New ampholytic microgels based on N-isopropylacrylamide and α-amino acid: changes in swelling behavior as a function of temperature, pH and divalent cation concentration
Marcin Mackiewicz,
Jan Romanski and
Marcin Karbarz*
Faculty of Chemistry, University of Warsaw, Pasteura 1, PL 02-093 Warsaw, Poland. E-mail: karbarz@chem.uw.edu.pl; Fax: +48-22-822-02; Tel: +48-22-822-02
First published on 22nd September 2014
Abstract
Several new microgels based on N-isopropylacrylamide and the amino acid L-ornithine were synthesized by means of surfactant free emulsion polymerization. N-δ-Acryloyl ornithine was copolymerized with N-isopropylacrylamide and N,N′-methylenebisacrylamide, leading to the synthesis of a new ampholytic microgel. The swelling behavior of the obtained microgels with respect to the amount of amino acid incorporated in the polymer network, temperature, concentration of ions and pH was investigated. The pH dependence of the microgel size, measured at a constant temperature, was found to exhibit a minimum; the pH range where the minimum appeared corresponded well with the pH range where zwitterions dominated. The temperature dependence of the swelling process obtained for different pH values showed that for the pH region where zwitterions dominated, the polymer networks collapsed more efficiently. The presence of free α-amino acid groups attached to the polymeric network of the microgels also enabled the complexation of some metal cations. The influence of the presence of two metal ions (Cu2+ and Ca2+), which differ significantly in their ability to form complexes with the α-amino-acid, on the swelling behavior was investigated. It was found that the presence of copper ions that form stable complexes with the α-amino acid strongly influenced the swelling behavior of the investigated microgels.
1. Introduction
Microgels are chemically cross-linked polymers, with a network structure, that are colloidal in size and swollen in a suitable solvent.1–3 With their low viscosities, very high surface areas and a rapid and large-magnitude volume phase transition in response to such external stimuli as a change in temperature, pH and ionic strength, microgels have great potential in controlled and regulated applications and therefore have great potential in such areas as controlled drug delivery,4–6 catalysis7 and sensors.8,9The environmentally sensitive gels that are most often investigated are thermo sensitive gels based on N-isopropylacrylamide (NIPA). Cross-linked poly(N-isopropylacrylamide) (pNIPA) hydrogels are known to exhibit a drastic volume phase transition at temperatures above 32 °C; this critical temperature increases with increasing quantity of crosslinking agent.10 At temperatures below 32 °C these gels are swollen, whereas above this temperature they dehydrate to the collapsed state. When groups able to change their charge in response to a change in pH (weak acid, base, or their salts) are introduced to the polymeric chains, the pNIPA microgel acquires new properties, such as sensitivity to a change in pH, enhancement of the size change during the volume phase transition, increase in the temperature at which the phenomenon starts, and the possibility of switching from discontinuous to continuous volume phase transition.
Microgels based on N-isopropylacrylamide are traditionally copolymerized with pH sensitive carboxylic acid comonomers like itaconic acid, vinylacetic acid or most commonly acrylic acid (AA), and with cationic comonomers like 4-vinylpyridine and 2-(aminoethyl)-methacrylatehydrochloride.11 Previous studies on temperature and pH sensitive microgels have also investigated polymers prepared from monomers that were either acidic or basic.12 For instance, Nayak and Lyon showed that microgels synthesized by copolymerization of NIPA, AA and N-(3-aminopropyl)methacrylamide exhibited zwitterionic behavior.13 Ogawa et al. used AA and 1-vinylimidazole as anionic and cationic monomers, respectively; both monomers were incorporated into the network of pNIPA cross-linked with N,N′-methylenebisacrylamide (BIS).14 Tan et al.15,16 studied pH-responsive polyampholyte microgels consisting of poly(methacrylic acid) and poly-(2-(diethylamino)ethyl methacrylate) (PMAA–PDEA). These microgel were more hydrophilic at low and high pH values and became compact between pH 4.0 and 6.0, near the isoelectric point.
