Preparation of defect-free nanocaterpillars via in situ nanoparticlisation of a well-defined polyacetylene block copolymer†
Ki-Young Yoon,
In-Hwan Lee and
Tae-Lim Choi*
Department of Chemistry, Seoul National University, Seoul, 151-747, Korea. E-mail: tlc@snu.ac.kr
First published on 25th September 2014
Abstract
Previously we reported a one-pot preparation of nano-objects resembling a caterpillar (nanocaterpillar) from polyacetylene (PA) diblock copolymers, via the in situ nanoparticlisation of conjugated polymers (INCP) approach. However, they showed the following limitations: micro-structure of PA block was stereo-random containing mixtures of E and Z polyenes, and their nanostructures were contaminated by nanosphere defects. Herein, we report strategies to prepare not only defect-free nanocaterpillars, but also the stereo-regular PA diblock copolymers. By altering the ROMP temperature, we can control the stereochemistry (E/Z) of the PA block, the degree of chain-transfer reaction and molar mass dispersity. As a result of a suppressed chain-transfer reaction at low temperature, ROMP of cyclooctatetraene (COT) at 0 °C produced defect-free nanocaterpillars containing cis-major PAs and they can be transformed into those containing trans-major PAs by thermal isomerisation. Compared to previously reported ill-defined block copolymers, these well-defined block copolymers lead to an increase in the length of nanocaterpillars and narrower length distribution.
Introduction
Self-assembly of block copolymers in solution has received great attention, because the block copolymers form not only spheres, cylinders, and vesicles, but also various intriguing nanostructures such as undulating cylinders,1 disklike micelles,2 and multicompartment structures.3 However, in most cases, these unique nanostructures were not directly formed after their synthesis because the intermolecular forces holding the unimers were not sufficient to form supramolecules in the reaction media. For this reason, post-synthetic treatments such as selective solvent addition, dialysis, temperature change, aging, or introduction of glue molecules were required to induce the self-assembly processes.1–4 Such additional processes may limit the mass production of polymeric nanostructures, narrowing their potential applications. This problem was overcome to some extent by polymerisation-induced self-assembly (PISA).5 Amphiphilic block copolymers prepared by aqueous dispersion/emulsion polymerisation could form supramolecules in situ during polymerisation. Nevertheless, these supramolecules are not thermodynamically stable adducts, and they are fragile when environment changes (for example, changes in temperature or solvents), because the driving force holding the unimers together is relatively weak. Chemists can take advantages of this by utilizing these supramolecules as smart materials.Recently, we reported an alternative approach known as in situ nanoparticlisation of conjugated polymers (INCP).6,7 The synthesis of polyacetylene (PA) diblock copolymers via ring-opening metathesis polymerisation (ROMP) of cyclooctatetraene (COT) is a good example of this process, because the introduction of insoluble PA due to effective π–π interaction between PA chains generate the strong attractive force to molecular self-assembly system. Furthermore, long PA blocks form nano-objects resembling caterpillars (nanocaterpillars in Fig. 1c), confirmed by atomic force microscopy (AFM), dry transmission electron microscopy (TEM), and cryo-TEM. Within the nanocaterpillar structures, the individual spheres were held tightly together by π–π interaction between PA segments, and these unique structures are very stable under heat and mechanical force.6
This process, however, suffered from following limitations.6 During the formation of nanocaterpillars, defects in the nanostructure were observed. At least more than 10% of the nanospheres failed to transform into nanocaterpillars. Also the PA core block consisted of non-uniform microstructures of polyenes comprised of a mixture of E and Z stereoisomers. These defects existed because at room temperature, a chain-transfer reaction catalysed by a highly active catalyst 2 inevitably occurred on the PA block. This resulted in the formation of not only stereo-random polyenes (Fig. 1a) but also PA blocks with broad molar mass dispersity (Đ), which produced block copolymers containing short PA chains. These nanospheres contaminated by the short PA core would not undergo the structural evolution to nanocaterpillars or prevent the elongation of nanocaterpillars by acting as end-cappers (Fig. 1b). In order to produce materials with well-defined nanostructures and microstructures, the underlying problem—the chain-transfer reaction on PA—should be suppressed (Fig. 1a and b). Herein, we report on our investigation of how reaction temperatures affect rates of ROMP, depolymerisation, chain-transfer reaction during ROMP of COT, and ratio of E and Z stereochemistry of the polyene. After optimising the ROMP conditions that suppress the chain-transfer reaction, we obtain longer nanocaterpillars containing either cis- or trans-major PA selectively without nanosphere defects. During the course of these investigations, evidences supporting the mechanism for the nanoparticlisation are obtained as well.
