Diisocyanate free and melt polycondensation preparation of bio-based unsaturated poly(ester-urethane)s and their properties as UV curable coating materials†
Lijing Han,
Jinyue Dai,
Lisheng Zhang,
Songqi Ma,
Jun Deng,
Ruoyu Zhang* and
Jin Zhu*
Ningbo Key Laboratory of Polymer Materials, Ningbo Institute of Material Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, P. R. China. E-mail: zhangruoy@nimte.ac.cn; jzhu@nimte.ac.cn
First published on 26th September 2014
Abstract
This paper reported the synthesis of bio-based unsaturated poly(ester-urethane)s via a nonisocyanate route, by metal-catalyzed melt polycondensation of itaconic acid with urethanediols. Three novel types of bio-based unsaturated poly(ester-urethane)s, namely, poly(urethanediol 2-itaconic acid), poly(urethanediol 4-itaconic acid) and poly(urethanediol 6-itaconic acid) (poly(U2-IA), poly(U4-IA) and poly (U6-IA) for short code, respectively), were prepared by a green synthetic route. The urethane linkage was formed by the reaction of two equivalent of ethylene carbonate with 1,6-hexanediamine, 1,4-butanediamine and 1,2-ethanediamine to form urethanediols. The urethanediols underwent polymerization with itaconic acid (IA) in the presence of metal catalyst dibutyltin dilaurate (DBTL) to produce low-molecular-weight bio-based unsaturated polyurethanes. Then, these bio-based unsaturated poly(ester-urethane)s were formulated with free radical photoinitiator and curing promoter to prepare UV curable polyurethane systems. After UV curing, the tensile properties, thermal properties and general coating properties of the three UV-cured polyurethane films were similar to that of UV cured polyurethane films prepared by polyurethane-acrylate (PUA). The results suggested that the obtained bio-based unsaturated polyurethanes could serve as coating materials.
Introduction
Due to its high elasticity, abrasive resistance, and other outstanding properties, polyurethane (PU) has been widely accepted by the market for a number of decades. Conventional polyurethane is mostly produced in the reaction of polyols with toxic diisocyanate, which is derived from the even more toxic phosgene. Recently, bio-based polyols, mainly synthesized from vegetable oils1,2 have been used for the production of polyurethanes. However, diisocyanate are still not bio-based and are considerably harmful for human health. From the standpoint of green chemistry, diisocyanate- and phosgene-free synthetic routes for the production of the next generation of polyurethanes are needed, especially, bio-based polyurethanes derived from renewable resources.A new class of poly(ester-urethane) (PEU), synthesized by the polyesterification of urethanediols with various diacids/diesters using lipase, is starting to attract researchers. Since the urethanediols were prepared by the reaction of diamines and cyclic carbonates, such as ethylene carbonate and trimethylene carbonate, from an ecological and economical point of view,3 this new PEU is much ‘greener’ than before. Interestingly, this kind of PEU has attracted strong interest in the fields of biomaterials and commodity plastics due in part to the fact that it exhibited similar mechanical properties compared with conventional polyurethanes.4,5
McCabe and Taylor reported the enzymatic synthesis using lipase of a novel polyester polyurethane with bis(hydroxyethyl)carbamates.6 Considering the desired physical properties of the finished polyester polyurethane, they used urethanediol with about 10% of the total diol. It has also been reported that poly(ester-urethane)s were prepared by the lipase-catalyzed direct polycondensation of urethane-containing diacids and diols.7 In the recent study, to produce a high-molecular-weight polyester-based polyurethane, the cyclic ester-urethane oligomer was firstly prepared by the transesterification of dimethyl adipate and urethanediol using Candida Antarctica lipase (lipase CA) in dilute anisole solution, followed by the ring-opening polymerization (ROP) of the cyclic ester-urethane oligomer in a more concentrated solution.8,9 However, a large amount of lipase was required in the process of the reaction so that the cost of the production was relatively high. Furthermore, the lipase-catalyzed polycondensation of a diol and diacid were time-consuming due to its lower reaction rate compared with metal-catalyzed polycondensation.
