89b | 0.89b | 0.78b | −0.11 | 0.99b | 0.99b | 0.99b | 0.98b | 0.63 | 0.83b | 0.82b | 0.81b | 0.40 | 0.24 | 0.94b | 0.93b | 0.95b | 0.87b | 0.82b |
| TB | — | — | 1.00 | 0.57 | 0.71a | 0.75a | 0.82b | 0.85b | 0.59 | 0.71a | 0.64 | 0.74a | 0.77a | 0.65 | 0.95b | 0.97b | 0.96b | 0.92b | 0.85b | 0.57 | 0.54 | 0.57 | 0.59 | 0.55 |
| Pellet | — | — | — | 1 | 0.91b | 0.89b | 0.82b | 0.70a | −0.40 | 0.94b | 0.95b | 0.93b | 0.86b | 0.32 | 0.72a | 0.70a | 0.67 | 0.18 | −0.40 | 0.99b | 1.00b | 1.00b | 0.97b | 0.91b |
| S1 | — | — | — | — | 1 | 0.99b | 0.90b | 0.78b | −0.46 | 0.93b | 0.90b | 0.93b | 0.85b | 0.41 | 0.86b | 0.84b | 0.83b | 0.29 | −0.24 | 0.94b | 0.92b | 0.92b | 0.81b | 0.72a |
| S2 | — | — | — | — | — | 1 | 0.92b | 0.81b | 0.38 | 0.90b | 0.88b | 0.91b | 0.85b | 0.52 | 0.86b | 0.85b | 0.84b | 0.44 | 0.26 | 0.92b | 0.90b | 0.90b | 0.80b | 0.72a |
| S3 | — | — | — | — | — | — | 1 | 0.82b | 0.33 | 0.87b | 0.86b | 0.91b | 0.90b | 0.74b | 0.84b | 0.85b | 0.86b | 0.65 | 0.62 | 0.83b | 0.82b | 0.84b | 0.78b | 0.76b |
| S4 | — | — | — | — | — | — | — | 1 | 0.77b | 0.75b | 0.72b | 0.76b | 0.83b | 0.68a | 0.83b | 0.83b | 0.81b | 0.77b | 0.73a | 0.69a | 0.66 | 0.70a | 0.71a | 0.74a |
| S5 | — | — | — | — | — | — | — | — | 1 | −0.33 | −0.33 | −0.21 | 0.41 | 0.67 | 0.31 | 0.39 | 0.38 | 0.75a | 0.80b | −0.49 | −0.50 | −0.43 | 0.19 | 0.46 |
| LB1 | — | — | — | — | — | — | — | — | — | 1 | 0.99b | 0.99b | 0.95b | 0.47 | 0.86b | 0.84b | 0.82b | 0.32 | −0.21 | 0.95b | 0.94b | 0.96b | 0.87b | 0.81b |
| LB2 | — | — | — | — | — | — | — | — | — | — | 1 | 0.98b | 0.96b | 0.52 | 0.79b | 0.77b | 0.75b | 0.20 | −0.29 | 0.96b | 0.95b | 0.97b | 0.89b | 0.83b |
| LB3 | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.96b | 0.61 | 0.86b | 0.85b | 0.84b | 0.42 | 0.20 | 0.94b | 0.94b | 0.95b | 0.87b | 0.81b |
| LB4 | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.77b | 0.83b | 0.82b | 0.82b | 0.56 | 0.51 | 0.86b | 0.85b | 0.88b | 0.82b | 0.78b |
| LB5 | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.48 | 0.54 | 0.60 | 0.71a | 0.81b | 0.28 | 0.28 | 0.36 | 0.35 | 0.44 |
| TB1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.99b | 0.98b | 0.74a | 0.62 | 0.75a | 0.72a | 0.73a | 0.62 | 0.57 |
| TB2 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.99b | 0.79b | 0.67 | 0.73a | 0.7a | 0.71a | 0.65 | 0.57 |
| TB3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.78b | 0.70a | 0.70a | 0.66 | 0.68a | 0.60 | 0.51 |
| TB4 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.96b | −0.16 | −0.2 | −0.2 | 0.43 | 0.48 |
| TB5 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | −0.46 | −0.48 | −0.42 | −0.14 | 0.31 |
| P1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 1b | 0.1b | 0.94b | 0.87b |
| P2 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.1b | 0.95b | 0.87b |
| P3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 1b | 0.94b | |
| P4 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 0.95b | ||
| P5 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | |||
 | ||
| Fig. 6 Correlations of CST and (a) DOM content in four sludge layers; (b) biopolymers located in different EEM regions. | ||
Additionally, it can be seen from Fig. 6(b) that CST correlated well with the aromatic protein I (R = 0.74, p < 0.05), aromatic protein II (R = 0.75, p < 0.05) and tryptophan protein (R = 0.87, p < 0.05) in soluble EPS, but not with humic acid and fulvic acid. Buntner et al. also suggested that SRF significantly positively correlated with protein content in soluble EPS.31 Lyko et al. also suggested the dewaterability of activated sludge was primarily dependent on concentration of dissolved macromolecular compounds,32 and proteins were always high MW substances. Excessive soluble EPS was found to be detrimental to cell cohesion and stability of sludge floc, leading to bad bioflocculation property, cell erosion and poor sludge–water separating performance. As mentioned above, it is interesting to note that the organic compositions in TB-EPS and pellet were relatively stable while that in soluble EPS and LB-EPS varied greatly under non-steady-state conditions.
