Microfluidic chip for monitoring Ca2+ transport through a confluent layer of intestinal cells†
C. Huang‡
*a,
Q. Ramadan§
*a,
J. B. Wacker¶
a,
H. C. Tekin||
a,
C. Ruffert**
a,
G. Vergèresb,
P. Silaccib and
M. A. M. Gijsa
aLaboratory of Microsystems, École Polytechnique Fédérale de Lausanne, Switzerland. E-mail: alramadanq@ime.a-star.edu.sg
bInstitute for Food Science, Agroscope, Federal Office of Agriculture, Berne, Switzerland
First published on 16th October 2014
Abstract
The absorption of dietary calcium through the intestinal barrier is essential for maintaining health in general and especially of the bone system. We propose a microfluidic model that studies free calcium (Ca2+) transport through a confluent monolayer of Caco-2 cells. The latter were cultured on a porous membrane that was positioned in between a top and bottom microfluidic chamber. Fresh cell culture medium was continuously supplied into the device at a flow rate of 5 nL s−1 and the culture progress of the cell monolayer was continuously monitored using integrated Transepithelial Electrical Resistance (TEER) electrodes. The electrical measurements showed that the Caco-2 monolayer formed a dense and tight barrier in 5 days. The transported free Ca2+ from the top microfluidic chamber to the basolateral side of the cell monolayer was measured using the calcium-sensitive dye fura-2. This is a ratiometric dye which exhibits an excitation spectrum shift from 340 nm to 380 nm, when it binds to Ca2+ with an emission peak at 510 nm. Therefore, the concentration of free Ca2+ is proportional to the ratio of fluorescence emissions obtained by exciting at 340 nm and 380 nm. The barrier function of the cell monolayer was evaluated by a measured rate of Ca2+ transport through the monolayer that was 5 times lower than that through the bare porous membrane. The continuous perfusion of cell nutrients and the resultant mechanical shear on the cell surface due to the fluid flow are two key factors that would narrow the gap between the in vivo and in vitro conditions. These conditions significantly enhance the Caco-2 cell culture model for studying nutrients bioavailability.
Introduction
Dietary calcium intake has an important impact on human health and chronic calcium deficiency resulting from inadequate intake or poor intestinal absorption is a major cause of reduced bone mass and osteoporosis. Calcium is absorbed in the mammal along two routes: (i) a transcellular mechanism, predominant in the duodenum and regulated by vitamin D, and (ii) a paracellular, concentration-dependent diffusional process that takes place throughout the length of the intestine.1 In normal conditions, calcium predominantly traverses the intestinal epithelium via the paracellular pathway,2 which is driven by the voltage gradient between the apical and basolateral side of the gut epithelium, and regulated by the tight junction proteins, which polymerize to form an array of channel-like paracellular pores.3 In vivo methods, which provide direct data of bioavailability, have been used to measure the bioavailability of a great variety of metabolites.4,5 Such studies imply the consumption of a certain dose of a nutrient by either humans or animals, and subsequently monitoring its concentration in the blood plasma over time and compare it to an equivalent nutrient dose found in a food source.6 However, in vivo studies are technically difficult, costly, and limited by ethical constraints. Another major drawback of in vivo data is the variability in physiological state of individuals and the possible interaction of the nutrient with other components in the diet.7 Therefore, alternative methods are needed.In vitro models of the human gastrointestinal tract (GIT), that closely mimic the physiological processes of absorption, may provide efficient tools for the bioavailability measurements, and therefore can show more systematic human tissue response to nutrients and significantly reduce experimentation expenses. The standard in vitro model used for studying the bioavailability of nutrients in food is a confluent layer of epithelial cells,8,9 with Caco-2 cells the most popular choice for modeling the human GIT.10,11 In this model, the cell monolayer is grown on a porous membrane, such as a Transwell insert (Millipore, USA), which is placed inside a culture well to form a double-compartment system. Such system allows accessing the cells from both the top/apical and bottom/basolateral side. When cultured on a micro-porous membrane, Caco-2 cells form dense intercellular junctional complexes, resulting in a tight epithelium and presenting a unique paracellular and transcellular barrier,12 thus providing a useful physiological tool to identify metabolites transport originating from a variety of digested food types.13 However, these in vitro methods require a large amount of cells, reagents and culture media, and, additionally, these tools are static and less suited to provide a dynamically controlled flow of cell nutrients and stimuli.14
Microfluidics-based cell culture systems have the potential to create dynamic in vivo-like cell microenvironments15,16 and therefore can be useful tools for screening of the physiological properties and Ca imaging.17,18 This paper introduces a technique to measure Ca2+ transport through a confluent monolayer of epithelial cells cultured in a double-layer microfluidic chip by using a ratio imaging method. Two pairs of Ag/AgCl TEER microelectrodes are integrated into the chip to continuously monitor the quality of the cell monolayer during the cell incubation, as well as throughout the Ca2+ transport experiments. Fig. 1 shows a conceptual schematic view of the Ca2+ transport microfluidic chip, which comprises a confluent monolayer of Caco-2 cells cultured on a porous membrane sandwiched in between two microfluidic chambers made in polydimethylsiloxane (PDMS) and clamped by two polymethylmethacrylate (PMMA) plates.