To the best of our knowledge, no reports has ever tried the synthesis of bio-based unsaturated polyurethanes by the melt polycondensation of urethanediols with diacid in the presence of metal catalyst using an isocyanate free method. Besides, in order to develop PEU coating materials, double bonds were introduced into the main chain of PEU. The widely used biomolecule “itaconic acid” (IA) possessing two carboxyl groups and one carbon–carbon double bond was selected here, which has been proved to be suitable for the synthesis of unsaturated polyesters.10 Then these bio-based unsaturated polyurethanes were formulated with free radical photoinitiator and curing promoter to prepare UV-curable polyurethane systems. After UV curing, the tensile and thermal properties of the cured films were evaluated using tensile, differential scanning calorimeter (DSC), and thermogravimetry (TGA). In addition, coating properties such as pencil hardness, flexibility, and adhesion were also investigated.
Experimental part
Synthesis of 1,2-bis(hydroxyethyloxycarbonylamino)ethane (urethanediol 2)
Ethylene carbonate (26.4 g, 0.3 mol), 1,2-ethanediamine (9.0 g, 0.15 mol) and 20 mL of methylene chloride were stirred in a 250 mL round-bottomed flask at room temperature until the absorption band at 1800 cm−1 in the IR spectrum disappeared. The white precipitate formed was filtered and further purified by recrystallization from acetone to give a white solid. The monomer urethanediol 2 was obtained after drying under reduced pressure (25.1 g, yield 71%).1H NMR (400 MHz; d6-DMSO; Me4Si): 3.01 (4H, d, CH2), 3.51 (4H, t, J = 5.2 Hz, CH2OH), 3.92 (4H, t, J = 5.2 Hz, CHO), 4.67 (2H, br, OH), 6.72 (0.4H, br, NH), 7.08 (1.6H, t, NH).
Synthesis of bio-based unsaturated poly(ester-urethane)s
The reaction mixture with itaconic acid/urethanediol molar ratio of 1.2 was charged into a 250 mL four-necked flask, equipped with a stirrer, nitrogen inlet, and Barrett type receiver. MEHQ (0.5 wt% relative to total reactants) and p-toluenesulfonic acid monohydrate (0.5 mol% relative to itaconic acid) were used as the free radical polymerization inhibitor and the catalyst for pre-polymerization, respectively. The mixture was precondensated at normal pressure at 110 °C for 4 h, with dry nitrogen bubbling through the mixture. Then DBTL (0.5 wt% relative to the total weights of reactants) was added as the polycondesation catalyst. Then the mixture was kept at 110 °C for 3 h under high vacuum. The synthetic route was shown in Scheme 1.The products were purified by dissolving in chloroform and precipitating in cold water. The polymer was filtered and dried in the oven at 35 °C to a constant weight. The Mn values of polymers were calculated from the acid value (AV) and hydroxyl value (OHV) according to eqn (1)
| Mn = 56.1 × 1000 × f/(AV + OHV) | (1) |
UV curing system with bio-based unsaturated poly(ester-urethane)s
The UV curable polyurethane system was formulated by mixing the bio-based unsaturated poly(ester-urethane) with 3 wt% photoinitiator DMPA and 2 wt% curing promoter triethanol amine (on the base of the oligomer' weight). The solution with 20% solid content was obtained. The resulting UV curable polyurethane system was poured into a stainless steel mould (with the grooves' size of 80 mm × 8 mm × 0.5 mm), followed by drying at 80 °C to get a constant mass and cured by UV irradiation for 30 min at room temperature (using a UV light source with a high-pressure mercury lamp (500 W) at 366 nm). The distance from lamp to the surface of samples was 15 cm. Besides, the system was cast on the tinplate with a thickness of about 40 μm by a drawdown bar. The UV-cured polyurethane films were stored in a dust free cabinet for 3 days before testing.Characterization
The details of characterization of oligomers and UV-cured polyurethane films can be found in the ESI,† and only the characterization of coating properties is briefly introduced here.The pencil hardness of coatings with the thickness of 40–50 μm on the tinplate was measured according to ASTM D 3363-00. Coated tinplates were placed on a firm horizontal surface. The pencil was held firmly against the film at a 45° angle and pushed away from the operator in a 6.5 mm stroke. The process was started with the hardest pencil and continued down the scale of hardness until the pencil will not scratch the film.
