Effect of the type of fluorofunctional organosilicon compounds and the method of their application onto the surface on its hydrophobic properties
H. Maciejewski*ab,
J. Karasiewiczb,
M. Dutkiewiczc,
M. Nowickid and
Ł. Majchrzyckic
aAdam Mickiewicz University, Faculty of Chemistry, Umultowska 89b, Poznań 61-614, Poland
bAdam Mickiewicz University Foundation, Poznań Science and Technology Park, Rubież 46, Poznań 61-612, Poland. E-mail: maciejm@amu.edu.pl; Fax: +48 61 8279754; Tel: +48 61 8279753
cWielkopolska Centre of Advanced Technologies, Umultowska 89c, Poznań 61-614, Poland
dInstitute of Physics, Poznań University of Technology, Nieszawska 13a, Poznań 60-965, Poland
First published on 1st October 2014
Abstract
Fluorofunctional silanes, polysiloxanes and silsesquioxanes were used for the modification of glass surfaces and their influence on hydrophobic properties were determined. To increase hydrophobicity of the surface, the modification was performed in two stages: (i) by a pretreatment using a silica sol (a rise in the surface roughness), and (ii) modification with the above silicon compounds. The hydrophobicity was determined by measuring the contact angle by drop profile tensiometry. The fluorocarbofunctional organosilicon derivatives examined were found to be good precursors for the synthesis of highly hydrophobic materials and coatings. In some cases the contact angles measured after surface modification exceeded 150°, i.e. they fell in the range characteristic of superhydrophobic surfaces.
Introduction
Non-wettable surfaces with high water contact angles (WCA) have attracted tremendous attention in recent years.1 Superhydrophobic coatings, i.e., the ones with WCA > 150°, have many spectacular applications based, particularly on their self-cleaning,2 anti-icing,3,4 oil repellent,5 electrowetting,6 and anticorrosive7 activities. The wettability of a surface depends on two factors: (i) its chemical composition and (ii) its structure (roughness). Different techniques for producing rough surfaces have been developed by imitating nature (biomimetics), such as wet chemical etching,8,9 inorganic or organic template method,10 electrospinning,11 phase separation12 and colloidal self-assembly.13,14 The last technique is very popular because the production and application of particles and nanoparticles on a surface allows easy control on its roughness.15–17 One of the ways to achieve this goal is the use of silica with different particle sizes obtained in the sol–gel process. The mild preparation conditions offer the possibility of incorporating a wide range of labile organic species into a glass composite. Moreover, sol–gel derived materials exhibit tunable porosity, transparency, hardness, and good thermal stability.18–24In the case of chemical modification, most efficient are the fluorine-containing compounds.25,26 In particular, the best effect is obtained by applying fluoro derivatives of organosilicon compounds, which combine the unique properties of the both components.27 Among this group of compounds, fluorofunctional trichloro- and trialkoxy-silanes,28–30 as well as fluorosilicones27,31,32 are applied most often, and recently, fluorofunctional silsesquioxanes33–35 have also been applied. Functional silsesquioxanes, owing to their unique properties, e.g., rigid nanoscale silicon–oxygen core with a diameter of about 1.5 nm, are often regarded as the smallest silica particle that can also influence the surface roughness and tunable properties of functional groups attached to silicon atoms. Therefore, the fluorofunctional silsesquioxanes are good precursors for the synthesis of highly hydrophobic materials.
In spite of many interesting properties, fluorofunctional silicon compounds are not commonly applied to this purpose mainly because of the difficulties in their synthesis, high price and poor availability of raw products. Our extensive experience of the process of hydrosilylation of different olefins has enabled the development of effective catalysts for processes,36 including fluorinated olefins. Owing to this, it was possible to perform simple syntheses of fluorofunctional silanes, polysiloxanes and silsesquioxanes in a one-pot process,37–39 and to use them later for surface modification. However, fluorocarbofunctional groups, in spite of their specific properties, surface properties in particular, are not reactive from a chemical point of view. To create a stable bond to a substrate, the presence of another reactive group is essential, as is the case of fluorofunctional trialkoxysilanes. This is why we developed a method of synthesis (based on consecutive hydrosilylation of two different olefins) of polysiloxanes and silsesquioxanes containing trimethoxysilyl or glycidyl group40 besides fluoroalkyl group. The reactivity of trimethoxysilyl and glycidyl groups causes that they can form stable bonds with the substrate as a result of a reaction with hydroxyl groups present on the surface.