Results and discussion
To prepare well-defined diblock copolymers containing a PA block with narrow Đ, we initially investigated the effect of reaction temperatures on chain-transfer reaction during the ROMP of COT. By using 1 as a monomer for the first block and a catalyst 2, the fundamental factors for the synthesis of the second block such as COT conversion and benzene formation (as a result of depolymerisation),8 were monitored by crude 1H NMR analysis at various temperatures (Table 1). As expected, the ROMP of COT was much faster at 40 °C than at room temperature, resulting in complete conversion in 4.5 h, whereas only 35% conversion was observed at 0 °C even after 24 h (Table 1, entries 1–3). Even using toluene, a relatively poor solvent for the ROMP of COT,6,8 the conversion reached 84% within 5 h at 55 °C. However, this condition produced a large amount of undesired benzene (34% at 55 °C and 21% at 40 °C), resulting in relatively short PA incorporated into the diblock copolymer, while the least amount of benzene (4%) was formed at 0 °C (Table 1, entries 1–4). In short, the reactions at higher temperatures not only had a positive influence on the PA synthesis by increasing the COT conversion but also a negative effect by promoting the depolymerisation that released the benzene at the same time. Clearly the benzene formation was a thermodynamically driven process. Thus, the fact that benzene formation was minimised at 0 °C implied that the chain-transfer reaction, another thermodynamically favoured process, could be minimised at 0 °C as well.| Entry | Solvent | Conca (M) | Temp (°C) | Time (h) | Convb [yield]c (%) | PhHd (%) | DPCOTe |
|---|---|---|---|---|---|---|---|
| a Based on [COT].b Conversion of COT.c Isolated yield after precipitation.d Yield of benzene formation calculated on the basis of the total number of double bonds from the converted COTs.e Degree of polymerisation of COT.f Ref. 6. | |||||||
| 1f | DCM | 0.1 | r.t. | 16 | 93 [92] | 12 | ∼25 |
| 2 | DCM | 0.1 | 0 | 24 | 35 [84] | 4 | ∼10 |
| 3 | DCM | 0.1 | 40 | 4.5 | 99 [89] | 21 | ∼24 |
| 4 | Toluene | 0.1 | 55 | 5 | 84 [88] | 34 | ∼17 |
To further investigate how temperature affected the degree of chain-transfer reaction, we determined the E/Z ratio on the PA block. In the absence of the chain-transfer reaction, PA produced by ROMP of COT should contain Z olefin as the major isomer, because only one out of four Z-olefins in COT undergoes the metathesis reaction, and three Z olefins remain untouched. Furthermore, since the Grubbs catalyst kinetically produces almost equal amounts of E and Z isomers for ROMP, we expected that the ideal ROMP of COT, in the absence of the chain-transfer reaction, would produce cis-major PA. In contrast, an increase in the degree of chain-transfer reaction on the PA block would produce more of the enthalpically favoured E olefins, even leading to the trans-major PA diblock copolymer with broader Đ at the end (Fig. 1a). Therefore, the investigation of the relationship between the temperature and E/Z ratio of PA would indicate the degree of chain-transfer reaction. To determine the E/Z ratio, we used cross-polarisation/magic-angle-spinning (CP/MAS) 13C solid-state NMR spectroscopy since liquid 1H NMR spectroscopy provided no information on the PA block, which spontaneously formed the unsolvated core.6 CP/MAS 13C solid-state NMR spectra showed that the trans-PA signal at 138 ppm increased and the cis-PA signal at 128 ppm decreased with the increase in the temperature (Fig. 2a).8 As a result, the PA block produced at 0 °C showed a very weak signal for E olefins, whereas that produced at 55 °C showed a weak signal for Z olefins. UV/vis spectroscopy provided another definitive conclusion on the stereoisomer for the PA blocks. PA diblock copolymer produced at 0 °C revealed a spectrum with the onset point at 630 nm, whereas that of the polymer synthesised at 55 °C was 800 nm (Fig. 2b). These onset points translated into band-gaps of 2.0 eV and 1.6 eV, respectively, and these values perfectly matched those of cis-PA and trans-PA.9 λmax showed good correlation with the degree of polymerisation (DP) of COT (See Table 1, entries 1–4). IR spectroscopy provided additional insight, since it vividly showed a sharp signal at 1010 cm−1, corresponding to trans-PA from the polymer synthesised at 55 °C, while that signal totally disappeared in the case of the polymer prepared at 0 °C (Fig. S2†).8 From these characterisations, we successfully verified that ROMP at 0 °C resulted in the cis-major PA diblock copolymer and that ROMP at 55 °C produced the trans-major PA. This conclusion implied that chain-transfer reaction on the PA or secondary metathesis reactions on the cis-major PA would be minimised at 0 °C.
