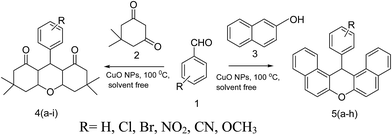
Recyclable CuO nanoparticles as heterogeneous catalysts for the synthesis of xanthenes under solvent free conditions†
Ganga Ram Chaudhary*,
Pratibha Bansal,
Navneet Kaur and
S. K. Mehta
Department of Chemistry & Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh-160014, India. E-mail: grc22@pu.ac.in; Fax: +91-172-2545074; Tel: +91-172 2534406
First published on 30th September 2014
Abstract
CuO nanoparticles (NPs) of 17–22 nm size have been synthesized in very high yield in 4 minutes using a microwave and water as medium. The obtained NPs have been characterized by FTIR, XRD and TEM. CuO NPs have been used as a nanocatalyst to carry out the synthesis of xanthenes in solvent free conditions. They exhibit good catalytic activity with excellent yield. The main features of CuO NPs as catalyst in the synthesis of xanthenes are reduced reaction time, higher yields, ease of product isolation, economic availability of catalyst, simple procedure, and no harmful byproducts. The spent heterogeneous catalyst has been recovered by simple filtration and reused for multiple cycles.
1. Introduction
Nanoparticles (NPs) have received significant attention as efficient catalysts in many organic reactions due to their high surface-to-volume ratio and coordination parts which provide a larger number of active sites per unit area in comparison with their heterogeneous counter sites.1 In the present report we have explored CuO (NPs) as a catalyst.Organic transformations using heterogeneous catalyst under solvent free condition has gained great importance, due to minimum pollution and easy work up conditions.2–5 In recent years, much attention has been given to the synthesis of xanthene derivatives.6–8
Xanthenes and its derivatives are very important class of heterocyclic compounds because of their wide range of biological and pharmaceutical properties.9 In addition, these compounds are widely used as dyes,10 fluorescent materials for sensing of biomolecules11 and for antiviral activity.12 These compounds are also utilized as antagonists for paralyzing action of zoxazolamine and in photodynamic therapy.13 Due to their wide range of applications, a wide variety of methods for the preparation of the xanthenes have been reported. Instead of Lewis acid14–17 as catalyst, which is associated with harsh experimental conditions such as anhydrous condition, high temperature, prolonged reaction time, expensive, harmful and difficult to handle reagents, low yield, difficult work up, use of nanocatalyst has been encouraged in recent times.18,19 There are successful attempts of xanthenes synthesis using ZnO18 and Fe3O4 nanocatalyst.19 However, in both cases quantity of catalyst required is large, moreover it takes more time to produce xanthenes with lesser yield.
Among various metal oxides NPs, in the present study we have chosen CuO NPs as promising candidate due to low cost, abundant resources, non-toxicity and easy preparation in various shapes of nanosized dimensions. CuO NPs has been widely used in electrochemical cells,20 gas sensors,21 photovoltaic cells,22 thermoelectric materials,23 nanofluids and for photocatalysis.24,25 The methods to synthesize CuO nanomaterials are diverse, such as electrochemical deposition, alcohothermal, solid-state reaction and sol–gel.26–29 However above mentioned methods for synthesis of CuO NPs had some disadvantage such as time consuming, expensive, pollution causing and low yields. To overcome all the problems we have chosen microwave (MW) synthesis over conventional synthesis. It takes just 4 min to synthesis CuO NPs of 17–22 nm size. This method is economical both in terms of energy consumption and time.
The purpose of the present work is to explore the utility of CuO NPs as catalyst in synthesis of xanthenes under solvent free conditions. In the present work, CuO NPs are acting as efficient heterogeneous catalyst in synthesis of xanthenes in terms of short reaction time, easy work up, excellent yield, enhanced energy efficiency, cost effective and no harmful by-products.