Most papers on hydrophobic properties reported the effect of only one type of organosilicon derivative, whereas there are no reports on the comparison of the effect of different types of silicon compounds. For this reason, this paper presents the results of our research on the modification of glass surfaces using fluorofunctional silanes, polysiloxanes and silsesquioxanes also containing the abovementioned reactive groups. In addition to octafluoropentyloxypropyl trimethoxysilane, we also used two siloxane copolymers of the same length of siloxane chain and the same number of octafluoropentyloxypropyl groups, but differing in the type of reactive groups (trimethoxysilylethyl or glycidoxypropyl ones), as well as two silsesquioxanes (POSS) with an analogous type of group. The choice of such derivatives was based on the results of earlier studies. Moreover, the present study was aimed at determining the effects of the modification method on the developed hydrophobic properties of the substrate. To meet this aim, modifications were performed using solutions of organosilicon derivatives only and by adding nanosilica.
Experimental
Materials
All commercially available chemicals were used as received, without any further purification. Poly(dimethyl-co-hydromethyl)siloxanes and vinyltrimethoxysilane were purchased from Gelest. Triethoxysilane and tetraethoxysilane were obtained from “Unisil” (Poland). Fumed silicas: Aerosil 130 (16 nm) and Aerosil300 (7 nm) were bought from Evonik. 3,3,3-Trifluoropropyltrimethoxysilane, 1H,1H,2H,2H-perfluorodecyltrimethoxysilane and the other reagents, i.e. allyl glycidyl ether, Karstedt catalyst and solvents, were supplied by Aldrich. All functionalized silicon compounds were obtained by hydrosilylation of a fluorinated olefin, namely 1,1,2,2,3,3,4,4-octafluoropentyl allyl ether, with triethoxysilane, poly(dimethyl-co-hydromethyl)siloxanes and octakis(hydrido, dimethylsiloxy)octasilsesquioxane. In the case of polysiloxane and silsesquioxane derivatives, an additional olefin was employed, namely vinyltrimethoxysilane or allyl glycidyl ether, in the process of consecutive hydrosilylation. Fluorofunctional silanes were synthesized according to the procedure described in ref. 37 and polysiloxanes and silsesquioxanes containing mixed functional groups – according to the procedure presented in ref. 38–40. 1,1,2,2,3,3,4,4-Octafluoropentyl allyl ether was synthesized by the Williamson reaction using octafluoropentanol and allyl chloride.41 Rhodium siloxide complex, [{Rh(OSiMe3)(cod)}2] was synthesized using the method mentioned in one of the previously studied report.42 The glass plates were purchased from Thermo Scientific.Physico-chemical characterization
1H NMR (300 MHz), 13C NMR (75 MHz) and 29Si MNR (59 MHz) spectra were recorded on a Varian XL 300 spectrometer at room temperature using CDCl3 or C6D6 as the solvent. FT-IR spectra were recorded on a Bruker Tensor 27 Fourier transform spectrometer equipped with a SPECAC Golden Gate diamond ATR unit. In all cases, 16 scans at a resolution of 2 cm−1 were collected for a spectrum. Measurements of the contact angle were carried out using a Krüss GmbH instrument model DSA 100 Expert. The method of measurement is based on an analysis of the drop shape and it enables measurements at a rate of up to 2000 f s−1. The instrument was equipped with a fully automatic dosing system.Acoustic AC mode atomic force microscopy (AAC-AFM) was applied to surface characterization. The measurements were performed on a scanning probe microscope Agilent 5500 equipped with silicon cantilevers BudgetSensors AllinOne working at a frequency of 150–400 kHz and dynamic mode with phase contrast imaging. The results of the measurements were analyzed using WSxM 5.0 Develop 6.5 software.43 The sample roughness was estimated based on the arithmetic mean roughness (Ra parameter) and root mean square (Rq parameter).