 | ||
| Fig. 2 (a) CP/MAS 13C solid-state NMR spectra and (b) UV/vis spectra in chloroform for PA diblock copolymers synthesised at various temperatures. | ||
Another supportive analysis for determining the degree of chain-transfer reaction is by measuring Đ by size exclusion chromatography (SEC). However, SEC traces for the diblock copolymers showed two sets of traces: the major trace corresponding to the self-assembled nanoparticles with high molecular weight and the minor trace corresponding to the single chains of diblock copolymers, which were disassembled under the shear pressure of SEC conditions (see Fig. S3† for the details).6,7 Although the major traces of SEC offered no information on the degree of chain-transfer reaction because the signals were from the collections of supramolecules, we could analyse the minor traces to infer the dispersity in the block copolymers. SEC analysis of PN50-b-PA40 (10 equiv. of COT) produced at 55 °C showed the broadest minor trace, while that produced at room temperature showed a narrower minor trace. Finally, PN50-b-PA40 synthesised at 0 °C showed the narrowest and unimodal minor trace (Fig. S3†). All these analyses demonstrated that the degree of chain-transfer reaction depended on the temperature, and as a result, ROMP of COT at 0 °C resulted in minimal chain-transfer reaction.
Based on the conclusions from several analyses, ROMP of COT at 0 °C was optimised to obtain the diblock copolymers containing a long PA block with narrow Đ, because the polymers with the actual COT DP of at least 30 would have sufficient driving force to form well-defined nanocaterpillars.6 To increase the DP of COT, a large feed ratio of COT (>100 eq.) was used to compensate for the conversion penalty at 0 °C: the DP doubled to 19 (Table 2, entry 1). In order to optimise ROMP of COT, we changed to more active catalyst 3.10 Indeed, model kinetic studies conducted at room temperature confirmed that the ROMP of COT using 3 was much faster than 2 (Fig. 3). Likewise, changing from 2 to 3 enhanced the rate of ROMP at 0 °C with shortened reaction time (24 h → 12 h) (Table 2, entry 2). By further increasing the COT concentration to 1 M and monomer-to-initiator (M/I) ratio to 200, and by optimising the solvent system by adding a co-solvent, chloroform, which increased the polymer solubility, diblock copolymers containing a long PA block of up to 52-mers of COT was obtained (Table 2, entries 3–5). After characterising the polymer by the same methods described above, we concluded that these polymers contained a long cis-major PA block with narrow Đ (Table S1, Fig. S2 and S4†).
| Entry | Cat. | Y | Solvent | Conca (M) | Temp (°C) | Time (h) | Convb [yield]c (%) | PhHd (%) | DPCOTe |
|---|---|---|---|---|---|---|---|---|---|
| a Based on [COT].b Conversion of COT.c Isolated yield after precipitation.d Yield of benzene formation calculated on the basis of the total number of double bonds from the converted COTs.e Degree of polymerisation of COT. | |||||||||
| 1 | 2 | 100 | DCM | 0.9 | 0 | 24 | 22 [60] | 12 | ∼19 |
| 2 | 3 | 100 | DCM | 0.9 | 0 | 12 | 32 [70] | 9 | ∼29 |
| 3 | 3 | 200 | DCM | 1.0 | 0 | 12 | 20 [45] | 11 | ∼35 |
| 4 | 3 | 200 | DCM/CHCl3 = 1 | 1.0 | 0 | 12 | 27 [47] | 12 | ∼46 |
| 5 | 3 | 200 | DCM/CHCl3 = 4 | 1.0 | 0 | 12 | 30 [52] | 12 | ∼52 |
 | ||
Fig. 3 Kinetic studies for the ROMP of COT at room temperature using 2 and 3 (feeding ratio, [NB]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [2 or 3] [2 or 3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COT] = 50 [COT] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). 50). | ||
With the successful synthesis of PA diblock copolymers with narrow Đ, their nanostructures were imaged by atomic force microscopy (AFM), showing the well-defined nanocaterpillars without any post-treatment (Fig. S5†). Compared to the nanocaterpillars with ill-defined polymers (synthesised at r. t.), the well-defined nanocaterpillars with well-defined polymers (synthesised at 0 °C, Table 2, entry 5) showed almost no nanospheres (Fig. 4a vs. b). Furthermore, the average lengths (Ln) of the two sets of nanocaterpillars were calculated using statistics (Fig. S6†). Ln for well-defined nanocaterpillars was greater than that of nanocaterpillars prepared at room temperature (154 nm vs. 101 nm) with the smaller standard deviation (σ/Ln, 0.411 vs. 0.561). Length distribution (Lw/Ln) was also narrower (1.17 vs. 1.32, Fig. 4a and b). Moreover, the AFM height profile showed that the average height of nanocaterpillars prepared at 0 °C statistically had a lower standard deviation (13.9% vs. 16.1%) at 90% confidence level, indicating that the PA blocks with narrow Đ were likely to form the core with more uniform sizes (Fig. S7c†). Lastly, dynamic light scattering (DLS) analysis of nanoparticles in chloroform revealed that the polymers with broader Đ showed two distinct populations of average hydrodynamic diameter (Dh) for nanospheres (21 nm) and nanocaterpillars (148 nm), while a definite unimodal population of well-defined nanocaterpillars (167 nm) was observed (Fig. 4c, see ESI† for the detailed condition). Because results from both AFM and DLS analyses were in excellent agreement, one can conclude that the polymers with the narrow Đ undergo self-assembly to form longer nanocaterpillars with narrower length distribution without nanosphere defects, thus supporting the models in Fig. 1b.11 Some divarications of nanocaterpillars were observed in Fig. 4a. We believe that there are due to stacking of individual nanocaterpillars because their heights were much higher than others (Fig. S8a and b†).
Since well-defined nanocaterpillars containing cis-major PA were prepared, our next efforts focused on producing the well-defined nanocaterpillars containing trans-major PA core, because trans-PA has different electronic properties such as much higher conductivity.8 However, diblock copolymers containing trans-major PA block with high DP and narrow Đ could not be prepared directly using ROMP of COT at 55 °C, because it would enhance both benzene formation, thereby lowering DP, and chain-transfer reaction, thereby broadening Đ. Hence, we chose an alternative strategy to detour to a trans-major PA by isomerisation of cis-major PA.12 Heating the polymer in toluene at 100 °C for 20 min successfully produced trans-major PA, and its stereochemistry was confirmed by CP/MAS 13C solid-state NMR and spectroscopic analyses (Fig. 5a and b and S2†).