3.5. Correlation of biopolymers composition located in four sludge layers
Table 5 showed a strong correlation between EEM regions I and II (R > 0.99, p < 0.01) for all sludge samples, indicating that their chemical composition were very similar to each other. Regions I and II were related to simple aromatic proteins such as tyrosine. Again, the fluorescent intensity (FI) of regions I and II could represent biochemical oxygen demand (BOD) of WWTP influent. Furthermore, it is worthy to note that both regions I and II were correlated well to region IV (R > 0.77, p < 0.05). It can be explained by the fact that more soluble EPS was produced at higher BOD concentrations. As mentioned earlier, water temperature negatively correlated well with the EPS content. As depicted in Fig. 5, regions I, II, IV and V were dominant fractions and accounted for more than 90% of total organic content in all floc layers. From April to July, the FRI of regions I and II decreased from 60% to 30% while that of regions IV and V was increased from 30% to 70% respectively. It is well known that the microbial activity was greatly influenced by water temperature. Since the degradation rates of BOD were lower for activated sludge at lower temperature (before April), the aromatic protein could not be rapidly utilised by microorganisms and as a result it will accumulate in sludge floc. After June, the sludge activity was improved with increase in temperature, thereby aromatic protein was more efficiently mineralized or converted into the humic substances through hydrolytic process under anaerobic condition.4. Change in structural model of activated sludge with operating time
The soluble EPS is weakly attached to cells and dispersed, moving freely among the flocs. By contrast, bound EPS is sludge fraction firmly binding to microbial cells which were only can be removed by physiochemical methods. According to experimental results, we proposed a model of change in structural properties of activated sludge floc with time (Fig. 7). Temperature was found to be the major factor affecting EPS properties. At first, the EPS content reduced with increase in temperature and reached minimum in the summer, consequently the sludge floc strength was improved. Meanwhile, no obvious change in chemical composition pellet and TB-EPS were observed, while soluble EPS was highly changeable. Proteins and polysaccharide were reduced and humic acid greatly increased in the summer. Since the dewaterability was manly influenced by chemical characteristics of soluble EPS, the dynamic variation in soluble EPS resulted in fluctuation of dewatering behavior of activated sludge.5. Technological implications
In general, the distribution and composition of EPS were significantly influenced by process flow, influent quality, operating conditions (hydraulic retention time (HRT), sludge retention time (SRT), temperature). The mass load of chemical oxygen demand (COD) to the plant showed a seasonal pattern with higher loading during the winter months. Correspondently, the composition of the activated sludge also showed a seasonal change and higher concentrations of extractable EPS, and the protein content of the total sludge and EPS also increased significantly during the winter.23 Wang et al. also found that a seasonal variation of bound EPS concentrations with highest values at low temperature periods.33 Higher COD/N ratios would result in increase of the content and decrease of protein content in EPS.34 As mentioned above, sludge dewaterability negatively correlated with total organic and protein concentration of soluble EPS which