The flexibility of the coatings was measured by T-bend test according to ASTM D4145-10. Coated tinplates were bent 180° around progressively more thicknesses of metal, the end point being when failures no longer occur. This test was a way of evaluating the ability of a coating system to withstand the stresses of fabrication.
The adhesion of the UV-cured films on the tinplate was evaluated using the ASTM D3359-09 crosshatch adhesion method. A lattice pattern with eleven cuts in each direction was made in the film to the tinplate, pressure-sensitive tape was applied over the lattice and then removed, and adhesion was evaluated by comparison with descriptions.
Results and discussion
Three novel types of bio-based unsaturated poly(ester-urethane)s containing ester linkages, namely poly(U2-IA), poly(U4-IA), and poly(U6-IA), were designed and synthesized by a green synthetic route without the use of diisocyanates (Scheme 1).(The detailed synthesis conditions and the materials used are illustrated in ESI.†) The urethane linkage was formed by the reaction of diamines and ethylene carbonate that produced urethane-containing diols excluding hazardous monomers such as phosgene and isocyanates. Also, ester linkages and double bonds were periodically introduced into the polyurethane chain.
The synthetic procedure contained two steps as illustrated in Scheme 1. In the first step, a series of urethanediols, as the hard segment of the polyurethane, were prepared by the reaction of two equivalent of ethylene carbonate with 1,6-hexanediamine, 1,4-butanediamine, 1,2-ethanediamine.11 The reaction formed urethane bond quickly at room temperature in the absence of any catalyst, and gave high yields (above 70%). In the second step, the urethanediol underwent melt polycondensation with itaconic acid in the presence of metal catalyst DBTL to produce low-molecular-weight polyurethane. Fig. 1 represents the 1H NMR spectra of poly(U2-IA), poly(U4-IA) and poly(U6-IA). The 1H NMR spectra of synthesized bio-based unsaturated poly(ester-urethane)s are confirmed with signals of double bond at about 5.8 ppm and 6.3 ppm. (See detail analysis in ESI†) from the 1H NMR analysis we can conclude that the targeted bio-based unsaturated polyurethanes were successfully prepared. Their hydroxyl value (OHV) and the acid value (AV) were also measured by titration in order to further quantify the chemical structures. All bio-based unsaturated poly(ester-urethane)s exhibited high acid value and relatively low hydroxyl value, which was in accordance with the feeding ratio of urethanediol and itaconic acid. (See Table S1 in ESI†) the Mn values of the bio-based unsaturated poly(ester-urethane)s calculated from the AV and OHV were 758 g mol−1 for poly(U2-IA), 1336 g mol−1 for poly(U4-IA) and 1111 g mol−1 for poly(U6-IA). It was found that poly(U2-IA) exhibited the lowest Mn. It may be due to the fact that urethanediol 4 and urethanediol 6 were slightly more reactive toward itaconic acid than urethanediol 2.9 We could see that the average double bond contents within each bio-based unsaturated poly(ester-urethane) chain were about 2.3, 3.7 and 2.9 for poly(U2-IA), poly(U4-IA) and poly(U6-IA), respectively. Since the double bond contents were all higher than that of curable PUA,12 the three bio-based unsaturated poly(ester-urethane)s could be used for UV curable system. Therefore, environmental friendly UV curable polyurethane systems could be prepared by using the bio-based unsaturated poly(ester-urethane)s.