2. Results and discussion
2.1 Characterization of CuO NPs
Fig. 1 displays FTIR spectra of CuO NPs. The broad band at 3200 cm−1 and small band at 1637 cm−1 correspond to the physical adsorbed water on the sample. The absorbance band at 1637 cm−1 is due to bending vibration involved in H–O–H angle and 3200 cm−1 band is due to stretching vibration of O–H bond.30 Absorption band between 417 cm−1 and 1030 cm−1 was attributed to the asymmetric and symmetric stretching frequency of Cu–O–Cu vibrational bands respectively.31 All the FTIR peaks of CuO NPs were slightly shifted to higher frequency than that of bulk CuO, which indicates the formation of small sized particles. Further, all the peaks of CuO NPs were relatively broad, which represent more symmetrical structure.XRD is powerful technique to analyze the structure of the material and weather the substance is crystalline or amorphous, as for crystalline substance well defined peaks are observed in XRD. Diffraction peaks for CuO NPs were obtained with (hkl) values as (110), (−111), (111/200), (−202), (020), (202), (−113), (022/−311), (113/220), (311), (004/−222) which are found to be same for single phase CuO NPs with a monoclinic (card JCPDS 72-0629)32 as shown in Fig. 2. No peaks of impurity was found in XRD pattern. Particle size was calculated from FWHM of reflection (111/200) of monoclinic CuO structure using Debye Scherrer formula (eqn (1)). The particle size was found to be ∼18 nm.
For determining the morphology, TEM micrograph of CuO NPs was taken. As illustrated from the Fig. 3, NPs were almost spherical in shape, well dispersed and narrow range of size distribution (17–22 nm).
The particle size distribution of synthesized CuO NPs has been estimated by particle size analyzer (PSA). Fig. 4 shows the typical particle size distribution graph which reveals that the average diameter of synthesized CuO NPs is 25 nm. The polydispersity index (PDI) of synthesized nanoparticles is found to be 0.20 that confirms the monodispersity of synthesized NPs.
2.2 Catalytic activity towards synthesis of xanthenes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a model substrate in the presence of CuO NPs as catalyst under solvent free conditions at 100 °C as a heterogeneous catalyst (Scheme 1). The results in Table 1 confirm that the yield of 5a xanthenes increased with increase in the amount of catalyst from 2 mg to 7 mg. Further increase in the amount of catalyst showed no improvement in yield.
1 ratio as a model substrate in the presence of CuO NPs as catalyst under solvent free conditions at 100 °C as a heterogeneous catalyst (Scheme 1). The results in Table 1 confirm that the yield of 5a xanthenes increased with increase in the amount of catalyst from 2 mg to 7 mg. Further increase in the amount of catalyst showed no improvement in yield.| R = H, Cl, Br, NO2, CN, OCH3 |
| S.no. | Amount of catalyst (mg) | Time (min)/% yield |
|---|---|---|
| 1. | Nil | 60/nil |
| 2. | 2 | 25/60 |
| 3. | 3 | 22/68 |
| 4. | 4 | 20/72 |
| 5. | 5 | 18/80 |
| 6. | 6 | 17/95 |
| 7. | 7 | 16/95 |
| 8. | 8 | 14/89 |
| 9. | 8 | 16/95 |
| S.no. | Benzaldehyde | Product (xanthenes) | Time (min) | Yield (%) | Melting point | Ref. |
|---|---|---|---|---|---|---|
| a Their melting points are compared with reported values. Amount catalyst used was 7 mg. | ||||||
| 4a |  |
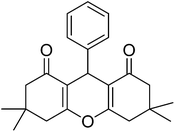 |
14 | 89 | 202–204 | 42 |
| 4b |  |
 |
18 | 92 | 230–231 | 42 |
| 4c |  |
 |
15 | 87 | 241–243 | 40 |
| 4d |  |
 |
9 | 90 | 219–221 | 42 |
| 4e |  |
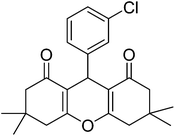 |
12 | 89 | 183–184 | 40 |
| 4f |  |
 |
13 | 90 | 230–232 | 40 |
| 4g |  |
 |
13 | 89 | 240–241 | 40 |
| 4h |  |
 |
9 | 95 | 261–262 | 40 |
| 4i |  |
 |
20 | 85 | 214–215 | 40 |
| 5a |  |
 |
16 | 95 | 181–183 | 40 |
| 5b |  |
 |
23 | 84 | 227–229 | 40 |