The chip was fabricated of two individually molded/micro patterned PDMS layers, which are termed here as the ‘apical’ and the ‘basolateral’ layers, respectively. These two layers sandwich a polyethylene terephthalate (PET) membrane with pore size of 0.4 μm. The assembled chip was sandwiched in between two PMMA layers and firmly mechanically clamped to prevent fluidic leakage. The PMMA fitting also facilitates inserting the TEER probes, as well as connecting the chip to the external fluidic apparatus for cell loading and continuous infusion of culture media. To maintain the optical path length, the base of the basolateral chamber was made of a glass cover slip with thickness of 170 μm. Our device allowed leakage-free operation during the experiments, as tested by injecting colored solutions in the microfluidic chip. For epithelial cell layer culture, 0.1 mL of a Caco-2 cells suspension at a density of 2 × 106 cells per cm2 was loaded into the chip using a syringe pump at a flow rate of 10 nL s−1. After cell loading, the chip was inserted into the incubator at 37 °C with 5% CO2 for cell sedimentation and proliferation. Fresh cell medium flow was continuously supplied at 5 nL s−1 during incubation. The cells formed a fully confluent monolayer after 5–6 days of culture. Caco-2 cells were also cultured in parallel in Transwell devices for comparison.
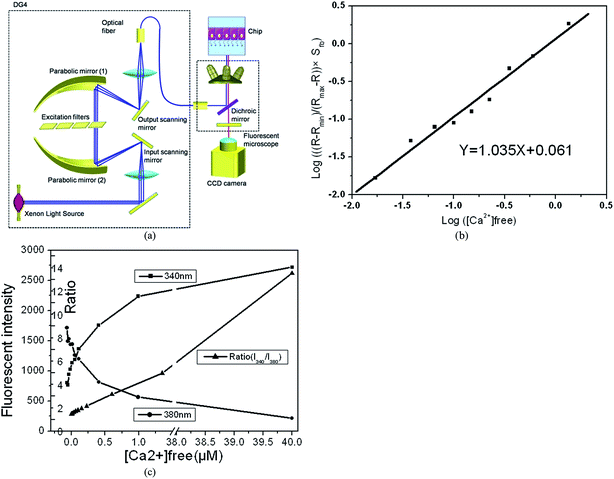
Several fluorescent probes show a spectral response upon binding to Ca2+, which in principle enables investigating changes in transport and absorption of Ca2+ using fluorescent microscopy. Fura-2 is the most popular dye for ratio-imaging microscopy: it is an ultraviolet (UV)-excitable Ca2+indicator that undergoes a shift in absorption upon binding to Ca2+.13 The dye is excited at a wavelength of 340 nm and 380 nm and the ratio of the emissions monitored at ∼510 nm corresponding to those wavelengths can be correlated to the amount of calcium in solution. The use of the ratio allows canceling out confounding variables such as variable dye concentration and liquid volumes. The setup used for detecting the calcium transport includes a dedicated imaging system which relies on the ratio imaging technique. Fig. 2a shows a schematic of the ratio imaging apparatus (Visitron, Germany), which was integrated onto an inverted microscope (Axio Observer, Zeiss). An illumination system (Lambda DG4, Sutter instruments, USA) with a 300 W xenon lamp and rapid wavelength switching function was used to excite the fura-2 dye. The dual galvanometer design of this system allowed switching between the two excitation filters within 2 milliseconds, which facilitates the ability to follow fast changes in Ca2+ concentrations. The Ca2+ images were captured using an integrated CCD camera (ORCA-D2, Hamamatsu, Japan) and the Ca2+ ratio was calculated using VisiView Premier Image acquisition Software (Visitron, Germany).