The introduction of amino acid moieties may result in new microgel properties such as ampholytic behavior, catalytic activity, sorption properties, sensitivity to pH and ionic strength. The ability to complex metal ions makes polymeric microgels very attractive as metal adsorbents. Amino acid moieties also may serve as a means to facilitate further chemical reactions to incorporate molecules for improved targeting.
We recently reported the synthesis of new polymeric gels with free α-amino-acid groups based on modified L-ornithine and L-lysine, and optionally N-isopropylacrylamide.17–20 The presence of α-amino acid in those polymers made it possible to control the charge sign and the excess of charge in the polymeric network by changing pH. The synthesized gels showed an interesting swelling behavior in response to changes in temperature, pH and concentration of divalent metal ions.
In the present study, we modified the synthesis of the gels based on N-isopropylacrylamide and N-δ-acryloyl ornithine to obtain polymeric microparticles not exceeding 1 μm in size. This investigation focused on how the swelling behavior of the synthesized microgels is influenced by the amount of α-amino acid groups incorporated into the polymer network, temperature, pH and the presence of divalent ions with differing ability to complex with the polymer network.
2. Experimental
2.1. Chemicals
N-Isopropylacrylamide (NIPA, 97%), N,N′-methylenebisacrylamide (BIS, 99%), potassium persulfate (KPS, 99.99%) and N,N,N′,N′-tetramethylethylenediamine (TEMED, 99.5%) were purchased from Aldrich. Sodium hydroxide (NaOH, 99%), hydrochloric acid (HCl, 35–38%), copper(II) sulfate pentahydrate (CuSO4·5H2O, 98%) and calcium(II) sulfate (CaSO4·2H2O, 99%) were purchased from POCh. N-δ-Acryloyl ornithine was synthesized according to the procedure described previously.20All chemicals were used as provided by manufacturer except for NIPA, which was recrystallized twice from benzene–hexane mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). All solutions were prepared using high purity water obtained from a Hydrolab/HLP purification system (water conductivity: 0.056 μS cm−1).
1). All solutions were prepared using high purity water obtained from a Hydrolab/HLP purification system (water conductivity: 0.056 μS cm−1).
2.2. Synthesis of poly(N-isopropylacrylamide-co-N-δ-acryloyl ornithine) NIPA-AcOrn microgel
Microgels were obtained by surfactant free emulsion polymerization.21 Various amounts of NIPA monomer, BIS and AcOrn were dissolved in 95 mL of deionized water in a three-neck flask equipped with a magnetic stirrer (set at 500 rpm during the entire polymerization), reflux condenser, inlet and outlet of inert gas. The total concentration of NIPA, amino acid derivative and BIS was kept constant at 150 mM. The microgels were synthesized with the mole fraction of the amino acid derivative equal to Ysolution in the pre-gel solution, defined as:
 | (1) |
In this study, Ysolution = 0, 2, 8, 20 and 25%. The mole fraction of BIS was kept constant at 1%. However, for 25% NIPA-AcOrn a lack of well-formed particles according to SEM and TEM, and no reliable measurements by DLS were observed.
The monomer solution was purged with argon for 0.5 h to remove any dissolved oxygen and heated up in an oil bath thermostated at 70 °C. Then KPS (0.1 g dissolved in 5 mL of deionized and degassed water) and 50 μL of TEMED were added to initiate the polymerization. The reaction continued for 8 h under an argon blanket, after which the solution was cooled down to room temperature. The schemes of the synthesis and the structure of the polymeric network are shown in Fig. 1. The microgels so obtained were purified by placing them into a dialysis tube with a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da molecular weight cutoff (Spectra/Por® 7 Dialysis Membrane), thus removing the oligomers and unreacted substrates. The microgels were dialyzed against 5 L of water for three weeks at room temperature, with the water changed daily. The final conductivity of the water used was close to the initial value (conductivity measurements were carried out using a Radiometer, model CDM230 conductometer). Before further proceeding the microgel solution was filtered via a syringe inline glass filter with pore size 1–2 μm.