| 5c |  |
 |
25 | 82 | 203–204 | 40 |
| 5d |  |
 |
14 | 93 | 310–312 | 40 |
| 5e |  |
 |
15 | 86 | 211–212 | 42 |
| 5f |  |
 |
15 | 83 | 287–289 | 40 |
| 5g |  |
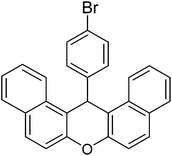 |
16 | 87 | 296–297 | 40 |
| 5h |  |
 |
13 | 90 | 218–219 | 40 |
 | ||
| Fig. 5 Recycling ability of CuO NPs for 5a in Table 2. | ||
2.3 Comparison with other catalyst present in literature
Earlier work18,19,36–43 carried out for synthesis of xanthenes showed that reactions with different catalysts required either higher amount of catalyst or longer reaction time. In some cases reactions were performed in di chloro methane (DCM) which results in difficult work up and environment hazards. A comparison of the use of CuO NPs in present work with some of the other reported catalysts for synthesis of xanthenes has been listed in Table 3. As evident from the Table 3 the size of CuO NPs is smaller than the other catalysts viz. ZnO NPs18 and Fe3O4 NPs19 for the synthesis of xanthenes. These results are in consistent with the fact that smaller the size of nano-catalyst more will be the catalytic activity (Table 3).| Name of catalyst | Amount of catalyst (mg) | Time/yield in (%) | Solvent/condition | Ref. |
|---|---|---|---|---|
| K5CoW12O40·3H2O | 64 | 2 (h)/91 | Solvent free/125 °C | 36 |
| Succinimide-N-sulfonic acid | 10 | 35 (min)/92 | Solvent free/80 °C | 37 |
| Sulfamic acid | 9.7 | 8 (h)/93 | Solvent free/125 °C | 38 |
| Tungstophosphoric acid/zirconia composites | 50 | 1 (h)/99 | Solvent free/130 °C | 39 |
| Iodine | 25.38 | 2.5 (h)/90 | Solvent free/90 °C | 40 |
| Fe(HSO4)3 | 35 | 4 (h)/85 | DCM | 41 |
| Amberlyst-15 | 10 | 2 (h)/94 | Solvent free/125 °C | 42 |
| Functionalized mesoporous materials | 20 | 6 (h)/80 | DCM/25 °C | 43 |
| ZnO NPs (24 nm) | 10 | 28 (min)/87 | Solvent free/80 °C | 18 |
| Fe3O4 NPs (40–50 nm) | 20 | 30 (min)/88 | Solvent free/80 °C | 19 |
| CuO NPs (17–22 nm) | 7 | 16 (min)/95 | Solvent free/80 °C | This work |
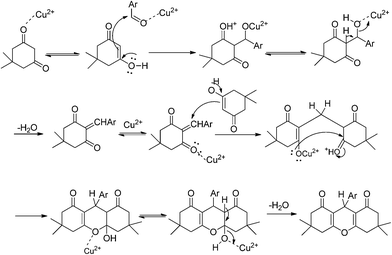
2.4 Plausible reaction mechanism
Copper site in CuO NPs behaves as Lewis acid, they have great tendency to co-ordinate with different functional group such as carbonyl (–CO), nitrile (–CN), hydroxyl (–OH), thiol (–SH) etc. As shown in Scheme 2 dimedone or aldehyde undergo chemical adsorption by interaction with acidic surface of metal sites. Because of interaction between the carbonyl group of substrate with CuO NPs, the carbonyl group of aldehyde was activated for nucleophilic attack in next step, leading to speed up the rate of reaction. Finally the product was obtained and the CuO NPs being released for further reactions. Similar mechanism is in case of naphthol and aldehyde.3. Conclusion
CuO NPs have been synthesized in high yield by using water as solvent. Due to the use of high power MW, time taken for synthesis is 4 minutes and the size obtained is also very small (17–22 nm). The as prepared CuO NPs have been employed as a catalyst in the synthesis of xanthenes with varied substitution pattern using conventional heating source under solvent free conditions. It has proved to be very efficient as compared to other catalysts that have already been used for the synthesis of xanthenes. Attractive features such as reduced reaction time, higher yields, ease of product isolation, economic availability of catalyst, simple procedure and solvent free condition combined with easy recovery, no harmful byproduct and reuse of this catalyst 4 times without much effect in product yield, makes it one of the best catalysts for synthesis of xanthenes.4. Experimental
4.1 General remarks
X-Ray Diffraction (XRD) spectra were recorded on Panalytical X'Pert Pro X-ray diffractometer equipped with Cu-kα radiation (1.5406 Å) operating at 40 kV, with scanning speed of 8° min−1 to examine the crystalline phase of the sample. Size is calculated by using Debye Scherrer equation (eqn (1))
