A green and facile approach to obtain 100 nm zeolitic imidazolate framework-90 (ZIF-90) particles via leveraging viscosity effects†
Wei-Shang Loa,
Szu-Mam Liub,
Shao-Chun Wanga,
Hsiu-Pen Lina,
Nianhan Ma*b,
Hsi-Ya Huangc and
Fa-Kuen Shieh*a
aDepartment of Chemistry, National Central University, 300 Jhong-Da Road, Chung-Li 32001, Taiwan. E-mail: fshieh@ncu.edu.tw
bInstitute of Systems Biology and Bioinformatics, National Central University, 300 Jhong-Da Road, Chung-Li 32001, Taiwan. E-mail: manhlin@ncu.edu.tw
cDepartment of Chemistry, Chung Yuan Christian University, 200 Chung Pei Road, Chung-Li, 32023, Taiwan
First published on 16th October 2014
Abstract
For the first time, ∼100 nm zeolitic imidazolate framework-90 (ZIF-90) particles were produced by a water–alcohol-based system, taking advantage of viscosity effects using an optimized H2O/tert-butanol/glycerol/PVP system. Furthermore, an in vitro cytotoxicity assay revealed the half-maximal effective concentration (EC50) of ZIF-90 nanoparticles to be ∼92 μg ml−1.
In past decades, porous materials such as metal–organic frameworks (MOFs)1 have attracted immense attention owing to their potential for producing a large variety of unique structures. In particular, porous materials offer sustainable solutions for issues, such as greenhouse gas reduction,2 industrial catalysis,3 pollutant treatment,4,5 disease diagnosis and therapy,6 and efficient electronics7 and gas fuel storage,8 owing to a variety of superior properties including a unique pore structure and large specific surface area.9 For the realization and advancement of more demanding applications such as sensors,10 catalysis,11 and drug delivery,12 porous materials miniaturized to the nanoscale, or even smaller, may offer significantly altered properties, such as higher reactivity, compared with bulk material. This shift occurs since increasing textural porosity and external surfaces removes or diminishes mass transfer limits.13 Therefore, strategies for the synthesis of nanosized porous materials, i.e., nanoMOFs, are attracting steady interest in the materials science field.14 In general, most conventional protocols for nanoMOF synthesis require minimal chemical reaction control (addition of modifiers or surfactants, or altering reactant ratios), but solely rely on growth control via physical parameters, such as the type of energy supply, reaction time, and temperature.14 Although a number of methodologies have been reported9,14 for the synthesis of nanosized MOFs, routes for their efficient synthesis with desirable size, shape, and surface morphology are still under investigation. Furthermore, most nanoMOFs are usually synthesized by systems containing toxic organic solvents such as dimethylformamide (DMF)15 and tetrahydrofuran (THF).16 However, in addition to reduced particle size, the use of porous solids for industrial or biomedical applications also requires a more environmentally friendly synthesis or biocompatible composition—i.e., a low-toxicity system for synthesis and less cytotoxicity. So far, unfortunately, such routes for synthesizing nanoMOFs are very scarce.