000 Da molecular weight cutoff (Spectra/Por® 7 Dialysis Membrane), thus removing the oligomers and unreacted substrates. The microgels were dialyzed against 5 L of water for three weeks at room temperature, with the water changed daily. The final conductivity of the water used was close to the initial value (conductivity measurements were carried out using a Radiometer, model CDM230 conductometer). Before further proceeding the microgel solution was filtered via a syringe inline glass filter with pore size 1–2 μm.
 | ||
| Fig. 1 Scheme of synthesis and structure of microgel based on N-isopropylacrylamide-co-δ-acrylic ornithine polymer crosslinked with N,N′-methylenebisacrylamide, and 1H NMR spectrum of microgel. | ||
2.3. Determining the amount of N-δ-acryloyl ornithine incorporated into the polymeric network of NIPA-AcOrn microgels
The amount of amino acid incorporated into the polymeric network of the gels was estimated from 1H NMR spectra, obtained at 500 MHz using a Varian Unity Plus spectrometer. The gel samples for the NMR experiments were prepared by swelling the dry microgels in D2O in NMR tubes. The 1H NMR spectra were recorded one day after the swelling. A typical 20% NIPA-AcOrn microgel spectrum is shown in Fig. 1. All signals in the spectrum are assigned to the appropriate protons, except protons G from the cross-linker. The signal from protons G is expected at 4.2–4.5 ppm,18 the absence of a signal in this region is caused by the low level of mol fraction of the linker (1%). Signal C (representing the α-protons of ornithine) and signal B (representing the protons from the isopropyl group) were used for estimating the amino acid loading content into the network:where 〈B〉 and 〈C〉 denote the areas of the corresponding signals. Fortunately, the error is very small, as the mol fraction of the linker was kept at a low level. The results so obtained are shown in Table 1 and compared with the molar fraction of acryloyl ornithine used in the polymerization.
| 2% NIPA-AcOrn | 8% NIPA-AcOrn | 20% NIPA-AcOrn | |
|---|---|---|---|
| a Value below the detection limit. | |||
| Ysolution | 2.0% | 8.0% | 20.0% |
| Ygel | — | 4.8% | 13.0% |
2.4. Instrumental examinations
3. Results and discussion
Selected SEM micrographs of microgels containing various amounts of AcOrn are shown in Fig. 2a. As can be seen, all the microgels formed spherical particles. However, the higher the content of amino acid in the polymeric network (8 and 20%), the more the spherical shape of the particles was deformed. The micrographs obtained with a transmission electron microscope (see Fig. 2b) confirmed that an increase in the amount of AcOrn in the microgels led to a greater deformation in the spherical shape of the particles, probably due to increasing self-adhesion of microgels with increasing amino acid content. During the drying process the microgel particles became strongly deformed. Moreover, analysis of the SEM and TEM micrograms leads to a conclusion that microgel size decreases with increasing AcOrn content.The influence of temperature on the swelling behavior of the gels containing different amounts of amino acid is shown in Fig. 3. The temperature dependencies of the equilibrium swelling ratio for the NIPA-AcOrn microgels were constructed using the hydrodynamic diameters of the microgels. The hydrodynamic diameters were determined using the dynamic light scattering method (DLS). Assuming that the particles exhibit random Brownian motion, their diffusion coefficient could be obtained from the decay of the autocorrelation function. Then the hydrodynamic diameter (Dh) of the microgel particles can be calculated using the Stokes–Einstein equation21,22
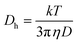 | (2) |
 | ||
| Fig. 3 Change in hydrodynamic diameter of microgels with various amount of amino acid as a function of temperature. | ||
Based on the swelling behavior of the microgels, two general trends were observed. An increase in the content of amino acid leads to: (a) a decrease in microgel size (Dh values at 25 °C were 1086, 1010, 895 and 605 nm for NIPA, 2% NIPA-AcOrn, 8% NIPA-AcOrn and 20% NIPA-AcOrn, respectively), this is in good agreement with the data obtained by SEM and TEM, and (b) an increase in the volume phase transition temperature (VPTT). pNIPA microgel was shown to have a VPTT consistent with the existing literature, i.e. around 32 °C,23 whereas for 20% NIPA-AcOrn microgel the VPTT was ca. 35 °C.