 | (1) |
where K is shape factor, λ is the X-ray wavelength, β is the full width at half the maximum intensity (FWHM), θ is Braggs angle and D is the mean size of particle. Fourier transform infrared (FTIR) spectra was obtained on a Perkin Elmer FTIR spectrophotometer in the frequency range of 4000–1000 cm−1 using KBr plates with 100 number scans and 4 cm−1 spectral resolution. Transmission electron microscope (TEM) micrograph was obtained by analyzed using Hitachi (H-7500) electron microscope operating at 80 kV. To check the particle size distribution, particle size analyser (PSA) was performed using Malvern Zetasizer nanoseries (Nano-S90). 1H and 13C NMR spectra were measured on a model advance II (Bruker) instrument with frequency 300 MHz for 1H NMR and 100 MHz frequency for 13C NMR using TMS as the internal standard and CDCl3 as solvent. Microwave IFB 20PG2S, with power output-800 watt, operational frequency-2450 MHz has been used for synthesis of CuO NPs.
Dimedone and naphthol was supplied by Sigma Aldrich, copper chloride was obtained from Glaxo laboratory India and sodium hydroxide (NaOH) and benzaldehyde was supplied by Qualigens. p-Chlorobenzaldehyde, m-chlorobenzaldehyde, o-anisaldehyde, p-anisaldehyde, o-nitrobenzaldehyde and p-nitrobenzaldehyde, m-nitrobenzaldehyde, p-bromobenzaldehyde, p-cyanobenzaldehyde, p-methyl benzaldehyde were purchased from HiMedia. Ethanol was supplied by Changshu, Yangyuan Chemicals China. Purity of all chemicals was more than 98% and was used without further purification. Doubly distilled water was used for the synthesis of CuO NPs.
4.2 Preparation of CuO NPs
In a typical reaction, 100 ml of 0.01 M solution of CuCl2·2H2O and 100 ml of 0.03 M solution of NaOH was prepared. Both the prepared solutions were mixed together and microwaved for 4 minutes. The formation of brown precipitates appeared. The final product was separated out by filtration, washed with distilled water and dried under room temperature. To optimise the synthesis, we have changed reaction time and found that there was no complete conversion of reactant into product before 4 minutes as peaks of impurities were obtained in XRD [Fig. S2 ESI†]. These impurity peaks are labelled as 1 and 2 in Fig. S2 ESI,† which are due to monoclinic phase of Cu2(OH)3Cl.44 To verify the reproducibility of synthesis of CuO NPs by MW, we have performed the synthesis of NPs three times at the interval of 24 hours. XRD results for the three samples are reproducible with almost same size [Fig. S3 ESI†].4.3 Synthesis of xanthenes
A mixture of aldehyde (1 mmol), 2-naphthol (2 mmol) or dimedone (2 mmol) and CuO NPs (0.007 g) was heated with stirring at 100 °C in an oil bath. The progress of reaction was monitored by TLC. After cooling, the reaction mixture was dissolved in dichloromethane (DCM) and the mixture stirred for 5 min. The suspended solution was filtered and then heterogeneous nanocatalyst was recovered. The dichloromethane was evaporated and the crude product was recrystallized from ethanol to give the pure product. The isolated catalyst was washed with ethanol and dried at room temperature for over night, catalyst was reused at least 4 times without an appreciable decrease of yield. All of the pure products were characterized by comparison of their physical (melting point) and spectral data (1H and 13C NMR) with those of authentic samples.33–35 Also the flash chromatography has been done to purify the products by using elutant (ethylacetate–hexane in ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). It has been seen that product got by recrystallization and flash chromatography results came out to be same in terms of yield and purity (1H NMR) [Fig. S4 ESI†].