Results and discussion
Prior to running the ratio measurements, the ratio imaging apparatus had to be calibrated. Samples with different concentrations of Ca2+, ranging from 0 to 40 μM, diluted in a calcium calibration buffer #1 (prepared from 50 mL of 10 mM K2EGTA and 50 mL of 10 mM CaEGTA stock solution, which can be blended to make buffers having free Ca2+ ranging from 0 μM to 39 μM), containing fura-2 were prepared and the ratio of the fluorescence emission intensity at the two excitation wavelengths were measured. Fig. 2b shows the calibration line of the free calcium containing 0–40 μM in 10 μM fura-2 on the chip. The emission intensities obtained at the two excitation wavelengths are defined as I340 and I380 for the excitation at 340 nm and 380 nm, respectively. The Ca2+ concentration has been obtained from the equation:19
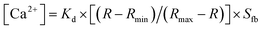 | (1) |
For comparing the experiments on confluent layer cell culture in our microfluidic device, Caco-2 cells with a concentration of 5.5 × 104 cells per cm2 were also seeded into Transwell devices and incubated. In parallel, 0.1 mL of Caco-2 cells suspension with a cell density of 2 × 106 cells per cm2 were loaded onto the chip at a flow rate of 10 nL s−1 and continuously supplied with DMEM cell media (Dulbecco's Modified Eagle's Medium) at a flow rate of 10 nL s−1 for 6 days. Successful cell loading into the chip was enhanced by blocking the fluidic ports at the apical side (except the cell loading port) and allow fluid aspiration from the basolateral side. All the introduced cells were observed to aggregate in the vicinity of the PET membrane pores. Fully confluent monolayers of Caco-2 cells were observed after 6 days in both the Transwell device and the microfluidic chip (see Fig. 3). The Ca2+ transport through the Caco-2 cell monolayer was carried out only after the monolayer reached 100% confluence, as confirmed by the TEER measurements. TEER measurements were normalized to account for the membrane surface. TEER increases during the cell incubation and reaches a maximum value after 6 days after cell seeding and then maintains a stable high value during further culture. However, the Caco-2 monolayer on the chip displayed a 7-fold higher value of TEER compared to that of a monolayer on a Transwell device (Fig. 4).
 | ||
| Fig. 4 Typical TEER values of a Caco-2 cell monolayer cultured (a) in a Transwell device and (b) on our microfluidic chip. All experiments were performed three times. | ||
For studying the Ca2+ transport through a confluent Caco-2 cell layer, DMEM cell media with a free Ca2+ concentration of 33 μM was used as a source of Ca2+ and was perfused through the apical chamber at a flow rate of 10 nL s−1 for 10 h. At the same time, the lower compartment was fluidically isolated; therefore, the liquid from the apical to the basolateral compartment could be only transported through diffusion. As a control experiment, Ca2+ transport measurements through a cell-free PET membrane were also conducted (see Fig. S1 of the ESI†). The emission intensities in the basolateral chamber obtained at the two excitation wavelengths, I340 and I380, were measured at different time intervals (see Fig. 5a). A notable feature is the opposite trend of the two intensities: while I340 increases over time, I380 decreases with increasing transport of the Ca2+ through the membrane. This also is further reflected in the ratio curve of Fig. 5b. We used this ratio to calculate the free Ca2+ concentration in the basolateral compartment (see Fig. 5c). Moreover, this figure shows also the obtained Ca2+ concentration for our control experiment, in which we only have the membrane in the device and not the Caco-2 cells. Ca2+ transport through the Caco-2 monolayer was ∼5 times slower than that through the bare membrane. While the latter show sharp increase of Ca2+ concentration after 8 hours of Ca2+ supplement injection, the Ca2+ concentration significantly increased only after 11 hours in the basolateral chamber beneath the cell monolayer, effectively demonstrated the barrier function of the epithelial cell layer. 4–5 μM of free Ca2+ originating from the DMEM cell media was transported through the Caco-2 monolayer and was detected after 19 h in the basolateral chamber. This corresponds to 14% of the concentration of free Ca2+ in the cell culture medium in the apical chamber. The corresponding basolateral to apical ratio of the calcium concentration measured over 19 hours is shown in Fig. 5d.
 | ||
| Fig. 5 (a) Fluorescent intensities in the basolateral compartment of the microfluidic chip: I340 is increasing with time and I380 shows the opposite trend; (b) calculated ratio I340/I380; (c) calculated Ca2+ concentration from the data of (b) by using eqn (1) (circles); for comparison, the measurement of the concentration in the basolateral compartment of microfluidic chip is presented, when just using the porous membrane, but not the cultured cells (triangles). (d) The basolateral/apical ratio of the calcium concentration measured with a continuous supply of Ca at a concentration of 33 μM. | ||
Conclusions
Our microfluidic device enabled monitoring the Ca2+ transport through a confluent intestinal cell monolayer over prolonged periods of time, while the integrated TEER measurements provided a continuous feedback on the quality of the cell monolayer. Confluent cell monolayers were reached after 5 day of cell seeding, as indicated by TEER. Fura-2 was used as an indicator of the free Ca2+ and a ratio imaging technique was employed to measure the transported free Ca2+. Significant Ca2+ transport was observed 11 hours after applying the Ca2+ supplement-containing cell culture media in the apical chamber and we could check the barrier function of the epithelial cell layer. From these results, we envision wider applicability of our device for measuring the bio-availability and transport of several nutrients. Also we could perform real-time measurements at the cellular level, by incorporating a live-cell imaging system, which comprises a cell incubation system that is directly integrated with the ratio imaging setup. Such system could also be used to differentiate the paracellular and the transcellular transport of calcium.References
- F. Bronner, D. Pansu and W. D. Stein, Am. J. Physiol., 1986, 250, G561–G569 CAS.