For further investigation, we selected 20% NIPA-AcOrn microgels, which demonstrate the greatest sensitivity to a change in pH. Fig. 4B shows the size of 20% NIPA-AcOrn microgel as a function of pH. The measurements were taken at 25 °C, a temperature much lower than the phase transition temperature. The hydrodynamic diameter decreased rapidly in the pH region from 1.5 to 3. Then, in the region 3 < pH < 8, the dependence was fairly stable. The microgel size again started to grow considerably at pH 8. The changes seen in Fig. 4B may be related to the acid–base equilibrium of the amino acids built into the polymer chains. Three amino acid species exist in the microgels: I (cation, protonated amino group), II (neutral form, dissociated carboxylic groups and protonated amino group, zwitterions), and III (anion, dissociated carboxylic group). The molar fractions (XI, XII, and XIII) of each form of the amino acid can be calculated using the following equations:
The values of Ka1 and Ka2 needed for the calculations were taken as those for free aliphatic amino acid (leucine): pKa1 = 2.36 and pKa2 = 9.60.24 The plots shown in Fig. 4B exhibit a minimum, characteristic for ampholytic polymer networks. The pH range over which the minimum in Fig. 4B is spread corresponds well to the pH distribution of the XII form shown in Fig. 4A. In this range, almost all amino acid groups are in the form of zwitterions. Here, the van der Waals and hydrophobic interactions contribute significantly to the collapse of the ampholytic polymer networks. Additionally, in this region there exist both electrostatic repulsions between the groups similarly charged and Coulombic attractions between the positive and negative charges, which may lead to collapse as well. At low and high pH, on the other hand, the behavior of the ampholytic networks is determined by the ionized forms (XI or XIII), which create an osmotic pressure in the network and therefore prompt the swelling process. Also, the charges on the ionized groups generate electrostatic repulsive forces between the polymer chains, which leads to further swelling of the network.
 | ||
| Fig. 4 Calculated distribution of amino acid forms as a function of pH, pKa1 = 2.36 and pKa2 = 9.60 (A). pH dependence of hydrodynamic diameter for 20% NIPA-AcOrn microgel measured at 25 °C (B). | ||
To further characterize the swelling behavior of the microgels at a given excess charge, the temperature dependence of the swelling ratio was examined at eight selected values of pH and is represented in a three-dimensional diagram in Fig. 5. As can be seen, temperature and pH strongly influence the microgel size and the swelling behavior. In the pH region where zwitterions dominate, the microgel size is the smallest both before and after the phase transition. The microgel achieves a completely collapsed state at temperatures higher than 35 °C. At the pH extremes, the microgels exhibited a larger diameter before and after phase transition and the discontinuous volume phase transition can be interpreted in terms of an incomplete collapse. For temperatures higher than 35 °C, in the pH region where zwitterions dominate, the surface is nearly flat and the gel achieves a completely collapsed state. At the pH extremes, the surface is distinctly curved.
 | ||
| Fig. 5 Three-dimensional diagrams of hydrodynamic diameter as functions of pH and temperature for 20% NIPA-AcOrn microgels. | ||
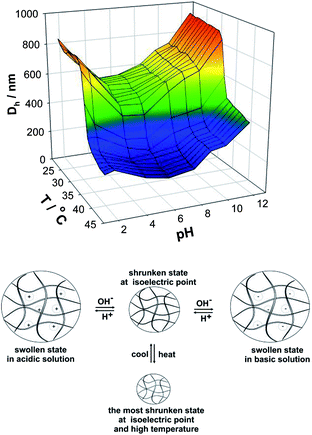
Next, we investigated the influence of the presence of metal ions on the swelling behavior of 20% NIPA-AcOrn microgels. For this purpose two kinds of ions were selected: calcium(II) and copper(II). The temperature dependencies of the swelling ratio were examined for several selected values of metal-ion concentration and are presented as three-dimensional diagrams in Fig. 6. In the case of calcium ions (Fig. 6A), at constant ionic strength, the temperature of phase transition does not change significantly over a wide range of calcium ion concentration. Only sensitivity to changes in temperature is exhibited. Microgels achieved completely shrunken state at temperatures higher than 35 °C and at any pCa value.