6). It has been seen that product got by recrystallization and flash chromatography results came out to be same in terms of yield and purity (1H NMR) [Fig. S4 ESI†].
Table 2, 4b; 1H NMR (300 MHz, CDCl3); δH (ppm): 0.85 (6H, s, 2 × CH3), 1.00 (6H, s, 2 × CH3), 1.97–2.10 (4H, q, 2 × CH2), 2.21–2.37 (4H, q, 2 × CH2), 3.67 (1H, s, OCH3), 4.70 (1H, s, CH), 6.60–7.26 (4H, m, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 26.89, 29.37, 29.63, 32.10, 41.06, 50.75, 55.11, 110.60, 113.95, 120.48, 127.77, 130.52, 132.21, 157.53, 162.21, 195.45.
Table 2, 4c; 1H NMR (300 MHz, CDCl3); δH (ppm): 0.97 (6H, s, 2 × CH3), 1.08 (6H, s, 2 × CH3), 2.10–2.17 (4H, q, 2 × CH2), 2.40–2.42 (4H, q, 2 × CH2), 3.70 (3H, s, OCH3), 4.65 (1H, s, CH), 6.69–7.17 (4H, dd, Ar–H). 13C NMR (300 MHz, CDCl3); δC (ppm): 27.40, 29.35, 30.96, 32.20, 40.93, 50.78, 55.02, 113.50, 115.90, 129.31, 136.42, 158.03, 161.83, 196.10.
Table 2, 4d; 1H NMR (300 MHz, CDCl3); δH (ppm): 0.98 (6H, s, 2 × CH3), 1.11 (6H, s, 2 × CH3), 2.10–2.24 (4H, q, 2 × CH2), 2.46 (4H, s, 2 × CH2), 4.76 (1H, s, CH), 7.41–8.08 (4H, dd, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 27.41, 29.42, 32.30, 40.99, 50.61, 114.75, 123.44, 129.39, 146.71, 151.23, 162.45, 195.43.
Table 2, 4e; 1H NMR (300 MHz, CDCl3); δH (ppm): 0.86 (6H, s, 2 × CH3), 0.99 (6H, s, 2 × CH3), 2.04–2.06 (4H, q, 2 × CH2), 4.59 (1H, s, CH), 7.24–7.39 (4H, m, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 27.43, 29.44, 32.28, 32.52, 41.00, 50.63, 114.84, 129.35, 131.95, 162.37, 195.42.
Table 2, 4f; 1H NMR (300 MHz, CDCl3); δH (ppm): 0.93 (6H, s, 2 × CH3), 1.06 (6H, s, 2 × CH3), 2.10–2.11 (4H, q, 2 × CH2), 2.37 (4H, s, 2 × CH2), 4.57 (1H, s, CH), 7.05–7.08 (2H, d, Ar–H), 7.23–7.26 (2H, d, Ar–H).13C NMR (100 MHz, CDCl3); δC (ppm): 27.50, 29.52, 31.58, 32, 27, 41.04, 50.70, 115.45, 120.49, 130.24, 131.22, 143.07, 161.77, 195.10.
Table 2, 4g; 1H NMR (300 MHz, CDCl3); δH (ppm): 0.97 (6H, s, 2 × CH3), 1.09 (6H, s, 2 × CH3), 2.13 2.15 (4H, q, 2 × CH2), 2.40–2.41 (4H, s, 2 × CH2), 4.62 (1H, s, CH), 7.13–7.15 (4H, m, Ar–H). 13C NMR (300 MHz, CDCl3); δC (ppm): 27.49, 29.53, 31.48, 32.27, 41.04, 50.70, 115.53, 128.29, 129.84, 132.31, 142.54, 161.75, 195.12.
Table 2, 4h; 1H NMR (300 MHz, CDCl3); δH (ppm): 1.03 (6H, s, 2 × CH3), 1.13 (6H, s, 2 × CH3), 2.20–2.21 (4H, q, 2 × CH2), 2.47 (4H, q, 2 × CH2), 4.69 (1H, s, CH), 7.09–7.27 (4H, m, Ar–H). 13C NMR (100 MHz, CDCl3; δC (ppm)): 27.51, 29.41, 31.75, 32.29, 41.00, 50.72, 115.30, 126.80, 127.15, 128.25, 129.24, 134.04, 146.04, 162.08, 195.45.
Table 2, 4i; 1H NMR (300 MHz, CDCl3); δH (ppm): 1.04 (6H, s, 2 × CH3), 1.10 (6H, s, 2 × CH3), 2.14–2.15 (4H, d), 2.24 (3H, s, CH3), 2.29 2.41 (4H, d), 4.62 (1H, s, CH), 6.94–6.97 (2H, d, Ar–H), 7.08 7.10 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 27.55, 29.61, 31.46, 32.30, 41.09, 50.80, 51.12, 116.07, 128.39, 128.86, 135.59, 141.15, 161.37, 163.06, 195.18.
Table 2, 5a; 1H NMR (300 MHz, CDCl3); δH (ppm): 6.44 (1H, s, CH), 7.24 (2H, s, Ar–H), 7.37–7.50 (9H, m), 7.74–7.79 (4H, m), 8.33–8.36 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 38.10, 117.39, 118.05, 122.73, 124.22, 126.77, 128.26, 128.54, 128.85, 131.14, 131.58, 145.02, 148.80.
Table 2, 5b; 1H NMR (300 MHz, CDCl3); δH (ppm): 2.12 (3H, s, CH3), 6.39 (1H, s, CH), 6.90–6.93 (2H, d. Ar–H), 7.33–7.38 (4H, m), 7.43–7.46 (2H, d, Ar–H), 7.50–7.53 (2H, d, Ar–H), 7.55–7.78 (4H, m), 8.32–8.35 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 21.02, 37.72, 117.50, 118.03, 122.79, 124.17, 126.71, 128.13, 128.74, 128.82, 129.25, 131.16, 131.59, 135.67, 142.18, 148.72.
Table 2, 5c; 1H NMR (300 MHz, CDCl3); δH (ppm): 3.54 (3H, s, OCH3), 6.37 (1H, s, CH), 6.58–6.60 (2H, d, Ar–H), 7.30–7.35 (4H, m), 7.38–7.40 (2H, d, Ar–H), 7.47–7.51 (2H, t, Ar–H), 7.69–7.75 (4H, m), 8.29–8.31 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 37.08, 55.05, 113.84, 117.53, 117.99, 122.67, 124.19, 126.74, 128.70, 128.78, 129.13, 131.07, 131.41, 148.69, 157.84.
Table 2, 5d; 1H NMR (300 MHz, CDCl3); δH (ppm): 6.53 (1H, s, CH), 7.34–7.38 (2H, t, Ar–H), 7.42–7.44 (2H, d, Ar–H), 7.50–7.54 (2H, t, Ar–H), 7.59–7.62 (2H, d, Ar–H), 7.75–7.79 (4H, m), 7.91–7.93 (2H, d, Ar–H), 8.19–8.22 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 37.86, 115.78, 118.06, 122.03, 123.86, 124.58, 127.19, 128.96, 129.06, 129.60, 131.09.
Table 2, 5e; 1H NMR (300 MHz. CDCl3); δH (ppm): 6.36 (1H, s, CH), 6.85–6.88 (1H, d), 6.95–6.99 (1H, t), 7.30–7.40 (6H, m), 7.47–7.51 (2H, t), 7.69–7.71 (4H, m), 8.21–8.23 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 37.75, 116.58, 118.08, 122.40, 124.38, 126.40, 126.74, 126.96, 128.33, 128.90, 129.16, 129.60, 131.06, 131.27, 134.42, 146.88, 148.79.
Table 2, 5f; 1H NMR (300 MHz, CDCl3); δH (ppm): 6.46 (1H, s, CH), 7.08–7.10 (2H, d, Ar–H), 7.39–7.48 (8H, m), 7.78–7.83 (4H, m) 8.29–8.32 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 37.37, 116.76, 118.01, 122.40, 124.36, 126.91, 128.63, 128.90, 129.08, 129.48, 131.08, 131.26, 132.09, 143.45, 148.72.