were strongly dependent on operating conditions and influent qualities of WWTP. Thus, the operator can control the operating parameters to reduce the production of soluble EPS and provide the sludge with good dewaterability. For instances, Barker and Stuckey suggested that it appeared to decrease to a minimum and then increase again, indicating the existence of an optimal SRT and HRT for minimizing the soluble EPS production.35 Furthermore, the low molecular weight polymers were mainly produced by bacteria in the logarithmic growth phase, which are representative components of the LB-EPS. Conversely, high MW polymers, the components of TB-EPS, are primarily formed in the stationary and decline phases. Therefore, the LB-EPS content decreased, and the TB-EPS content increased with increased SRTs. Biopolymers with high MW in soluble EPS were the determining factors of sludge dewaterability. Multivalent cations exhibited excellent binding capacity for EPS, hence they could absorb the sticky soluble EPS from water phase and enhance the floc strength of activated sludge through electrical neutralization and bridging. Many studies reported that sludge dewatering and filtration performance can be effectively improved by dosing inorganic salt coagulants, such as ferric and aluminum salts.19,366. Conclusion
This study focused on discussing the effects of floc properties, especially dynamic variation of EPS distribution and composition in sludge from five WWTPs on sludge dewatering behavior. The following conclusions can be drawn from the experimental results:• Overall, sludge dewatering property was not significantly influenced by the charge property, VSS/TSS ratio and pH of sludge system. Furthermore, there was only moderate correlation between CST and the amount of fine particles.
• The total EPS content of activated sludge was decreased significantly in the summer. The evolution in fluorescence characteristic of four sludge fractions from different WWTPs showed a very similar pattern with operating time. Additionally, the chemical composition of TB-EPS and pellet was relatively stable and protein-like compounds are the dominant fractions. Soluble EPS and LB-EPS also mainly contained protein-like substances before May while the protein content was decreased and humic-like substances began to appear and increased significantly in the summer.
• Sludge dewaterability negatively correlated significantly with concentration of protein-like substances and/or soluble EPS rather than chemical characteristics of other sludge fractions. Therefore, fluctuation of sludge dewatering performance in full-scale WWTP was primarily dependent on dynamic variation characteristics of soluble EPS during operation.
Acknowledgements
This study was financially supported by the State Water Project for Integrated Water Supply Sludge Quality and Depth of Dewatering Technology (2012ZX07408001-05) and National Natural Science Foundation of China (no. 51025830, 41201498).References
- H. Liu, J. Yang, N. Zhu, H. Zhang, Y. Li, S. He, C. Yang and H. Yao, J. Hazard. Mater., 2013, 258, 144 CrossRef PubMed.
- M. Niu, W. Zhang, D. Wang, Y. Chen and R. Chen, Bioresour. Technol., 2013, 144, 337 CrossRef CAS PubMed.
- Y. Liu and H. H. Fang, Crit. Rev. Environ. Sci. Technol., 2003, 33, 237 CrossRef CAS.
- W. R. Knocke and D. L. Wakeland, Virginia Water Resources Research Center, Virginia Polytechnic Institute and State University, 1982 Search PubMed.
- B. Jin, B. M. Wilén and P. Lant, Chem. Eng. J, 2004, 98, 115 CrossRef CAS PubMed.
- L. H. Mikkelsen and K. Keiding, Water Res., 2002, 36, 2451 CrossRef CAS.
- X. Li and S. Yang, Water Res., 2007, 41, 1022 CrossRef CAS PubMed.
- G. H. Yu, P. J. He and L. M. Shao, Water Res., 2010, 44, 797 CrossRef CAS PubMed.
- G. H. Yu, P. J. He, L. M. Shao and P. P. He, Environ. Sci. Technol., 2008, 42, 7944 CrossRef CAS.
- J. I. Houghton, J. Quarmby and T. Stephenson, Water Sci. Technol., 2001, 44, 373 CAS.
- M. J. Higgins and J. T. Novak, Water Environ. Res., 1997, 225–232 CrossRef CAS.
- S. N. Murthy and J. T. Novak, Water Environ. Res., 1999, 71, 197 CrossRef CAS.
- T. L. Poxon and J. L. Darby, Water Res., 1997, 31, 749 CrossRef CAS.
- S. F. Yang and X. Y. Li, Process Biochem., 2009, 44, 91 CrossRef CAS PubMed.
- C. Park and J. T. Novak, Water Res., 2007, 41, 1679 CrossRef CAS PubMed.
- A. Apha, Standard methods for the examination of water and wastewater, American Public Health Association, American Water Work Association, Water Environment federation (Wef), Washington DC, 20th edn, 1998, vol. 252 Search PubMed.
- X. H. Wang, Z. C. Wu, Z. W. Wang, X. Yin and X. X. Du, Bioresour. Technol., 2009, 100, 39–45 Search PubMed.
- C. P. Dancey and J. Reidy, Statistics without Maths for Psychology: using SPSS for Windows, Pearson Education, London, 2004 Search PubMed.
- M. R. Mehrnia, H. Azami and M. H. Sarrafzadeh, Colloids Surf., B, 2013, 109, 90 CrossRef CAS PubMed.
- B. Liao, D. Allen, G. Leppard, I. Droppo and S. Liss, J. Colloid Interface Sci., 2002, 249, 372 CrossRef CAS PubMed.
- Z. Zhu, T. Li, D. Wang and C. Yao, Chin. J. Environ. Eng., 2009, 2, 1599 Search PubMed.
- P. R. Karr and T. M. Keinath, J.-Water Pollut. Control Fed., 1978, 50, 1911 Search PubMed.
- B. M. Wilén, D. Lumley, A. Mattsson and T. Mino, Water Res., 2008, 42, 4404 CrossRef PubMed.
- P. Lembre, C. Lorentz and P. Di Martino, The Complex World of Polysaccharides, InTech, p. 371, 2012 Search PubMed.
- F. Meng, S. R. Chae, A. Drews, M. Kraume, H. S. Shin and F. Yang, Water Res., 2009, 43, 1489 CrossRef CAS PubMed.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701 CrossRef CAS.
- G. P. Sheng and H. Q. Yu, Water Res., 2006, 40, 1233 CrossRef CAS PubMed.
- L. Xi, N. Lv, H. Zhang and B. Sun, CIESC J., 2013, 64, 3003 CAS.
- Z. Wang, Z. Wu and S. Tang, Water Res., 2009, 43, 2504 CrossRef CAS PubMed.
- F. Meng, H. Zhang, F. Yang, S. Zhang, Y. Li and X. Zhang, Sep. Purif. Technol., 2006, 51, 95 CrossRef CAS PubMed.
- D. Buntner, H. Spanjers and J. van Lier, Water Res., 2014, 51, 284 CrossRef CAS PubMed.
- S. Lyko, T. Wintgens, D. Al-Halbouni, S. Baumgarten, D. Tacke, K. Drensla, A. Janot, W. Dott, J. Pinnekamp and T. Melin, J. Membr. Sci., 2008, 317, 78 CrossRef CAS PubMed.
- X. Wang, Z. Wu, Z. Wang, X. Yin and X. Du, Bioresour. Technol., 2009, 100, 1937 CrossRef CAS PubMed.
- B. Durmaz and F. Sanin, Water Sci. Technol., 2001, 44, 221 CAS.
- D. J. Barker and D. C. Stuckey, Water Res., 1999, 33, 3063 CrossRef CAS.
- W. Zhang, P. Xiao, Y. Liu, S. Xu, F. Xiao, D. Wang and C. W. Chow, Sep. Purif. Technol., 2014, 132, 430 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13379j |
| This journal is © The Royal Society of Chemistry 2015 |