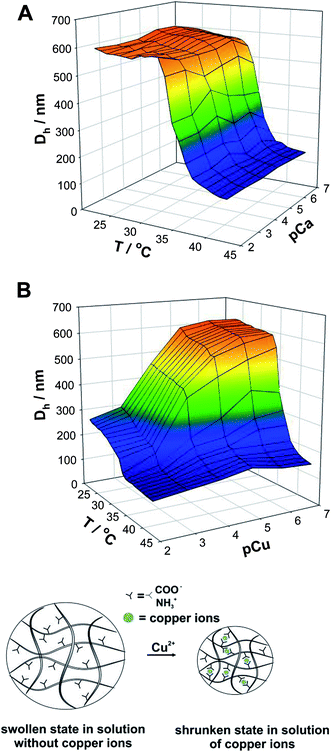 | ||
| Fig. 6 Three-dimensional diagrams of hydrodynamic diameters as functions of concentration of calcium (A) and copper ions (B) and temperature for 20% NIPA-AcOrn microgels. | ||
In the case of copper ions (Fig. 6B) the microgels exhibited the sensitivity to both temperature and ion concentration. A decrease in the swelling ratio with an increase in the concentration of Cu2+ was observed. For smaller concentrations of copper ions the 20% NIPA-AcOrn microgel underwent the volume phase transition at about 35 °C, while for higher Cu2+ concentrations we observed the transition at lower temperature, at approx. 33 °C. The temperature induced change in the swelling ratio was not very significant when pCu was smaller than 3. A more significant change in the swelling ratio was observed for pCu higher than 4. A drastic drop in the swelling ratio was observed when pCu was changed from 5 to 3 at temperature below volume phase transition.
The observed behavior of the microgels could be explained in terms of the formation of complexes between metal ions and amino acid groups attached to the polymeric chains of the microgel networks. It is known that amino acids can form stable complexes with some metal cations. Usually, two complexes of stoichiometry 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and can be formed.25–28
2 and can be formed.25–28
The presence of such complexes in the gel may influence its swelling ratio. The first complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) should expand the polymer network by introducing an excessive positive charge to the polymeric chains, which leads to an increase in the osmotic pressure between the solution and the gel. The second (1
1) should expand the polymer network by introducing an excessive positive charge to the polymeric chains, which leads to an increase in the osmotic pressure between the solution and the gel. The second (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) complex increases the overall cross-link density of the gels and leads to the shrinking of the polymer network. Copper ions form complexes of relatively high stability constants. The values of the stability constants for the complexes with glycine (unbound to the gel) are log
2) complex increases the overall cross-link density of the gels and leads to the shrinking of the polymer network. Copper ions form complexes of relatively high stability constants. The values of the stability constants for the complexes with glycine (unbound to the gel) are log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βML = 8.1 and log
βML = 8.1 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βML2 = 15.3. The weak influence of the calcium ions on the swelling ratio could be explained in terms of the formation of very weak complexes with only one stoichiometry: 1
βML2 = 15.3. The weak influence of the calcium ions on the swelling ratio could be explained in terms of the formation of very weak complexes with only one stoichiometry: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (log
1 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βML = 1.4). The mean values of the stability constants between the metal ions and glycine were taken from ref. 26.
βML = 1.4). The mean values of the stability constants between the metal ions and glycine were taken from ref. 26.
4. Conclusions
New ampholytic microgels based on N-isopropylacrylamide and modified natural amino acid L-ornithine were synthesized by surfactant free emulsion polymerization. The microgels with 20% content of amino acid showed an interesting swelling behavior in response to changes in temperature, pH and concentration of metal ions. The amino acids made it possible to control the excess of either positive or negative charge on the polymeric network of that microgel. The temperature dependencies of the swelling process obtained for different pH values showed that in the pH region where zwitterions dominate, the polymer networks collapse more efficiently. Metal ions that have the ability to form complexes with α-amino acid caused the microgels to shrink.The metal ion sorption ability of the presented microgels and the temperature dependence of their swelling process make the synthesized microgels interesting materials in terms of the temperature triggered swinging of their heavy-metal binding strength. Additionally, the presence of free α-amino acid groups attached to the polymer network offers the possibility of further modification of the microgel, i.e. through binding to these α-amino acid groups. The bound compounds could be easily released from the microgel by the appropriate change in pH and temperature; the swelling state, the hydrophilicity/hydrophobicity balance and the stability of the peptide/ester bonds will be affected. Thus microgels with free amino acid groups are also interesting from the standpoint of drug delivery systems.
