Synthesis of heterocycle-tethered acylbenzofurans and benzodifurans from odorless and recyclable organoseleno polystyrene resin†
Abstract
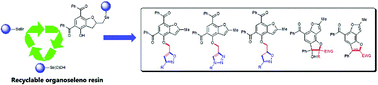
Organoseleno polystyrene resin-supported synthesis provided quick access to a series of acylbenzofuran derivatives tethered to isoxazoles, triazoles and isoxazolines as well as benzodifurans. Although this methodology proceeded through multiple steps such as the seleno-induced attachment, nucleophilic substitutions, 1,3-dipolar cycloadditions, and syn-selenoxide eliminations from organoseleno resin, the overall product yields were generally good. The concise and safe procedures, wide application scope, lack of odor, stability and recyclability of the organoseleno resin, and the good yields and high purity of products are the advantages of this work over the more traditional solution-based chemistry.

 Please wait while we load your content...
Please wait while we load your content...