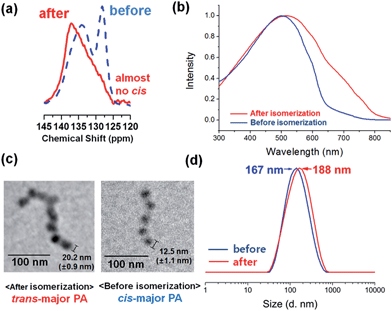 | ||
| Fig. 5 (a) CP/MAS 13C solid-state NMR spectra, (b) UV/vis spectra, (c) TEM images, and (d) DLS intensity profiles of the well-defined nanocaterpillars before and after cis/trans isomerisation. | ||
The change in stereochemistry by isomerisation to trans-PA altered the nanostructure. Firstly, the isomerisation increased the average core size of nanocaterpillars because trans-PA was more stretched than cis-PA. Transmission electron microscopy (TEM) analysis, which showed the nano-objects resembling caterpillars by revealing the electron-rich PA core only even without staining, confirmed that the average core diameter for cis-major PA (12.5 nm) increased to 20.2 nm after isomerisation (Fig. 5c). This change induced slight increase in the overall size of the nanocaterpillars as well. Measuring Ln by AFM revealed the increase in the length from 154 nm to 170 nm, while the length dispersity barely changed as 1.19 (Fig. S7†). DLS analysis in chloroform also showed that Dh of nanocaterpillars also increased by isomerisation from 167 nm to 188 nm (Fig. 5d).
Similarly, the nanocaterpillars from a diblock copolymer with shorter DP of COT (30) showed the same trend with the previous one with the longer PA block (DP of COT 52, Fig. S8c–e†). The nanocaterpillars prepared at 0 °C showed a unimodal trace under the DLS analysis while those prepared at r.t. showed a bimodal trace. Also, isomerisation increased the Dh measured by DLS from 130 nm to 155 nm. All these observations clearly supported that thermal isomerisation produced well-defined nanocaterpillars containing trans-PA whose core size and length slightly increased as a result of the elongated PA block.
Conclusions
In summary, we have synthesised the PA diblock copolymers containing long cis-major PA cores with narrow Đ by ROMP of COT at 0 °C. Suppressing the chain-transfer reaction resulted in the formation of longer nanocaterpillars without nanosphere defects. Furthermore, by thermal isomerisation, well-defined nanocaterpillars containing long trans-major PA cores with narrow Đ were also prepared. This cis-to-trans conversion on PA increased both the core size and length of the nanocaterpillars. These results demonstrate how one can control not only the stereochemistry on PA and the dispersity of the PA block but also the core size, nanoparticle length, and electronic properties, such as band gap, by manipulating the reaction conditions of the catalysis.Acknowledgements
The financial support from Basic Science Research Program, the Nano-Material Technology Development Program, and BRL through NRF is acknowledged. We thank NCIRF and NICEM at SNU for generous support of the TEM, EA, and Solid-state NMR experiments. K.-Y.Y. thanks Hyun Ju Song and Jihye Lee for help with illustration.Notes and references
- (a) H. Cui, Z. Chen, S. Zhong, K. L. Wooley and D. J. Pochan, Science, 2007, 317, 647 CrossRef CAS PubMed; (b) Z. Li, J. Ma, N. S. Lee and K. L. Wooley, J. Am. Chem. Soc., 2011, 133, 1228 CrossRef CAS PubMed; (c) S. Jain and F. S. Bates, Science, 2003, 300, 460 CrossRef CAS PubMed; (d) Q. Ma, E. E. Remsen, C. G. Clark, T. Kowalewski and K. L. Wooley, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5058 CrossRef CAS PubMed.
- L. Yin and M. A. Hillmyer, Macromolecules, 2011, 44, 3021 CrossRef CAS.
- (a) A. O. Moughton, M. A. Hillmyer and T. P. Lodge, Macromolecules, 2011, 45, 2 CrossRef; (b) Z. Li, E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 306, 98 CrossRef CAS PubMed; (c) A. H. Gröschel, F. H. Schacher, H. Schmalz, O. V. Borisov, E. B. Zhulina, A. Walther and A. H. E. Müller, Nat. Commun., 2012, 3, 710 CrossRef PubMed; (d) B. Fang, A. Walther, A. Wolf, Y. Xu, J. Yuan and A. H. E. Müller, Angew. Chem., Int. Ed., 2009, 48, 2877 CrossRef CAS PubMed; (e) J. Dupont and G. Liu, Soft Matter, 2010, 6, 3654 RSC.