Currently, our research is focused on the development of an efficient, rapid approach and environmentally friendly synthetic methods for precisely miniaturizing zeolitic imidazolate framework (ZIF) to realize particles with sizes from the micrometer to nanometer scale.17 In addition, we investigate their potential biomedical applications.18 ZIFs, a subclass of MOFs, have an extended 3D structure consisting of tetrahedral metal ions (M = Zn or Co) bridged by imidazolate. Moreover, the M–Im–M angle is similar to the Si–O–Si angle (145°) preferred in zeolites. ZIF-90, zinc(imidazolate-2-carboxyaldehyde(ICA))2, is with the carbonyl group in the 2-position of the imidazole linker and the resulting structure has also been reported to have a high thermal and solvent stability.19 Therefore, it is an ideal composition for use in gas separation or structural patches20 composed of thin films or membranes.21
Water-miscible alcohols (as trigger solvent in the synthetic precipitation method), e.g., ethanol (C = 2), propan-2-ol (C = 3), and tert-butanol (C = 4), have been used in the water-based synthetic system, and their effects on synthesized ZIF-90 were studied in our recent report.17 Remarkably, we found that a smaller ZIF-90 particles were produced with increasing viscosity (i.e., increased number of carbons in the alcohol of the synthetic solution). More specifically, the synthetic system of tert-butanol + water/polyvinyl-pyrrolidone polymer (PVP, MW = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) produced ∼280 nm ZIF-90 particles. Thus, the alcohol viscosity of the applied water-based system appears to be a vital factor for controlling the particle size of ZIF-90. In order to further reduce particle size, in this study, we adopted a water–alcohol-based system primarily modeled on that of the aforementioned study. In this case, for obtaining smaller ZIF particles as well as for the study of viscosity effects on particle size, we examined the volume-ratio change with glycerol (OH = 3) as a modifier. We then applied a synthetic system using a mixed tert-butanol/H2O/PVP solvent. It is worth noting that ZIF-90 nanoparticles (denoted as nanoZIF-90 hereafter) were well-dispersed and had a narrowed particle size distribution, ∼100 nm, when they were produced by this high-viscosity synthetic system with the concentrations of glycerol at 25 v/v% and that of PVP at 0.2 wt% (Scheme 1). In addition, in vitro toxicity of nanoZIF-90 was also established around 90 μg ml−1 of half-maximal effective concentration (EC50). To the best of our knowledge, this is the smallest-diameter ZIF-90 particles synthesized by a water-related system so far as well as this nanoscaled material obtained here is with low cytotoxicity. Notably, although some reports22,23 had shown that ZIF-90 particles with sizes of one hundred nanometers or less can be successfully synthesized, those nanoscaled ZIF-90 particles produced by using the DMF solvent carry the risk of the toxic solvent still being present within the particles. Thus, we should note once again that a particle size distribution in this range (∼100 nm) as well as nanoscaled ZIF-90 synthesized by water-related systems are needed for improved membrane-type patches20 such as transdermal patches20 or for applications as nanocarriers for cell penetration24 or gas-separation films.19
000) produced ∼280 nm ZIF-90 particles. Thus, the alcohol viscosity of the applied water-based system appears to be a vital factor for controlling the particle size of ZIF-90. In order to further reduce particle size, in this study, we adopted a water–alcohol-based system primarily modeled on that of the aforementioned study. In this case, for obtaining smaller ZIF particles as well as for the study of viscosity effects on particle size, we examined the volume-ratio change with glycerol (OH = 3) as a modifier. We then applied a synthetic system using a mixed tert-butanol/H2O/PVP solvent. It is worth noting that ZIF-90 nanoparticles (denoted as nanoZIF-90 hereafter) were well-dispersed and had a narrowed particle size distribution, ∼100 nm, when they were produced by this high-viscosity synthetic system with the concentrations of glycerol at 25 v/v% and that of PVP at 0.2 wt% (Scheme 1). In addition, in vitro toxicity of nanoZIF-90 was also established around 90 μg ml−1 of half-maximal effective concentration (EC50). To the best of our knowledge, this is the smallest-diameter ZIF-90 particles synthesized by a water-related system so far as well as this nanoscaled material obtained here is with low cytotoxicity. Notably, although some reports22,23 had shown that ZIF-90 particles with sizes of one hundred nanometers or less can be successfully synthesized, those nanoscaled ZIF-90 particles produced by using the DMF solvent carry the risk of the toxic solvent still being present within the particles. Thus, we should note once again that a particle size distribution in this range (∼100 nm) as well as nanoscaled ZIF-90 synthesized by water-related systems are needed for improved membrane-type patches20 such as transdermal patches20 or for applications as nanocarriers for cell penetration24 or gas-separation films.19
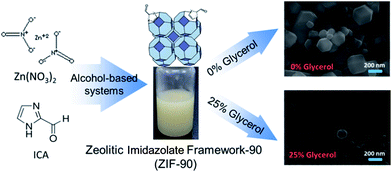 | ||
| Scheme 1 Synthesis of ZIF-90 nanoparticles with ∼100 nm particle size prepared by changing the viscosity using glycerol in a water–alcohol-based system. | ||
The detailed experimental procedure is described in the Experimental section (ESI†). Briefly, nanoZIF-90 were synthesized as follows: zinc nitrate (Zn(NO3)2) was added to the tert-butanol solution termed the trigger solution (solution A). Another aqueous solution containing ICA and 0.2% polyvinylpyrrolidone (PVP, MW = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was also prepared as a glycerol modifier (in varying amounts) (solution B). In general, the volume ratio of solution A to B was fixed at 1. The reactant molar ratio of Zn to ICA was maintained at 1
000) was also prepared as a glycerol modifier (in varying amounts) (solution B). In general, the volume ratio of solution A to B was fixed at 1. The reactant molar ratio of Zn to ICA was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in the final solution. After mixing solutions A and B at ambient temperature for a few minutes, the resultant powder was collected by centrifugation, washed with excess methanol, and vacuum dried at 50 °C.