- N. Thongon, L. I. Nakkrasae, J. Thongbunchoo, N. Krishnamra and N. Charoenphandhu, Am. J. Physiol.: Cell Physiol., 2009, 296, C1373–C1382 CrossRef CAS PubMed.
- H. Fujita, K. Sugimoto, S. Inatomi, T. Maeda, M. Osanai, Y. Uchiyama, Y. Yamamoto, T. Wada, T. Kojima, H. Yokozaki, T. Yamashita, S. Kato, N. Sawada and H. Chiba, Mol. Biol. Cell, 2008, 19, 1912–1921 CrossRef CAS PubMed.
- S. Hollingworth, K. R. Gee and S. M. Baylor, Biophys. J., 2009, 97, 1864–1872 CrossRef CAS PubMed.
- J. S. Busse and J. P. Palta, Physiol. Plant., 2006, 128, 313–323 CrossRef CAS PubMed.
- K. J. Yeum and R. M. Russell, Annu. Rev. Nutr., 2002, 22, 483–504 CrossRef CAS PubMed.
- J. Parada and J. M. Aguilera, J. Food Sci., 2007, 72, R21–R32 CrossRef CAS PubMed.
- M. Shimizu, Biosci., Biotechnol., Biochem., 2012, 74, 232–241 CrossRef PubMed.
- A. P. Au and M. B. Reddy, J. Nutr., 2000, 130, 1329–1334 CAS.
- F. Leonard, E. M. Collnot and C. M. Lehr, Mol. Pharmaceutics, 2010, 7(6), 2103–2119 CrossRef CAS PubMed.
- V. Gupta, N. Doshi and S. Mitragotri, PLoS One, 2013, 8(2), e57136, DOI:10.1371/journal.pone.0057136.
- L.-S. L. Gan and D. R. Thakker, Adv. Drug Delivery Rev., 1997, 23, 77–98 CrossRef CAS.
- J. R. Kanwar and R. K. Kanwar, BMC Immunol., 2009, 10, 19 CrossRef PubMed.
- M. B. Esch, T. L. King and M. L. Shuler, in Annu. Rev. of Biomed. Eng., Annual Reviews, Palo Alto, vol. 13, pp. 55–72 Search PubMed.
- H. J. Kim, D. Huh, G. Hamilton and D. E. Ingber, Lab Chip, 2012, 12, 2165–2174 RSC.
- Q. Ramadan, H. Jafarpoorchekab, C. B. Huang, P. Silacci, S. Carrara, G. Koklu, J. Ghaye, J. Ramsden, C. Ruffert, G. Vergeres and M. A. M. Gijs, Lab Chip, 2013, 13, 196–203 RSC.
- T. V. Chokshi, D. Bazopoulou and N. Chronis, Lab Chip, 2010, 10, 2758–2763 RSC.
- M. Ghannad-Rezaie, X. Wang, B. Mishra, C. Collins and N. Chronis, PLoS One, 2012, 7(1), e29869, DOI:10.1371/journal.pone.0029869.
- G. Grynkiewicz, M. Poenie and R. Y. Tsien, J. Biol. Chem., 1985, 260, 3440–3450 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09370d |
| ‡ College of Chemical Engineering, Nanjing Forestry University (NFU), Nanjing, 210037, P. R. China. |
| § Bioelectronics Department, Institute of Microelectronics, A*STAR, Singapore. |
| ¶ CSEM, Rue Jaquet-Droz 1, CH-2002 Neuchâtel, Switzerland. |
| || Stanford University School of Medicine, Canary Center Early Cancer Detection, Stanford, CA 94305-5101, USA. |
| ** Institut fuer Mikroproduktionstechnik, Produktionstechnisches Zentrum, Leibniz Universitaet Hannover, 30823 Garbsen, Germany. |
| This journal is © The Royal Society of Chemistry 2014 |