Modification of glass plates
Results and discussion
The glass surfaces were modified with fluorofunctional organosilicon compounds, representing the three basic types of derivatives, i.e., silanes, polysiloxanes and silsesquioxanes. Considering that the hydrophobic properties are influenced to a significant extent by fluoroalkyl chain length, all the derivatives employed had the same substituent, namely an octafluoropentyloxypropyl group. Moreover, each of the compounds had a reactive group that, by a reaction with hydroxyl groups present of glass surface, forms a stable bond with the substrate. Our earlier research showed that, besides the commonly used trialkylsilyl groups, glycidyl groups are also highly reactive. The former, via hydrolysis to silanols, undergoes easy condensation with the surface OH groups to form stable siloxane bonds, while in the latter case, their oxirane ring undergoes rupture under the influence of a hydroxyl group, forming a bond to the substrate. All derivatives were obtained by the hydrosilylation of allyl octafluoropentyl ether with triethoxysilane and hydrosilylation of vinyltrimethoxysilane or allyl glycidyl ether with poly(hydrimethyl-co-dimethyl)siloxane or octakis(hydridodimethylsiloxy)octasilsesquioxane in definite stoichiometric ratios. Five derivatives, the formulas of which are presented in Fig. 1, were chosen for the study. Moreover, for a comparison of the hydrophobic properties, the silanization of glass plates was performed using two commercially available fluorofunctional silanes, i.e., 3,3,3-trifluoropropyltrimethoxysilane (TFS), and 1H,1H,2H,2H-perfluorodecyltrimethoxysilane (PFS).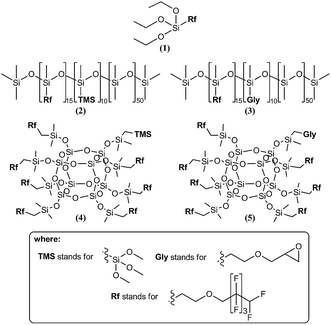 | ||
| Fig. 1 Fluorofunctional organosilicon compounds employed for the modification of glass plate surfaces. | ||
In the first stage of the study, glass plate surfaces were subjected to modification with acidified 5% ethanolic solutions of the abovementioned compounds. After modification, the plates were subjected to drying at 120 °C for 1 hour and then the water contact angle (WCA) was measured. Initially, modification was carried out using three methods: (i) by immersing a plate several times in a modifier solution, (ii) by immersing a plate in an analogous way, but under the action of ultrasound, and (iii) by vapor phase deposition. However, the obtained results showed that the differences in the water contact angles on surfaces modified by fluorofunctional polysiloxanes or silsesquioxanes using the aforementioned methods were insignificant, which indicates that the modification method has practically no effect on the hydrophobic properties of the surfaces. Only in the case of modification with fluorofunctional silanes was an increase in WCA observed when the surface was modified by vapor phase deposition. The increase was caused by the greater volatility of this compound compared to polysiloxanes and silsesquioxanes, which resulted in a higher concentration of this derivative in the vapor phase and this was reflected by the higher degree of surface coverage. However, to compare hydrophobic properties of different compounds it was necessary to create identical conditions of their application, which would be impossible in the case of poorly volatile polysiloxanes and silsesquioxanes. For this reason, only a dip-coating under the action of ultrasound (ultrasound helped with a better and more uniform distribution of compounds on the surface) was used at the next stage of the study. The WCA values for the surfaces modified with all fluorofunctional silicon derivatives synthesized in this study as well as with two commercially available silanes (TFS and PFS), using the aforementioned method, are shown in Table 1.
The obtained results show that modifications with all compounds employed in this study induced the formation of hydrophobic surfaces. The lowest WCA values were observed for the surfaces treated with fluorofunctional silanes. Furthermore, the WCA value is influenced by the length of the fluoroalkyl chain (thereby by fluorine content in a compound). From among the surfaces modified with different fluorofunctional silanes, the highest WCA values were observed for the surface modified with PFS, which has the longest fluoroalkyl chain. However, considering that the abovementioned compound contains more than twice as many of fluorine atoms as silane (1), the difference in the WCA values of the discussed surfaces (by 6°) is relatively small. The other commercial silane (TFS), which contains only three fluorine atoms, was characterized by the lowest WCA. In the latter case, however, the WCA value was influenced not only by the lowest fluorine content, but also by the length of the chain, because the longer the alkyl chain (including fluoroalkyl one), the greater are the hydrophobic properties. Considering that the differences in WCA for the silanes appeared to be small, as well as that the present study was aimed at determining the effect of the silicon compound structure with the same fluoroalkyl substituents, the further part of this study was carried out with compounds 1–5 as the surface modifying agents. An analysis of the results shown in Table 1 suggests that WCA is influenced not only by the number of fluoroalkyl groups in a molecule, but also by the structure of the fluorine-containing compound. The highest WCA was observed for the surfaces modified with silsesquioxanes (4 and 5). A comparison of the structure of different types of silicon compounds suggests that modification with fluorofunctional silane (1) results in linking this compound to a point on a glass plate surface, which in the case of favorable conditions is followed by the condensation of a few silane molecules at the surface of the plate (see Fig. 2a). The molecules are linked to the surface in a rigid manner. In case of fluorofunctional polysiloxane, the surface is covered with a siloxane layer. Taking into account the flexibility of the siloxane chain and the statistical distribution of the functional groups (Fig. 2b), one can expect that the chain is close to the surface only at the point of its linking, whereas its other part undergoes undulation, which will expose fluoroalkyl groups on the surface. Moreover, the polysiloxane layer will cover the free hydroxyl groups on the surface, thus blocking them. In the case of silsesquioxanes, their linking to the surface occurs only via one group. Therefore, the silsesquioxane cage is flexible and seven fluoroalkyl groups can be very well exposed on the surface (Fig. 2c).
Moreover, an analysis of the WCA values for various polysiloxane and silsesquioxane derivatives showed higher values for the derivatives containing glycidoxypropyl groups (Gly) compared to those containing trimethoxysilylethyl groups (TMS). This is because of the length of the chain that links a reactive group with the main siloxane chain or silsesquioxane cage. In the case of glycidyl derivatives, this chain is longer, which results in greater flexibility of the attachment to the surface, thereby providing a better possibility for the orientation of fluoroalkyl groups. Therefore, greater surface diversification occurs, which results in an increase in WCA. An example of epoxy group-containing polysiloxane linking to the surface is shown in Fig. 3.
 | ||
| Fig. 3 Exemplary way of linking a glass substrate and epoxy group-containing fluorofunctional polysiloxane. | ||
Examples of drop shapes for selected systems (A–C) are presented in Fig. 4.
It is well-known that maximum water contact angle on a perfectly smooth surface is 120°, and a micro/nano structure must be developed to achieve superhydrophobic properties.30 Synthetic superhydrophobic surfaces have been produced through various approaches, including the creation of a rough surface followed by a coating with low surface energy substances. Many methods have been developed to obtain rough surfaces. Among these methods, colloid assembly is most suitable.
Employing the procedure described previously studied reports,21 we have coated glass plates using a silica sol that was obtained by mixing aqueous ammonia with tetraethoxysilane (TEOS) in ethanol. After stirring for two hours at 60 °C, nanosilica (Aerosil 300) was added. To determine the optimal concentration of silica added to the sol, we prepared several solutions, which were then placed in contact with the glass plates. The silica was immersed in the sol for 5 minutes before the first dip-coating, and 5 seconds before the subsequent dip-coating. After each coating, the substrate was dried at room temperature for 5 min, and this process was repeated 5 times. The modification was ultrasound-aided to obtain a better dispersion of silica in the silica sol. The coated plates were then heated at 200 °C for 1 h. For comparison, another series was made in an analogous manner with the additional use of ultrasounds. At the next stage, plates prepared in this way were immersed in an acidified solution of a suitable fluorofunctional compound using the dip-coating method. The contact angles on surfaces modified by fluorofunctional silane (1) and polysiloxane (2) derivatives are presented in Table 2.
| Compound | WCA [°] on the surface modified with silica sol mixed with Aerosil 300 at concentrations given below | |||
|---|---|---|---|---|
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2% 2% |
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2% 1.2% |
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6% 0.6% |
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3% 0.3% |
|
| 1 | 91 | 106 | 95 | 95 |
| 2 | 111 | 125 | 121 | 110 |
The results presented in Table 2 show that the highest water contact angle was achieved for the surface pretreated with a 5% solution of the silica sol mixed with 1.2% Aerosil 300. This is significantly higher than the concentration used in further studies. The abovementioned WCA values were considerably higher than the ones observed for surfaces modified with solutions of fluorofunctional silicon compounds only. For this reason, at the next stage of this study, the glass plates were modified with silica sol according to the procedure described above using two types of nanosilica: Aerosil 300 (particle size of 7 nm) and Aerosil 130 (particle size 16 nm). Such prepared glass substrates were treated with solutions of all types of fluorofunctional derivatives. All modified surfaces were subject to WCA measurements, results of which are presented in Table 3.
The obtained results explicitly show that Aerosil 130, i.e., the silica of a greater particle size (16 nm), creates greater surface roughness, which results in a considerable increase in the WCA after modification with the organosilicon compound. Considering the kind of organosilicon compound employed for modification, one can notice that the tendency to a change in hydrophobic properties was maintained, as in the case of surface modification with solutions of organosilicon derivatives only, i.e., the lowest values were obtained for fluorofunctional silane (1) and the highest were obtained for silsesquioxane derivatives (4 and 5). Noteworthy is also the surface modified with polysiloxane (3) that was earlier covered with Aerosil 130. In this case, a superhydrophobic surface was also obtained. However, the preparation of polysiloxane containing mixed functional groups as well as the price of the derivative are considerably more advantageous compared to silsesquioxane derivatives. Examples of the drop shapes for the selected systems (D–F) are presented in Fig. 5.
This study showed that surfaces subjected to the preliminary modification with silica of a definite particle size, followed by a further modification with fluorofunctional silicon compound that can be stably bound to the substrate, become strongly hydrophobic. Bonding of silsesquioxanes with a cage structure to preliminary modified glass surface results in its greatest diversification and the best hydrophobic properties (the highest WCA values), particularly if they are linked via a long group originating from the glycidoxypropyl substituent (Gly). However, the application of polysiloxane (3) containing the same substituents causes (due to the great flexibility of the siloxane chain) an appropriate arrangement of siloxane on the rough surface and the best orientation of fluoroalkyl groups outside the layer. Only for this derivative and for functionalized silsesquioxanes one can observe the greatest increase in WCA (by 46 degree, 43 degree and 48 degree, respectively) caused by the diversification of the surfaces by silica (Fig. 6).
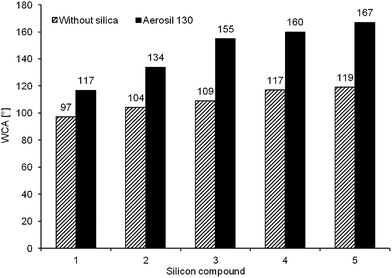 | ||
| Fig. 6 Change in the WCA for the surfaces modified with solutions of organosilicon compounds alone and in addition with the use of silica Aerosil 130, while using ultrasound-aided immersion method. | ||
The morphology of the surfaces of the created coatings was also studied by atomic force microscopy, and results at its initial stage were quite surprising. Measurements of a non-treated plate and a silsesquioxane-covered plate showed that the non-treated plate has comparable (and sometimes even higher) roughness than the modified one. The measurements were repeated several times also using the new plates modified with the same compound, and results were similar. To determine how the roughness will be affected by silsesquioxanes having another number of functional groups, two derivatives with different contents of Rf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMS groups were prepared, i.e., 6
TMS groups were prepared, i.e., 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 4
2 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The abovementioned compounds were the subject of our earlier research.44 A comparison of the results obtained for non-treated plate and a series of plates covered by functionalized silsesquioxanes with different ratios of functional groups, Rf
4. The abovementioned compounds were the subject of our earlier research.44 A comparison of the results obtained for non-treated plate and a series of plates covered by functionalized silsesquioxanes with different ratios of functional groups, Rf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMS, showed that the non-treated plate was characterized by a slightly greater roughness than the plates modified using POSS-containing 4 fluoroalkyl groups and 4 trimethoxysilyl groups and 6 fluoroalkyl and 2 trimethoxysilyl groups. Modification of the plate surface by the derivative containing seven fluoroalkyl and one trimethoxysilyl group brought about a small increase in surface roughness. The root mean square deviation of surface Rq was 0.35 nm for the non-treated substrate and 0.23, 0.37 and 0.41 nm for substrates modified using POSS whose ratios of functional groups Rf
TMS, showed that the non-treated plate was characterized by a slightly greater roughness than the plates modified using POSS-containing 4 fluoroalkyl groups and 4 trimethoxysilyl groups and 6 fluoroalkyl and 2 trimethoxysilyl groups. Modification of the plate surface by the derivative containing seven fluoroalkyl and one trimethoxysilyl group brought about a small increase in surface roughness. The root mean square deviation of surface Rq was 0.35 nm for the non-treated substrate and 0.23, 0.37 and 0.41 nm for substrates modified using POSS whose ratios of functional groups Rf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMS were 4
TMS were 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 6
4, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 7
2 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Differences in the surface morphology are presented in Fig. 7.
1, respectively. Differences in the surface morphology are presented in Fig. 7.
 | ||
Fig. 7 AFM images of (a) non-treated glass plate, (b)–(d) glass plates modified with silsesquioxanes of Rf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMS group ratios of 4 TMS group ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 6 4, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 7 2 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. 1, respectively. | ||
The measurements of the roughness (Rq) correlate well with the results of the water contact angle (WCA) carried out on the same substrates. We have established that the WCA values increase with increasing number of fluoroalkyl groups present in a molecule of a modifier compound, as well as with increasing surface roughness. The effect of surface smoothing that was observed for substrates modified with the POSS derivative with a ratio of functional groups of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 should be explained by the possibility of forming multidimensional structures on the glass surface. The structures with a low degree of organization of fluoroalkyl chains are the products of the condensation of trimethoxysilyl groups (present in POSS) not only with the substrate but also between the modifier molecules. This causes surface smoothing and reduces the number of accessible surface fluoroalkyl groups. The POSS derivative containing six fluoroalkyl and two trimethoxysilyl groups can still form extended surface structures, but their effects on the reduction in the surface roughness is slightly less, which in combination with a greater content of fluoroalkyl groups, results in an increase in surface hydrophobicity. In the case of the surface modified with functionalized organosilicon derivatives only, the highest wetting angle (117°) was observed for the substrate treated with the derivative containing only one trimethoxysilyl and seven fluoroalkyl groups. This can be explained by the fact that the above derivative can be linked to the support via only one trimethoxysilyl group. This is why silsesquioxane molecules have a great freedom of orientation and the possibility of forming a homogeneous surface layer, in which seven fluoroalkyl substituents can be well arranged on the surface, causing strong hydrophobization. Moreover, silsesquioxanes that are well-defined regular nanomolecules can level scratches occurring on a glass plate by partially filling them. This is proved by the average distances between the local maxima observed on surfaces of the untreated glass plates. This is highlighted by the roughness profile presented in Fig. 8 and in that the abovementioned maximum is 225 nm on average.
4 should be explained by the possibility of forming multidimensional structures on the glass surface. The structures with a low degree of organization of fluoroalkyl chains are the products of the condensation of trimethoxysilyl groups (present in POSS) not only with the substrate but also between the modifier molecules. This causes surface smoothing and reduces the number of accessible surface fluoroalkyl groups. The POSS derivative containing six fluoroalkyl and two trimethoxysilyl groups can still form extended surface structures, but their effects on the reduction in the surface roughness is slightly less, which in combination with a greater content of fluoroalkyl groups, results in an increase in surface hydrophobicity. In the case of the surface modified with functionalized organosilicon derivatives only, the highest wetting angle (117°) was observed for the substrate treated with the derivative containing only one trimethoxysilyl and seven fluoroalkyl groups. This can be explained by the fact that the above derivative can be linked to the support via only one trimethoxysilyl group. This is why silsesquioxane molecules have a great freedom of orientation and the possibility of forming a homogeneous surface layer, in which seven fluoroalkyl substituents can be well arranged on the surface, causing strong hydrophobization. Moreover, silsesquioxanes that are well-defined regular nanomolecules can level scratches occurring on a glass plate by partially filling them. This is proved by the average distances between the local maxima observed on surfaces of the untreated glass plates. This is highlighted by the roughness profile presented in Fig. 8 and in that the abovementioned maximum is 225 nm on average.
A totally different situation has been observed for surfaces modified with functionalized polysiloxane (2), as shown in Fig. 9.
In this case, a considerable increase in surface roughness was observed (Rq is 26.8 nm). The surface coverage with polysiloxane results in the appearance of irregular oval structures. This can be explained by the great flexibility of the siloxane chain and the statistical distribution of functional groups in the chain. The orientation of the abovementioned groups on the surface results in the creation of a kind of domains. A long polysiloxane chain can, due to linking to several alkoxysilyl groups, undergo additional bending that results in the over-exposure of fluoroalkyl groups. This is also a confirmation of the model presented in Fig. 2. This causes the greater surface roughness and, even though polysiloxane (2) does not contain considerable fluoroalkyl substituents as silsesquioxane (4), the water contact angle of the polysiloxane-modified surface is also high and equals to 109°.
Preliminary modification of glass plate surface certainly brings about an increase in its roughness and additional chemical modification with fluorofunctional organosilicon derivatives leads to the formation of superhydrophobic surfaces. Fig. 10 shows AFM images of the surface pretreated with a silica sol and then modified with polysiloxane (2) and silsesquioxane (4).
 | ||
| Fig. 10 AFM images of glass plates modified with silica sol and then with (a) polysiloxane (2), and (b) silsesquioxane (4). | ||
In both cases Rq is almost the same at 58.2 nm. In the case of the plate covered with silica sol and then with silsesquioxane the layer is considerably thicker, which results in some crack formation, but generally the surfaces are homogeneous.
Conclusions
The surfaces of glass plates were modified with five different organosilicon derivatives (silanes, polysiloxanes and silsesquioxanes) that contained the same fluoroalkyl substituents (octafluoropentyloxypropyl, Rf) and groups enabling the formation of bonds to the substrate (trialkoxysilyl TMS or glycidoxypropyl Gly) and the hydrophobic properties of the surfaces were compared. Initially, the modification was performed using three methods; however, due to their insignificant effect on hydrophobic properties, a further study was carried out using an ultrasound-aided dip-coating method. In each case, hydrophobic surfaces were produced, but the best effect (the highest values of wetting angle) was obtained for the modification using silsesquioxanes (4 and 5). Compared to other compounds studied, silsesquioxanes are characterized by the greatest number of fluoroalkyl substituents (although the highest fluorine content occurs in the case of silane (1)) that are well exposed on the surface. The cage structure of silsesquioxane enables it to be linked to the substrate by one corner and the remaining seven corners with fluoroalkyl substituents contribute to the high hydrophobicity. Initially, it was supposed that the cage structure will lead to an increase in surface roughness, however, AFM showed that the cage dimensions are small enough to result in an opposite effect to that expected, i.e., the modification with silsesquioxanes caused a reduction in the surface roughness. To increase the surface roughness, two silicas, Aerosil 130 and Aerosil 300, were applied to the plates from the silica sol. Modification of such prepared surfaces with the abovementioned silicon compounds made it possible to create superhydrophobic surfaces in the case of compounds 3–5. The best effect was obtained for the modification with silsesquioxanes (WCA = 167°), however, polysiloxane also produced a superhydrophobic surface (WCA = 155°). These results can be explained by the great flexibility of the siloxane chain that enables a good orientation of fluoroalkyl chains on the surface. In the case of both polysiloxanes and silsesquioxanes, it was found that a longer substituent that links them to the substrate had higher surface hydrophobicity because the distance between the molecules and the surface is greater, which means that the surface roughness is also greater. Considering that the synthesis of fluorofunctional polysiloxane is simpler and cheaper than that of silsesquioxane, as well as the fact that the hydrophobizing activity of polysiloxanes is considerably more effective than that of most frequently applied fluorofunctional silanes, this group of derivative appears to be the most promising from the point of view of practical applications.Acknowledgements
We gratefully acknowledge the financial support from the Ministry of Science and Higher Education (Poland) for the project no. N N209 765640.Notes and references
- X. Gao and L. Jiang, Nature, 2004, 432, 36 CrossRef CAS PubMed.
- V. A. Ganesh, H. K. Raut, A. S. Nair and S. Ramakrishna, J. Mater. Chem., 2011, 21, 16304 RSC.
- M. Farzaneh and D. K. Sarker, J. Adhes. Sci. Technol., 2009, 23, 1215 CrossRef.
- S. A. Kulinich and M. Farzaneh, Langmuir, 2009, 25, 8854 CrossRef CAS PubMed.
- T. Darmanin and F. Guitted, J. Mater. Chem., 2009, 19, 7130 RSC.
- J. A. Taylor, T. N. Krupenkin, T. M. Schneider and S. Yang, Langmuir, 2004, 20, 3824 CrossRef.
- Y. Zhu, J. Zhang, J. Zheng, Z. Huang, L. Feny and L. Jiang, Adv. Funct. Mater., 2006, 16, 568 CrossRef CAS.
- Z. Guo, W. Liu and B. L. Su, J. Colloid Interface Sci., 2011, 353, 335 CrossRef CAS PubMed.
- Y. Xiu, L. Zhu, D. W. Hess and C. D. Wong, Nano Lett., 2007, 7, 3388 CrossRef CAS PubMed.
- Q. F. Xu, J. N. Wang, I. H. Smith and K. D. Sanderson, J. Mater. Chem., 2009, 19, 655 RSC.
- L. Valtola, A. Koponen, M. Karesoja, S. Hietala, A. Laukkanen, H. Tenhu and P. Denifl, Polymer, 2009, 50, 3103 CrossRef CAS.
- N. Zhao, Q. D. Xie, L. H. Weng, S. Q. Wang, X. Y. Zhang and J. Xu, Macromolecules, 2005, 38, 8996 CrossRef CAS.
- G. Zhang, D. Y. Wang, Z. Z. Gu and H. Möhwald, Langmuir, 2005, 21, 9143 CrossRef CAS PubMed.
- L. Xu and J. He, Langmuir, 2012, 28, 7512 CrossRef CAS PubMed.
- L. Mammen, X. Deng, M. Untech, D. Vijayshanker, P. Papadopoulos, R. Berger, E. Riccardi, F. Leroy and D. Vollmer, Langmuir, 2012, 28, 15005 CrossRef CAS PubMed.
- X. Du, X. Li and J. He, ACS Appl. Mater. Interfaces, 2010, 2, 2365 CAS.
- X. Du and J. He, ACS Appl. Mater. Interfaces, 2011, 3, 1269 CAS.
- Y. Xiu, F. Xiao, D. W. Hess and C. P. Wong, Thin Solid Films, 2009, 517, 1610 CrossRef CAS.