4 in the final solution. After mixing solutions A and B at ambient temperature for a few minutes, the resultant powder was collected by centrifugation, washed with excess methanol, and vacuum dried at 50 °C.
To evaluate effects of viscosity on particle size, we performed a series of nanoZIF-90 syntheses from mixtures with a range of viscosities, by using different percentages of glycerol. In other words, we precisely tuned the particle size by controlling the glycerol modifier in a range of volume ratios from 0% to 25% in the final solution (Fig. 1). As expected, the particle size decreased from 280 nm to 110 nm as the volume ratio of glycerol increased from 0% to 25%. This trend is consistent with our assumption that the viscosity of the applied water–alcohol-based system solution is a key factor for controlling the nanoZIF-90 particle size. Additionally, the SEM images in Fig. 1b and c show that particles obtained with higher viscosity have a regular morphology. This indicates once again that glycerol plays an important role in the control of particle size and shape by controlling the viscosity of the synthetic solution.
However, it is worth noting that particle size increased dramatically when the volume ratio of glycerol modifier was greater than 25% in the synthetic solution (i.e., 30% glycerol; Fig. S1, ESI†). We believe that glycerol is not homogenously dispersed throughout the solution if the volume ratio of the modifier is greater than 25%. Therefore, under this condition, the viscosity is no longer a dominant factor determining particle size.
The crystallinity and functional group of as-synthesized nanoZIF-90 obtained using this water–alcohol-based system were examined using power X-ray diffraction and FT-IR, respectively. As evidenced in the XRD and FT-IR patterns, they exhibit the same functional group and crystallinity, respectively, as those synthesized in solvothermal systems with N,N-dimethylformamide (DMF) as the solvent25 (Fig. 2a, b, S2 and S3†). This indicates that our new method can also produce phase-pure nanoZIF-90. The structure of the nanoZIF-90 particles was also investigated by the N2-adsorption isotherm method; these results are summarized in Fig. 2c and Table 1. Remarkably, the nanoZIF-90 particles (size ∼ 100 nm) had a Langmuir surface area (SL) of about 1183 m2 g−1, and total pore volume of 0.52 cm3 g−1 when produced at ambient temperature. The N2 adsorption isotherm shown in Fig. 2c indicates a typical type-I isotherm.26 The increase in the volume adsorbed at very low relative pressures is due to the presence of micropores; a second uptake at high relative pressure with a very small adsorption–desorption hysteresis loop indicates the existence of textural meso-/microporosity, caused by the packing of crystal particles.27 Additionally, the thermal properties of nanoZIF-90 were investigated by using thermogravimetric analysis (TGA). The TGA curves in Fig. 2d and S5† were found to be different from those reported for the synthesis of micrometer-sized ZIF-90 crystals prepared using DMF. However, this result is similar to one in our previous report,17 as the TGA trajectories of the sample prepared in DMF show a deep drop at two steps in the 50–320 °C range, which resulted from the entrapment of DMF within the synthesized ZIF-90.12,17