Gel content properties
After UV curing, the three bio-based unsaturated polyurethanes transformed into crosslinked polymer by a chain reaction initiated by free radicals, which were produced by UV irradiation.13 The kinetics of UV curing process was illustrated in Scheme 2. Firstly, free radicals were generated up on UV irradiation of free radical photoinitiator DMPA by hemolytic cleavage of C–C bonds. Then, once initiated, the chain reaction will develop very much like in a conventional thermal polymerization with the formation of insoluble crosslinked polymer.The gel content is proportional to the crosslinking density and can be used as a parameter to indicate the efficiency of curing.14 In order to study the crosslinking properties of the three UV cured polyurethane films, the gel content were obtained from the difference in the weights of the sample before and after acetone extraction. The un-crosslinked polymer would dissolve in the acetone while the crosslinked polymer remained. The gel contents of UV-cured poly(U2-IA), UV-cured poly(U4-IA) and UV-cured poly(U6-IA) were 78.90%, 86.68%, and 88.35%, respectively (as shown in Table 1). These gel content values may indicate that the UV curing system was highly, but not completely UV cured. Clearly, the UV-cured poly(U2-IA) exhibited the lowest gel content. This may be explained by the viscosity difference.15 The UV-curing system with poly(U2-IA) exhibited the lowest viscosity because poly(U2-IA) possessed the lowest Mn. Consequently, oxygen could more easily spread into the UV-curing system with poly(U2-IA) where more and more initiator radicals are scavenged,16 and then the odds of the light polymerization would be reduced leading to not fully UV cured. On the other hand, this result could also be affected by the fact that the vitrification occurred during curing can restrict the diffusion of PEU chains.17 Therefore, the residual double bonds in PEU were remained and could not be further cured at room temperature.
| Samples | Tensile strength (MPa) | Elongation at break (%) | Young's modulus (MPa) | Gel content (%) |
|---|---|---|---|---|
| UV-cured poly(U2-IA) | 1.37 | 51 | 16.1 | 78.90 |
| UV-cured poly(U4-IA) | 2.50 | 33 | 51.9 | 86.68 |
| UV-cured poly(U6-IA) | 0.78 | 53 | 2.3 | 88.35 |
Tensile properties
The tensile experiment was carried out by an Instron 5567 Electric Universal Testing Machine with gauge length of 50 mm at a cross-head speed of 5 mm min−1, and their tensile strength, elongation at break, and Young's modulus were summarized in Table 1. In some cases, the crosslinking density largely affect the elongation at break and tensile strength.18 It could be seen in this work that the UV-cured poly(U4-IA) film exhibited the lowest value of elongation at break among the three samples. This fact may be attributed to the reason that the elongation at break for cured films depends on not only the chemical structure of the prepolymers but also the crosslinking density of networks. On the other side, the UV-cured poly(U4-IA) film possessed the highest tensile strength (2.50 MPa), following by the UV-cured poly(U2-IA) film (1.37 MPa), while the UV-cured poly(U6-IA) film had the lowest strength. The results could be attributed to the fact that the aliphatic chain length of poly(U4-IA) was shorter than that of poly(U6-IA) and the crosslinking density of UV-cured poly(U4-IA) film was higher than that of UV-cured poly(U2-IA) film.In conclusion, the tensile properties of the three UV-cured polyurethane films were similar to that of UV-cured films prepared with PUA.15 Such data suggested that the obtained bio-based unsaturated poly(ester-urethane)s prepared by a green method that avoids the use of hazardous diisocyanates could be used for UV curable polyurethane system, instead of polyurethane-acrylate.
Thermal properties
The thermal behavior of each UV-cured polyurethane film was characterized by using DSC as shown in Fig. 2. It can been seen that the Tg values of the three cured polyurethane films were 28, 21 and 8 °C for poly(U2-IA), poly(U4-IA) and poly(U6-IA), respectively. Obviously, the Tg values decreased with the increase of diamine's aliphatic chain length in the UV-cured polyurethane film, which could be attributed to the reduction of the chain mobility with the increase of aliphatic chain length in diamine, as supported by the results of Ubaghs et al.19 And in this case, the effect of un-crosslinking components is minor. Such result clearly indicated that the Tg values depended on the chemical structure of the diamine used. | ||
| Fig. 2 DSC curves of the three UV-cured polyurethane films obtained from the second heating runs from −40 to 120 °C at a heating rate of 10 °C min−1. | ||
The TGA and DTG curves of the three UV-cured polyurethane films under nitrogen and air were showed in Fig. 3. The TGA curves of all the three UV-cured polyurethane films were similar under nitrogen, and all of them can be divided into three stages as shown in Fig. 3(a). In stage I, an insignificant weight loss at about 150 °C was thought to be mainly due to the loss of humidity in the films. Stage II showed onset temperatures from 255 to 273 °C and close peak temperatures from 285 to 298 °C for the samples. Thermal degradation of the urethane linkages and the formation of –NCO, primary amines, and secondary amines take place in this stage, as found in a previous study.20 Stage III, which occurred at approximately 390 to 401 °C and exhibited peak temperatures from 434 to 454 °C, may correspond to the thermal degradation of soft fragments, such as the ester bonds of alkyl chains, in good agreement with previous observation21 (see Table S2 in ESI†).