Table 2, 5g; 1H NMR (300 MHz, CDCl3); δH (ppm): 5.21 (1H, s, CH), 7.16–7.18 (2H, d, Ar–H), 7.29–7.40 (6H, m), 7.48–7.52 (2H, t, Ar–H), 7.71–7.76 (4H, m), 8.22–8.24 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 37.72, 115.89, 118.12, 121.69, 122.02, 122.71, 124.58, 127.24, 129.06, 129.49, 129.56, 131.04, 131.06, 134.2.
Table 2, 5h; 1H NMR (300 MHz, CDCl3); δH (ppm): 6.57 (1H, s, CH), 7.44–7.48 (4H, m), 7.50–7.53 (2H, d, Ar–H), 7.59–7.65 (4H, m), 7.84–7.88 (4H, m), 8.28–8.30 (2H, d, Ar–H). 13C NMR (100 MHz, CDCl3); δC (ppm): 26.03, 122.09, 124.65, 127.24, 128.92, 129.21, 129.66, 155.58.
Acknowledgements
GRC would like to acknowledge the financial support of the SERB-DST India (SB/EMEQ-166/2013) and PB is thankful to UGC, Delhi for the fellowships.References
- A. S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik and Sk. M. Islam, Appl. Catal., A, 2014, 469, 320–327 CrossRef CAS
.
- G. R. Chaudhary, P. Bansal and S. K. Mehta, Chem. Eng. J., 2014, 243, 217–224 CrossRef CAS
.
- E. Y. Yuzik-Klimova, N. V. Kuchkina, S. Sorokina, D. G. Morgan, L. Z. Nikoshvili, N. Lyubimova, V. G. Matveeva, E. M. Sulman, B. D. Stein, W. E. Mahmoud, A. A. Al-Ghamdi, A. Kostopoulou, A. Lappas, Z. B. Shifrina and L. M. Bronstein, RSC Adv., 2014, 4, 23271–23280 RSC
.
- M. Tajbakhsh, M. Farhang, R. Hosseinzadeh and Y. Sarrafi, RSC Adv., 2014, 4, 23116–23124 RSC
.
- S. Pathak, K. Debnath, M. R. Mollick and A. Pramanik, RSC Adv., 2014, 4, 23779–23789 RSC
.
- F. Shirini, A. Yahyazadeh and K. Mohammadi, Chin. Chem. Lett., 2014, 25, 341–347 CrossRef CAS
.
- H. Naeimi and Z. S. Nazifi, Appl. Catal., A, 2014, 477, 132–140 CrossRef CAS
.
- M. Mokhtary and S. Refahati, Dyes Pigm., 2013, 99, 378–381 CrossRef CAS
.
- J. M. Khurana, D. Magoo, K. Aggarwal, N. Aggarwal, R. Kumar and C. Srivastava, Eur. J. Med. Chem., 2012, 58, 470–477 CrossRef CAS PubMed
.
- W. Zhang and R. Xu, Int. J. Hydrogen Energy, 2012, 37, 17899–17909 CrossRef CAS
.
- A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, J. Am. Chem. Soc., 2008, 130, 12095–12101 CrossRef CAS PubMed
.
- H. N. Hafez, M. I. Hegab, I. S. Ahmed-Farag and A. B. A. El-Gazzar, Bioorg. Med. Chem. Lett., 2008, 18, 4538–4543 CrossRef CAS PubMed
.
- J. P. Tardivoa, A. D. Giglio, C. S. de Oliveirab, D. S. Gabrielli, H. C. Junqueirab, D. B. Tadab, D. Severinob, R. F. Turchiello and M. S. Baptista, Photodiagn. Photodyn. Ther., 2005, 2, 175–191 CrossRef
.
- K. R. Moghadam and S. C. Azimi, J. Mol. Catal. A: Chem., 2012, 363–364, 465–469 CrossRef
.
- A. Zare, A. R. Moosavi-Zare, M. Merajoddin, M. A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M. H. Beyzavi, E. Rostami, A. Arghoon and R. Roohandeh, J. Mol. Liq., 2012, 167, 69–77 CrossRef CAS
.
