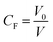Facile phase transfer of gold nanoparticles from aqueous solution to organic solvents with thiolated poly(ethylene glycol)†
A. M. Alkilany*a,
A. I. Bani Yaseena,
J. Parkb,
J. R. Ellerb and
C. J. Murphyb
aDepartment of Pharmaceutics & Pharmaceutical Technology, Faculty of Pharmacy, The University of Jordan, Amman 11942, Jordan
bDepartment of Chemistry, University of Illinois at Urbana-Champaign, 600 South Mathews Avenue, Urbana Il 61801, USA. E-mail: a.alkilany@ju.edu.jo; murphycj@illinois.edu
First published on 14th October 2014
Abstract
A simple approach for the efficient transfer of large gold nanoparticles from water to organic solvents using thiolated poly(ethylene glycol) as a phase transfer agent is presented. The addition of a common solvent during the phase transfer allowed for nanoparticles to be concentrated by a factor of 250 without aggregation.
Gold nanoparticles (GNPs) have attracted wide interest due to their fascinating optical properties and promising applications.1 Aqueous-based syntheses of GNPs are dominant, which allow precise shape/size control.1a However, stable GNPs in organic solvents are required for various applications such as preparing hydrophobic composites with water-insoluble polymers and to control assembly of nanoparticles on substrates upon evaporation from volatile solvents.2 Due to the difficulty of preparing GNPs with a range of sizes in organic solvents (the well-known Brust/Schriffin method works best for GNPs less than 5 nm),3 two approaches are employed to prepare hydrophobic nanoparticles in organic solvents: (1) ligand exchange with hydrophobic molecules that result in the precipitation of nanoparticles from aqueous medium followed by (possibly difficult) resuspension without aggregation in organic solvent; (2) phase transfer of GNPs from aqueous media to organic solvents across the liquid–liquid interface.4 However, the phase transfer of GNPs is experimentally size-dependent, with serious difficulties encountered in transferring large nanoparticles (>10 nm) from water to organic solvents.4d,h,i,5 Methods for phase transfer include the use of alkanethiol or alkylamine surface ligands,6 cationic surfactants in ionic liquids,4f transfer to a solid substrate followed by sonication,4c the use of a specialized bidentate thiol ligand that is not commercially available,4a and the use of mechanical force.4e Herein we report a simple and highly efficient protocol to transfer large GNPs from water to organic solvent (dichloromethane, DCM) using thiolated polyethylene glycol (PEG-SH) as a phase transfer agent, which is commercially available and used routinely to functionalize nanoparticles, in addition to a co-solvent.7 Moreover, we present a method to effectively concentrate the transferred nanoparticles in the DCM phase that enables broader application of these nanomaterials. We observed that citrate-capped GNPs (cit-GNPs, 12 nm in diameter) transfer from aqueous solution to DCM layer that contains PEG-SH upon the addition of methanol. Interestingly, the addition of an aqueous GNPs layer to a DCM layer containing PEG-SH followed by shaking did not induce phase transfer (Fig. 1a). The addition of a common solvent that is miscible in both water and DCM (e.g. methanol) was necessary for efficient and spontaneous transfer (Fig. 1b). Upon the addition of methanol, complete phase transfer was observed (Fig. 1b). The transferred GNPs exhibited a typical red-coloured solution and corresponding UV-vis spectrum, which indicated no nanoparticle aggregation. The LSPR band was red shifted (from 520 to 530 nm), without significant broadening, in agreement with the LSPR dependency on the refractive index of the medium (Fig. 1d).8 The attachment of PEG-SH at the surface of GNPs was confirmed by XPS analysis and solubility evaluation (Fig. S1 and S2†).
While PEG-SH efficiently transferred GNPs from water to DCM, other widely used phase-transfer agents (1-dodecanethiol or tetraoctylammonium bromide) did aggregate GNPs and failed to induce similar transfer at equal molar levels, which demonstrates the versatility of PEG-SH as a phase transfer agent (Fig. S3†). The remarkable ability of PEG-SH molecules to transfer GNPs from water to DCM layer relies on the unique hydrophilic–hydrophobic character of PEG molecules.7 Since PEG is soluble in both water and DCM, we hypothesize that well-solvated PEG molecules maintain thiol availability to cap the GNP's surface in both water and DCM phases, resulting in fast capping and thus stabilization that precedes GNPs aggregation and assembly at the liquid–liquid interface (Fig. S4†). A control study with non-thiolated PEG of similar molecular weight did not induce phase transfer, but rather resulted in nanoparticle aggregation and assembly at the liquid–liquid interface, thus highlighting the importance of the thiol moieties in PEG-SH molecules for phase transfer (Fig. 1c). Moreover, we found that our protocol works well with a wide range of molecular weight of PEG-SH. While a very small PEG-SH (MW of 350 Da) resulted in nanoparticle aggregation upon phase transfer, PEG-SH with larger molecule weights (MW of 1000, 5000, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) resulted in complete phase transfer without nanoparticle aggregation (Fig. S5†). Our results support the general trend of enhanced ability of sufficiently large PEG molecules to stabilize nanoparticles.7
000 Da) resulted in complete phase transfer without nanoparticle aggregation (Fig. S5†). Our results support the general trend of enhanced ability of sufficiently large PEG molecules to stabilize nanoparticles.7
The choice of the common solvent was critical to facilitate a complete phase transfer of GNPs without aggregation. Methanol, ethanol, isopropanol, DMF, and DMSO completely transferred GNPs to the DCM layer with no aggregation; however, acetone, acetonitrile and THF did not induce phase transfer (Fig. 2a and Fig. S6†). An explanation for this behaviour is not clear and should be the subject of future work. Interestingly, the final concentration of transferred GNPs in the DCM layer was affected by the type of common solvent. Fig. 2a shows that ethanol and isopropanol diluted transferred GNPs in DCM layer where methanol and DMSO concentrated them, as compared to the initial concentration of GNPs in water phase prior to transfer (Fig. 2a). DMF has no significant effect. Due to the volatility of methanol compared to DMSO, we considered methanol as a suitable common solvent for phase transfer in subsequent experiments. The effect of different common solvents on the concentration of transferred GNPs in the DCM layer could be related to the relative affinity of common solvents to both layers and should be dictated by the ternary phase diagram of biphasic system with three components (water, DCM and common solvent).
 | ||
| Fig. 2 (a) Phase transfer of cit-GNPs in water (2.0 mL) to DCM (2.0 mL) containing PEG-SH upon the addition of (2.0 mL) of various common solvents as labelled. The control vial represents the initial biphasic system prior to addition of common solvents. (b) Concentration degree as a function of volume added of common solvents to induce a complete phase transfer. After 5.0 mL of (methanol or DMSO) or 4.0 mL of (DMF, ethanol, or isopropanol) monophasic system was observed in all vials as per Fig. S6† and thus CF values were not calculated. CF values are calculated for systems that induced complete phase transfer as per Fig. S6.† | ||
To represent the ability of a common solvent to concentrate or dilute transferred GNPs in the DCM phase, we introduce a quantitative term “concentration factor, CF”:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GNPs solution
GNPs solution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM is 2
DCM is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Methanol increases the solubility of DCM in water, which decreases the final volume of the DCM layer containing GNPs resulting in an increased concentration of GNPs in the organic layer (Fig. S6†). The addition of excess methanol (2.5
1). Methanol increases the solubility of DCM in water, which decreases the final volume of the DCM layer containing GNPs resulting in an increased concentration of GNPs in the organic layer (Fig. S6†). The addition of excess methanol (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) results in the formation of monophasic system (Fig. S6†).
1) results in the formation of monophasic system (Fig. S6†).
To evaluate the ability of our protocol to phase transfer large GNPs with different surface chemistries, we prepared large GNPs using the hydroquinone method (∼23–93 nm, see ESI† for details and TEM images).9 Similar to cit-GNPs, complete transfer of GNPs without aggregation was observed as evident from the constant colour of GNP solutions and typical UV-vis spectra, before and after transfer (Fig. 3). Moreover, surfactant-capped gold nanorods with various dimensions (see ESI† for details and TEM images) were transferred readily, confirming the ability of our approach to phase transfer GNPs with various dimensions, shapes and surface chemistries to organic solvents. Similar to cit-GNPs, we did observe that methanol concentrates the transferred gold nanoparticles and nanorods in the DCM layer (data not shown). Addition of methanol to a similar control system (water–DCM) in absence of GNPs, maintains biphasic system and decreases the volume of DCM phase (Fig. S8†), suggesting that the “concentration effect” is a general phenomenon controlled by the phase diagram of the (methanol–water–DCM) system.
 | ||
| Fig. 3 Phase transfer of spherical GNPs prepared with the hydroquinone method with various core size (vials 1–6, left panel) and rod-shape GNPs with various dimensions (vial 7–10, right panel) from aqueous solution (2.0 mL) in panel (a) to (2.0 mL) of DCM containing PEG-SH in panel (b), upon the addition of methanol (2.0 mL). UV-vis spectra of spherical GNPs (c) and of rod-shape GNPs (d). The symbols B and A in both legends indicate GNPs in water prior to and after phase transfer, respectively. Details on nanoparticle dimensions and TEM images are available in the ESI.† | ||
The encouraging results prompted us to evaluate the scalability of our phase transfer approach. Cit-GNPs (150 mL, 1.0 nM) were completely transferred to the DCM layer (150 mL) by the addition of 150 mL methanol (Fig. 4). Remarkably, GNPs were concentrated in small organic droplet (∼0.6 mL and CF of ∼250) upon the addition of more methanol (350 mL total), without nanoparticle aggregation as shown in Fig. 4e and S9.† Upon drying of the highly concentrated organic layer, PEG-GNPs can be readily resuspended in various polar and hydrophobic organic solvents without nanoparticle aggregation as evident from red colour of GNP solutions and their UV-vis spectra (Fig. S10†). Transferred GNPs are stable in dichloromethane for at least eight months as evident from constant UV-vis spectra and solution color (data not shown).
 | ||
| Fig. 4 Phase transfer of cit-GNPs in water (150 mL) to DCM (150 mL initial) containing PEG-SH upon the addition of increasing amount of methanol (mL): (a) (0); (b) (150); (c) (225); (d) (300); (e) (350). UV-vis spectra are available in the ESI.† | ||
The necessity of the addition of a common solvent to induce nanoparticle transfer agrees with published reports for other phase transfer systems.4e,5,10 We hypothesize that common solvents reduce the interfacial tension at the liquid–liquid interface, which promotes efficient contact between GNPs in water and PEG-SH in DCM. Spontaneous assembly of PEG-SH at the surface of GNPs coupled to the fact that PEG molecules prefer the DCM phase over the aqueous phase,11 ensure efficient transfer of GNPs. The lipophilicity of PEG is well documented and its partition coefficient between DCM and water is ∼180 at a molecular weight of 5000 Da, which is similar to that used in this study.12 This surprising fact motivated us to confirm the reported preference of PEG for the DCM layer over water and calculate the partition coefficient of a fluorophore-tagged PEG (FITC-PEG-SH, 5000 Da) to be ∼80 (Fig. S11†). PEG can be extracted to certain organic solvents, which display hydrogen bond donating capacity such as dichloromethane and chloroform.12,13 Interestingly, we noted that GNPs transferred spontaneously and completely to DCM or chloroform, but no phase transfer was observed into hexane or toluene, where PEG has poor interaction and low solubility (Fig. S12†). In addition to acting as a phase transfer agent, PEG-SH is a known capping agent to stabilize nanoparticles in aqueous and organic media resulting in remarkable stability of GNPs during the phase transfer process and in the organic layer.7
Another possible hypothesis regarding the role of common solvent originates from the fact that PEG molecules may partition into the aqueous phase with spontaneous assembly at the surface of nanoparticles in water.7 It has been reported that the transfer of PEG molecules from water to DCM is an endothermic process due to the replacement of a more energetically favored PEG solvation in water for that in DCM.12 However, a significant gain in entropy of PEG in DCM drives the transfer from water to DCM.12 Bound PEG molecules on GNPs may behave differently than free PEG molecule in solution, and thus the entropy gain is not enough to drive efficient phase transfer of GNPs. Addition of common solvents to PEG-GNPs in water disrupts the hydrogen bonds between PEG molecules and water and thus decreases the energy barrier related to solvation in water. A significant decrease in the solvation energy barrier coupled to entropy gain in DCM drives the transfer of GNPs from water to DCM. In a control study, PEG-GNPs in water did not transfer to DCM layer until methanol was added (Fig. S13†), in agreement with previous report where PEG-GNPs in water did not transfer to chloroform until centrifugation was applied or ethanol was added to mechanically or chemically (respectively) peel the aqueous hydration shell and promote nanoparticle transfer.4e The detailed mechanism of the phase transfer of GNPs from water to DCM using our protocol is under investigation.
In conclusion, we have developed a simple, spontaneous, and efficient protocol to transfer GNPs of various sizes, shapes and surface-capping agents from water to organic solvents with the ability to remarkably concentrate GNPs in the organic layer and maintaining their colloidal stability. The described protocol is a convenient and cost-effective one in the functionalization and manipulation of nanoparticles.
Notes and references
- (a) E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740 RSC; (b) C. J. Murphy, A. M. Gole, S. E. Hunyadi, J. W. Stone, P. N. Sisco, A. Alkilany, B. E. Kinard and P. Hankins, Chem. Commun., 2008, 544 RSC; (c) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- R. A. Sperling and W. J. Parak, Philos. Trans. R. Soc., A, 2010, 368, 1333 CrossRef CAS PubMed.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Chem. Commun., 1994, 801 RSC.
- (a) M. Lista, D. Z. Liu and P. Mulvaney, Langmuir, 2014, 30, 1932 CrossRef CAS PubMed; (b) M. Karg, N. Schelero, C. Oppel, M. Gradzielski, T. Hellweg and R. von Klitzing, Chemistry, 2011, 17, 4648 CrossRef CAS PubMed; (c) H. J. Baik, S. Hong and S. Park, J. Colloid Interface Sci., 2011, 358, 317 CrossRef CAS PubMed; (d) W. Cheng and E. Wang, J. Phys. Chem. B, 2003, 108, 24 CrossRef; (e) M. Liu, W. C. Law, A. Kopwitthaya, X. Liu, M. T. Swihart and P. N. Prasad, Chem. Commun., 2013, 49, 9350 RSC; (f) G.-T. Wei, Z. Yang, C.-Y. Lee, H.-Y. Yang and C. R. C. Wang, J. Am. Chem. Soc., 2004, 126, 5036 CrossRef CAS PubMed; (g) J. M. McMahon and S. R. Emory, Langmuir, 2006, 23, 1414 CrossRef PubMed; (h) M. N. Martin, D. Li, A. Dass and S. K. Eah, Nanoscale, 2012, 4, 4091 RSC; (i) J. Yang, J. Y. Lee and J. Y. Ying, Chem. Soc. Rev., 2011, 40, 1672 RSC.
- M. N. Martin, J. I. Basham, P. Chando and S. K. Eah, Langmuir, 2010, 26, 7410 CrossRef CAS PubMed.
- (a) X. Wang, S. Xu, J. Zhou and W. Xu, J. Colloid Interface Sci., 2010, 348, 24 CrossRef CAS PubMed; (b) L. Li, K. Leopold and M. Schuster, J. Colloid Interface Sci., 2013, 397, 199 CrossRef CAS PubMed.
- A. S. Karakoti, S. Das, S. Thevuthasan and S. Seal, Angew. Chem., Int. Ed., 2011, 50, 1980 CrossRef CAS PubMed.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef CAS.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042 CrossRef CAS PubMed.
- N. Gaponik, D. V. Talapin, A. L. Rogach, A. Eychmüller and H. Weller, Nano Lett., 2002, 2, 803 CrossRef CAS.
- S. Zalipsky and J. M. Harris, in Poly(ethylene glycol), American Chemical Society, 1997, pp. 1–13 Search PubMed.
- M. Spitzer, E. Sabadini and W. Loh, J. Phys. Chem. B, 2002, 106, 12448 CrossRef CAS.
- A. G. Anselmo, R. C. Sassonia and W. Loh, J. Phys. Org. Chem., 2006, 19, 780 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedure on the synthesis of GNPs and phase transfer with transmission electron microscope images for prepared nanoparticles. X-ray photoelectron spectroscopy analysis of GNRs before and after phase transfer is provided. See DOI: 10.1039/c4ra11928b |
| This journal is © The Royal Society of Chemistry 2014 |