| Systema | SLa (m2 g−1) | SBETb (m2 g−1) | Vtotalc | Vmicrod | Particle sizee [nm] |
|---|---|---|---|---|---|
| a Langmuir surface area.b BET surface area.c Total pore volume.d t-Plot micropore volume.e Particle size calculated from SEM images. | |||||
| DMF25 | 1320 | 1270 | 0.58 | 0.48 | 1430 ± 311 |
| H2O/tert-butanol17 | 1011 | 766 | 0.45 | 0.33 | 276 ± 60 |
| H2O/glycerol(25%)/tert-butanol | 1183 | 897 | 0.52 | 0.38 | 110 ± 30 |
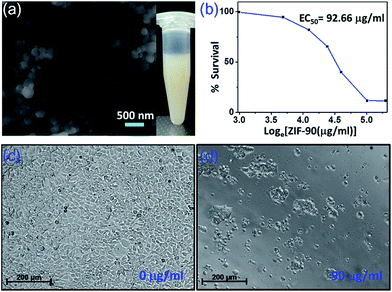
In order to gauge the potential of nanoZIF-90 for biomedical applications, their in vitro toxicity was established by the AlamarBlue assay, using a cell line of HEK-293 (Fig. 3). In order to maintain high dispersity in the high ionic strength of the cell medium,28 the nanoZIF-90 were capped with cetyltrimethylammonium bromide (CTAB) before performing the assays (Fig. 3a and S6†). As seen in Fig. 3b, the EC50 of our nanoZIF-90 was determined to be approximately 92 μg ml−1. This value is comparable to the reported EC50 values for gold nanoparticles (varies from 5 to 1000 μg ml−1), mesoporous silica nanomaterials (from 30 to 500 μg ml−1), ZIF-8 (from 100 to 140 μg ml−1), and MILs (57 ± 11 μg ml−1).29–32 The representative images in Fig. 3c and d show a cell line incubated with and without nanoZIF-90, respectively. This indicates that the HEK-293 cell line experienced cell death after incubation with 90 μg ml−1 nanoZIF-90 in 2 days.
In summary, we report the first synthesis of 110 nm size ZIF-90 nanoparticles, using a water–alcohol-based (H2O/PVP/glycerol/tert-butanol) system under ambient conditions. The effects of viscosity observed by the variation of glycerol concentrations on the properties of the synthesized ZIF-90 were also investigated. Further, the cytotoxicity of the nanoZIF-90 is moderate and comparable to that of other organic and inorganic drug carriers. This green-synthesis strategy is fast and reliable, compared to conventional organic-solvent-based systems. Thus, our water/polymer/alcohol system allows for the reduction in the size of ZIF-90 particles to ∼100 nm and the particles have a potential for use in industrial and biomedical applications owing to their less toxically synthetic method and low cytotoxicity.
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 103-2113-M-008-001; 103-2320-B-008-003-MY3) with special financial support from the Aim for the Top University Project by the Ministry of Education, Taiwan.Notes and references
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- M. Pera-Titus, Chem. Rev., 2013, 114, 1413–1492 CrossRef PubMed.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- M. M. Khin, A. S. Nair, V. J. Babu, R. Murugan and S. Ramakrishna, Energy Environ. Sci., 2012, 5, 8075–8109 CAS.
- A. Walcarius and L. Mercier, J. Mater. Chem., 2010, 20, 4478–4511 RSC.
- J. Cornella, C. Zarate and R. Martin, Chem. Soc. Rev., 2014 10.1039/c4cs00206g.
- A. Du, Z. Zhu and S. C. Smith, J. Am. Chem. Soc., 2010, 132, 2876–2877 CrossRef CAS PubMed.
- B. Fang, H. Zhou and I. Honma, J. Phys. Chem. B, 2006, 110, 4875–4880 CrossRef CAS PubMed.