Acknowledgements
This work was supported by Iuventus Grant no. IP2012 015272 from the Polish Ministry of Science and Higher Education.References
- G. Chen and A. S. Hoffman, Nature, 1995, 373, 49 CrossRef CAS PubMed.
- M. J. Snowden, B. Z. Chowdhry, B. Vincent and G. E. Morris, J. Chem. Soc., 1996, 92, 5013 CAS.
- B. R. Saunders and B. Vincent, Adv. Colloid Interface Sci., 1999, 80, 1 CrossRef CAS.
- J. Qian and F. Wu, J. Mater. Chem. B, 2013, 1, 3464 RSC.
- M. Varma, A. Kaushal and S. Garg, J. Controlled Release, 2005, 103, 499 CrossRef CAS PubMed.
- J. K. Oh, R. Drumright, D. J. Siegwart and K. Matyjaszewski, Prog. Polym. Sci., 2008, 33, 448 CrossRef CAS PubMed.
- C. Schunicht, A. Biffis and G. Wulff, Tetrahedron, 2000, 56, 1693 CrossRef CAS.
- T. Hoare and R. Pelton, Macromolecules, 2007, 40, 670 CrossRef CAS.
- M. Mackiewicz, T. Rapecki, Z. Stojek and M. Karbarz, J. Mater. Chem. B, 2014, 2, 1483 RSC.
- Z. Xiaoli, G. Xiangling, Z. Lina and K. Xiang-Zheng, Nanoscale Res. Lett., 2012, 7, 519 CrossRef PubMed.
- Y. Hertle and T. Hellweg, J. Mater. Chem. B, 2013, 1, 5874 RSC.
- S. E. Kudaibergenov, N. Nurajec and V. Khutoryanskiyd, Soft Matter, 2012, 8, 9302 RSC.
- S. Nayak and L. A. Lyon, Polym. Prepr., 2003, 44, 679 CAS.
- K. Ogawa, A. Nakayama and E. Kokufuta, J. Phys. Chem. B, 2003, 107, 8223 CrossRef CAS.
- B. H. Tan and K. C. Tam, Adv. Colloid Interface Sci., 2008, 136, 25 CrossRef CAS PubMed.
- B. H. Tan, P. Ravi and K. C. Tam, Macromol. Rapid Commun., 2006, 27, 522 CrossRef CAS.
- M. Karbarz, K. Pulka, A. Misicka and Z. Stojek, Langmuir, 2006, 22, 7843 CrossRef CAS PubMed.
- M. Karbarz, J. Romanski, K. Michniewicz, J. Jurczak and Z. Stojek, Soft Matter, 2010, 6, 1336 RSC.
- M. Karbarz, K. Pyrzynska, J. Romanski, J. Jurczak and Z. Stojek, Polymer, 2010, 51, 2959 CrossRef CAS PubMed.
- J. Romanski, M. Karbarz, K. Pyrzynska, J. Jurczak and Z. Stojek, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 542 CrossRef CAS.
- I. Galaev and B. Mattiasson, Smart Polymers, Applications in Biotechnology and Biomedicine, CRC Press, New York, 2nd edn, 2008 Search PubMed.
- T. Hoare and R. Pelton, Polymer, 2005, 46, 1139 CrossRef CAS PubMed.
- D. Gan and L. A. Lyon, J. Am. Chem. Soc., 2001, 123, 7511 CrossRef CAS PubMed.
- Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, New York, 78th edn, 1997 Search PubMed.
- L. D. Petit, Pure Appl. Chem., 1984, 56, 247 CrossRef.
- T. Kiss, I. Sovago and A. Gergely, Pure Appl. Chem., 1991, 63, 597 CrossRef.
- I. Sovago, T. Kiss and A. Gergely, Pure Appl. Chem., 1993, 65, 1029 CrossRef CAS.
- G. Berthon, Pure Appl. Chem., 1995, 67, 1117 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |