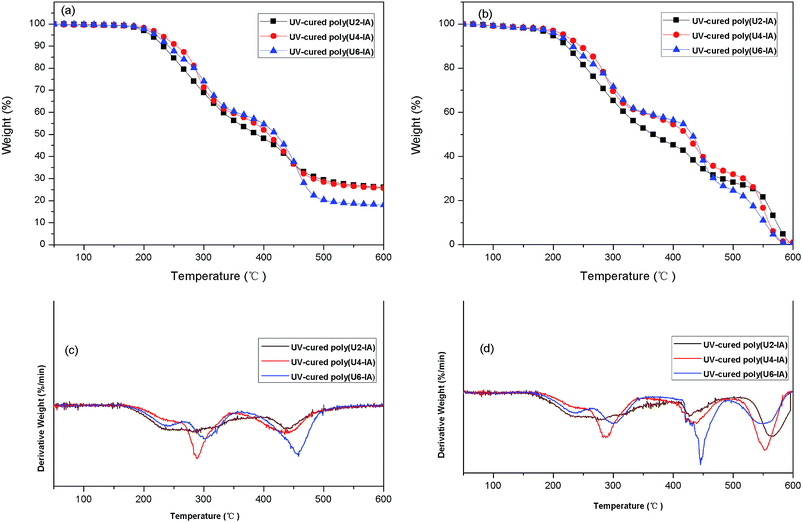 | ||
| Fig. 3 TGA curves (heating rate: 10 °C min−1) and DTG curves of the three UV-cured polyurethane films under nitrogen (a and c) and air (b and d). | ||
The TGA curves of samples under air atmosphere were also similar with each other, but four, instead of three, stages of thermal degradation were observed (Fig. 3(b)). Stages I, II and III can be corresponded to the decomposition behavior under nitrogen condition. The appearance of stage IV, with an onset temperature from 550 to 566 °C and a peak temperature from 589 to 593 °C, was observed, which could be attributed to the dehydrogenation and depolycondensation of alkyl groups of the UV-cured polyurethane films22 (see Table S3 in ESI†). Based on these results, we concluded that all UV-cured polyurethane films showed a similar thermal decomposition behavior and exhibited excellent thermal stability.
General coating properties
A summary of the UV-cured coating properties was found in Table 2. After the completion of UV curing, several films properties such as pencil hardness, flexibility and adhesion were evaluated. The three UV-cured polyurethane films showed the pencil hardness in the order UV-cured poly(U4-IA) > UV-cured poly(U2-IA) > UV-cured poly(U6-IA). It could be attributed to the fact that the hardness of the coatings depends on not only the chemical structure of the prepolymers but also the cross-linking density of networks. The T-bend test and tape test showed that all UV-cured polyurethane coatings on the tinplate substrate exhibited excellent flexibility and adhesion, similar to that of UV-curing coatings with PUA. The results further demonstrated that the obtained bio-based unsaturated poly(ester-urethane)s based on isocyanate-free chemistry could be used as supplementary for polyurethane-acrylate.| Samples | Pencil hardness | Flexibility | Adhesion |
|---|---|---|---|
| UV-cured poly(U2-IA) | H | 0T | 5B |
| UV-cured poly(U4-IA) | 2H | 1T | 4B |
| UV-cured poly(U6-IA) | 2B | 0T | 5B |
Conclusion
The bio-based unsaturated poly(ester-urethane)s containing two urethane linkages, two ester linkages and one double bond in each repeating unit were designed and synthesized by the metal-catalyzed melt polycondensation of itaconic acid with urethanediols as a diisocyanate-free green method. The average double bond contents within the three bio-based unsaturated poly(ester-urethane)s were higher than that of PUA (about 2.3, 3.7 and 2.9 for poly(U2-IA), poly(U4-IA) and poly(U6-IA), respectively), so that they could be used for UV curable polyurethane systems. After the completion of UV curing, the tensile properties, thermal properties and general coating properties of the three samples were found to be comparable to that of UV-cured PUA films. These results suggested that the bio-based unsaturated polyurethanes prepared by a green method that avoids the use of hazardous diisocyanates could be used for UV curable polyurethane systems, instead of polyurethane-acrylate. The UV-cured poly(U4-IA) showed the best tensile strength, thermal property, pencil hardness, flexibility, and adhesion among the three samples, which could be an potential candidate as oligomers for UV-curable coatings, plastics, inks and adhesives.Acknowledgements
The authors greatly thank the financial support from the National Natural Science Foundation of China (21204096).References
- A. Campanella, L. M. Bonnaillie and R. P. Wool, J. Appl. Polym. Sci., 2009, 112, 2567–2578 CrossRef CAS.
- S. D. Miao, S. P. Zhang, Z. G. Su and P. Wang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 243–250 CrossRef CAS.
- J. H. Clements, Ind. Eng. Chem. Res., 2003, 42, 663–674 CrossRef CAS.
- B. Ochiai and T. Endo, Prog. Polym. Sci., 2005, 30, 183–215 CrossRef CAS.
- B. Nohra, L. Candy, J. F. Blanco, C. Guerin, Y. Raoul and Z. Mouloungui, Macromolecules, 2013, 46, 3771–3792 CrossRef CAS.
- R. W. McCabe and A. Taylor, Green Chem., 2004, 6, 151–155 RSC.
- H. Hayashi, Y. Yanagishita and S. Matsumura, Int. J. Mol. Sci., 2011, 12, 5490–5507 CrossRef CAS PubMed.
- Y. Soeda, K. Toshima and S. Matsumura, Macromol. Biosci., 2005, 5, 277–288 CrossRef CAS PubMed.
- Y. Yanagishita, M. Kato, K. Toshima and S. Matsumura, ChemSusChem, 2008, 1, 133–142 CrossRef CAS PubMed.
- B. C. Guo, Y. W. Chen, Y. D. Lei, L. Q. Zhang, W. Y. Zhou, A. B. M. Rabie and J. Q. Zhao, Biomacromolecules, 2011, 12, 1312–1321 CrossRef CAS PubMed.
- G. Rokicki and A. Piotrowska, Polymer, 2002, 43, 2927–2935 CrossRef CAS.
- C. W. Chang and K. T. Lu, Prog. Org. Coat., 2012, 75, 435–443 CrossRef CAS.
- C. Decker, Prog. Polym. Sci., 1996, 21, 593–650 CrossRef CAS.
- S. B. Wu, J. D. Jorgensen, A. D. Skaja, J. P. Williams and M. D. Soucek, Prog. Org. Coat., 1999, 36, 21–33 CrossRef CAS.
- H. P. Xu, F. X. Qiu, Y. Y. Wang, W. L. Wu, D. Y. Yang and Q. Guo, Prog. Org. Coat., 2012, 73, 47–53 CrossRef CAS.
- C. Decker and A. D. Jenkins, Macromolecules, 1985, 18, 1241–1244 CrossRef CAS.
- X. J. Wang and M. D. Soucek, Prog. Org. Coat., 2013, 76, 1057–1067 CrossRef CAS.
- L. E. Nielsen, J. Macromol. Sci., Rev. Macromol. Chem., 1969, C3, 69 CrossRef.
- L. Ubaghs, N. Fricke, H. Keul and H. Hocker, Macromol. Rapid Commun., 2004, 25, 517–521 CrossRef CAS.
- I. Javni, Z. S. Petrovic, A. Guo and R. Fuller, J. Appl. Polym. Sci., 2000, 77, 1723–1734 CrossRef CAS.
- E. Hablot, D. Zheng, M. Bouquey and L. Averous, Macromol. Mater. Eng., 2008, 293, 922–929 CrossRef CAS.
- F. Gaboriaud and J. P. Vantelon, J. Polym. Sci., Part A: Polym. Chem., 1982, 20, 2063–2071 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08665a |
| This journal is © The Royal Society of Chemistry 2014 |