- B. J. Basu, V. Hariprakash, S. T. Aruna, R. V. Lakshni, J. Manasa and B. S. Shruthi, J. Sol-Gel Sci. Technol., 2010, 56, 278 CrossRef CAS.
- H. M. Shang, Y. Wang, S. J. Limmer, T. P. Chou, K. Takahashi and G. Z. Cao, Thin Solid Films, 2005, 472, 37 CrossRef CAS.
- Q. F. Xu, J. N. Wang and K. D. Sanderson, A General Approach for Superhydrophobic Coating with Strong Adhesion Strength, J. Mater. Chem., 2010, 20, 5961 RSC.
- Y. Xiu, D. W. Hess and C. P. Wong, J. Colloid Interface Sci., 2008, 326, 465 CrossRef CAS PubMed.
- A. V. Rao, S. S. Latthe, D. Y. Nadargi, H. Hirashima and V. Ganescan, J. Colloid Interface Sci., 2009, 332, 484 CrossRef PubMed.
- K.-Y. Yeh, K.-H. Cho and Li-J. Chen, Langmuir, 2009, 25, 14187 CrossRef CAS PubMed.
- M. Pagliaro and R. Ciriminna, J. Mater. Chem., 2005, 15, 4981 RSC.
- L. W. McKeen, Fluorinated Coatings and Finishes Handbook, Wiliam Andrew Publ., New York, 2006 Search PubMed.
- M. J. Owen, in Silicones and Silicone-Modified Materials, ed. S. J. Clarson, M. J. Owen, S. D. Smith and M. E. Van Dyke, ACS Publ., New York, 2010, ch. 9 Search PubMed.
- Y. Hayashi, T. Yoneda and K. Matsamoto, J. Ceram. Soc. Jpn., 1994, 102, 206 CrossRef CAS.
- N. Yoshino, Chem. Lett., 1994, 4, 735 CrossRef.
- B. Arkles, Y. Pan and Y. M. Kim, in Silanes and Other Coupling Agents, ed. K. L. Mittal, VSP, Utrecht, 2009, vol. 5 Search PubMed.
- C. Pasquet, C. Lonquet, S. Hamdami-Devarennes, B. Amedur and F. Ganachaud, in Silicone Surface Science, ed. M. J. Owen and P. R. Dvornic, Springer, Dordrecht, 2012, ch. 5 Search PubMed.
- F. Giuda-Pietrasanta and B. Boutevin, Adv. Polym. Sci., 2005, 179, 1 CrossRef.
- W. Choi, A. Tuteja, S. Chhatre, J. M. Mabry, R. E. Cohen and G. H. McKinley, Adv. Mater, 2009, 21, 2190 CrossRef CAS.
- A. Tuteja and J. M. Mabry, in Silicone Surface Science, ed. M. J. Owen and P. R. Dvornic, Springer, Dordrecht, 2012, ch. 6 Search PubMed.
- Application of Polyhedral Oligomeric Silsequioxanes, ed. C. Hartmann-Thompson, Springer, Dordrecht, 2011 Search PubMed.
- B. Marciniec, H. Maciejewski, C. Pietraszuk and P. Pawluć, Hydrosilylation A. Comprehensive Review on Recent Advances, Springer, Dordrecht, 2009 Search PubMed.
- H. Maciejewski, B. Marciniec and J. Karasiewicz, EP 2473514 (B1), 2012.
- H. Maciejewski, J. Karasiewicz and B. Marciniec, EP 2473551 (B1), 2013.
- H. Maciejewski, M. Dutkiewicz and B. Marciniec, EP 2473549 (B1), 2013.
- M. Dutkiewicz, H. Maciejewski, B. Marciniec and J. Karasiewicz, Organometallics, 2011, 30, 2149–2153 CrossRef CAS.
- H. Maciejewski, J. Karasiewicz and B. Marciniec, Polimery, 2012, 57, 449 CrossRef CAS.
- B. Marciniec, P. Krzyżanowski, E. Walczuk-Guściora and W. Duczmal, J. Mol. Catal., 1999, 144, 263 CrossRef CAS.
- I. Horcas, R. Fernandez, J. M. Gomez-Rodrigez, J. Colchero, J. Gomez-Herrero and A. M. Baro, Rev. Sci. Instrum., 2007, 78, 013705 CrossRef CAS PubMed.
- H. Maciejewski, J. Karasiewicz, M. Dutkiewicz and B. Marciniec, Silicon, 2014 Search PubMed , submitted for publication.
| This journal is © The Royal Society of Chemistry 2014 |