- V. Valtchev and L. Tosheva, Chem. Rev., 2013, 113, 6734–6760 CrossRef CAS PubMed.
- J. Liu, F. Sun, F. Zhang, Z. Wang, R. Zhang, C. Wang and S. Qiu, J. Mater. Chem., 2011, 21, 3775–3778 RSC.
- H.-L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351–3370 RSC.
- R. Ananthoji, J. F. Eubank, F. Nouar, H. Mouttaki, M. Eddaoudi and J. P. Harmon, J. Mater. Chem., 2011, 21, 9587–9594 RSC.
- F. Fajula, A. Galarneau and F. D. Renzo, Microporous Mesoporous Mater., 2005, 82, 227–239 CrossRef CAS PubMed.
- E. A. Flugel, A. Ranft, F. Haase and B. V. Lotsch, J. Mater. Chem., 2012, 22, 10119–10133 RSC.
- Y.-S. Li, F.-Y. Liang, H. Bux, A. Feldhoff, W.-S. Yang and J. Caro, Angew. Chem., Int. Ed., 2010, 49, 548–551 CrossRef CAS PubMed.
- V. M. Suresh, S. J. George and T. K. Maji, Adv. Funct. Mater., 2013, 23, 5585–5590 CrossRef CAS.
- F.-K. Shieh, S.-C. Wang, S.-Y. Leo and K. C. W. Wu, Chem.–Eur. J., 2013, 19, 11139–11142 CrossRef CAS PubMed.
- C.-Y. Sun, C. Qin, X.-L. Wang, G.-S. Yang, K.-Z. Shao, Y.-Q. Lan, Z.-M. Su, P. Huang, C.-G. Wang and E.-B. Wang, Dalton Trans., 2012, 41, 6906–6909 RSC.
- T.-H. Bae, J. S. Lee, W. Qiu, W. J. Koros, C. W. Jones and S. Nair, Angew. Chem., Int. Ed., 2010, 49, 9863–9866 CrossRef CAS PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2011, 112, 1232–1268 CrossRef PubMed.
- A. J. Brown, J. R. Johnson, M. E. Lydon, W. J. Koros, C. W. Jones and S. Nair, Angew. Chem., Int. Ed., 2012, 51, 10615–10618 CrossRef CAS PubMed.
- D. Hua, Y. K. Ong, Y. Wang, T. Yang and T.-S. Chung, J. Membr. Sci., 2014, 453, 155–167 CrossRef CAS PubMed.
- T. Yang and T.-S. Chung, J. Mater. Chem. A, 2013, 1, 6081–6090 CAS.
- S. Mayor and R. E. Pagano, Nat. Rev. Mol. Cell Biol., 2007, 8, 603–612 CrossRef CAS PubMed.
- W. Morris, C. J. Doonan, H. Furukawa, R. Banerjee and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 12626–12627 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 17 CrossRef.
- J. Cravillon, R. Nayuk, S. Springer, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2011, 23, 2130–2141 CrossRef CAS.
- J. Zhuang, C.-H. Kuo, L.-Y. Chou, D.-Y. Liu, E. Weerapana and C.-K. Tsung, ACS Nano, 2014, 8, 2812–2819 CrossRef CAS PubMed.
- Q. He, Z. Zhang, Y. Gao, J. Shi and Y. Li, Small, 2009, 5, 2722–2729 CrossRef CAS PubMed.
- Y. Pan, S. Neuss, A. Leifert, M. Fischler, F. Wen, U. Simon, G. Schmid, W. Brandau and W. Jahnen-Dechent, Small, 2007, 3, 1941–1949 CrossRef CAS PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- C. Tamames-Tabar, D. Cunha, E. Imbuluzqueta, F. Ragon, C. Serre, M. J. Blanco-Prieto and P. Horcajada, J. Mater. Chem. B, 2014, 2, 262–271 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10488a |
| This journal is © The Royal Society of Chemistry 2014 |