- (a) P. A. Rupar, L. Chabanne, M. A. Winnik and I. Manners, Science, 2012, 337, 559 CrossRef CAS PubMed; (b) W. Xiaosong, G. Guerin, W. Hal, W. Yishan, I. Manners and M. A. Winnik, Science, 2007, 317, 644 CrossRef PubMed; (c) S. K. Patra, R. Ahmed, G. R. Whittell, D. J. Lunn, E. L. Dunphy, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2011, 133, 8842 CrossRef CAS PubMed; (d) E. Lee, B. Hammer, J.-K. Kim, Z. Page, T. Emrick and R. C. Hayward, J. Am. Chem. Soc., 2011, 133, 10390 CrossRef CAS PubMed.
- (a) Y. Li and S. P. Armes, Angew. Chem., Int. Ed., 2010, 49, 4042 CrossRef CAS PubMed; (b) S. Sugihara, A. Blanazs, S. P. Armes, A. J. Ryan and A. L. Lewis, J. Am. Chem. Soc., 2011, 133, 15707 CrossRef CAS PubMed; (c) A. Blanazs, J. Madsen, G. Battaglia, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2011, 133, 16581 CrossRef CAS PubMed; (d) B. Charleux, G. Delaittre, J. Rieger and F. D'Agosto, Macromolecules, 2012, 45, 6753 CrossRef CAS; (e) W.-M. Wan and C.-Y. Pan, Polym. Chem., 2010, 1, 1475 RSC.
- K.-Y. Yoon, I.-H. Lee, K. O. Kim, J. Jang, E. Lee and T.-L. Choi, J. Am. Chem. Soc., 2012, 134, 14291 CrossRef CAS PubMed.
- (a) J. Kim, E.-H. Kang and T.-L. Choi, ACS Macro Lett., 2012, 1, 1090 CrossRef CAS; (b) I.-H. Lee, P. Amaladass, K.-Y. Yoon, S. Shin, Y.-J. Kim, I. Kim, E. Lee and T.-L. Choi, J. Am. Chem. Soc., 2013, 135, 17695 CrossRef CAS PubMed; (c) I.-H. Lee, P. Amaladass and T.-L. Choi, Chem. Commun., 2014, 50, 7945 RSC.
- (a) F. L. Klavetter and R. H. Grubbs, J. Am. Chem. Soc., 1988, 110, 7807 CrossRef CAS; (b) O. A. Scherman and R. H. Grubbs, Synth. Met., 2001, 124, 431 CrossRef CAS; (c) O. A. Scherman, I. M. Rutenberg and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 85105 CrossRef PubMed . For the previous synthesis of PA by two-step Durham methods, see;; (d) R. S. Saunders, R. E. Cohen and R. R. Schrock, Macromolecules, 1991, 24, 5599–5605 CrossRef CAS; (e) R. R. Schrock, S. Luo, J. C. Lee, N. C. Zanetti and W. M. Davis, J. Am. Chem. Soc., 1996, 118, 3883–3895 CrossRef CAS; (f) M. Buchmeiser and R. R. Schrock, Macromolecules, 1995, 28, 6642–6649 CrossRef CAS.
- K. Yoshino, Synth. Met., 1989, 28, 669 CrossRef.
- (a) J. A. Love, J. P. Morgan, T. M. Trnka and R. H. Grubbs, Angew. Chem., Int. Ed., 2002, 41, 4035 CrossRef CAS; (b) E.-H. Kang, I. S. Lee and T.-L. Choi, J. Am. Chem. Soc., 2011, 133, 11904 CrossRef CAS PubMed.
- It should be noted that the average Dh for the sample prepared at r. t. should be lower than 150 nm, because DLS clearly shows bimodal distribution, but it does not give the average Dh for the whole population. Also the dispersity in length is still inevitable due to intrinsic step-growth-like mechanism of the nanocaterpillar formation.
- T. Ito, H. Shirakawa and S. Ikeda, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 1943 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10325d |
| This journal is © The Royal Society of Chemistry 2014 |