- M. Mokhtary and S. Refahati, Dyes Pigm., 2013, 99, 378–381 CrossRef CAS
.
- N. G. Khaligh, Ultrason. Sonochem., 2012, 19, 736–739 CrossRef CAS PubMed
.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, Chin. Chem. Lett., 2012, 23, 1225–1229 CrossRef CAS
.
- M. A. Ghasemzadeh, J. Safaei-Ghomi and S. Zahedi, J. Serb. Chem. Soc., 2013, 6, 769–779 CrossRef
.
- M. Kong, W. Zhang, Z. Yang, S. Weng and Z. Chen, Appl. Surf. Sci., 2011, 258, 1317–1321 CrossRef CAS
.
- C. Yang, X. Su, J. Wang, X. Cao, S. Wang and L. Zhang, Sens. Actuators, B, 2013, 185, 159–165 CrossRef CAS
.
- C. C. Vidyasagar, Y. A. Naik, T. G. Venkatesh and R. Viswanatha, Powder Technol., 2011, 214, 337–343 CrossRef CAS
.
- Q. Zhang, K. Zhang, D. Xu, G. Yang, H. Huang, F. Nie, C. Liu and S. Yang, Prog. Mater. Sci., 2014, 60, 208–337 CrossRef CAS
.
- M. M. Sarafraz and F. Hormozi, Exp. Therm. Fluid Sci., 2014, 52, 205–214 CrossRef CAS
.
- A. N. Ejhieha and M. K. Shamsabadi, Appl. Catal., A, 2014, 477, 83–92 CrossRef
.
- N. Mukherjee, B. Show, S. K. Maji, U. Madhu, S. K. Bhar, B. C. Mitra, G. G. Khan and A. Mondal, Mater. Lett., 2011, 65, 3248–3250 CrossRef CAS
.
- Z. Hong, Y. Cao and J. Deng, Mater. Lett., 2002, 52, 34–38 CrossRef CAS
.
- S. Sohrabnezhad and A. Valipour, Spectrochim. Acta, Part A, 2013, 114, 298–302 CrossRef CAS PubMed
.
- J. Jayaprakash, N. Srinivasan and P. Chandrasekaran, Spectrochim. Acta, Part A, 2014, 123, 363–368 CrossRef CAS PubMed
.
- S. Y. Venyaminov and F. G. Prendergast, Anal. Biochem., 1997, 248, 234–245 CrossRef CAS PubMed
.
- B. Zhao, P. Liu, H. Zhuang, Z. Jiao, T. Fang, W. Xu, B. Lub and Y. Jiang, J. Mater. Chem. A, 2013, 1, 367–373 CAS
.
- D. Han, H. Yang, C. Zhu and F. Wang, Powder Technol., 2008, 185, 286–290 CrossRef CAS
.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, Chin. Chem. Lett., 2012, 23, 1225–1229 CrossRef CAS
.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, J. Saudi Chem. Soc., 2012 DOI:10.1016/j.jscs.2012.05.007
.
- B. Karami, S. J. Hoseini, K. Eskandari, A. Ghasemi and H. Nasrabadi, Catal. Sci. Technol., 2012, 2, 331–338 CAS
.
- L. Nagarapu, S. Kantevari, V. C. Mahankhali and S. Apuri, Catal. Commun., 2007, 8, 1173–1177 CrossRef CAS
.
- F. Shirini and N. G. Khaligh, Dyes Pigm., 2012, 95, 789–794 CrossRef CAS
.
- B. Rajitha, B. S. Kumar, Y. T. Reddy, P. N. Reddy and N. Sreenivasulu, Tetrahedron Lett., 2005, 46, 8691–8693 CrossRef CAS
.
- T. S. Rivera, A. Sosa, G. P. Romanelli, M. N. Blanco and L. R. Pizzio, Appl. Catal., A, 2012, 443–444, 207–213 CrossRef CAS
.
- B. Das, B. Ravikanth, R. Ramu, K. Laxminarayana and B. V. Rao, J. Mol. Catal. A: Chem., 2006, 255, 74–77 CrossRef CAS
.
- H. Eshghi, M. Bakavoli and H. Moradi, Chin. Chem. Lett., 2008, 19, 1423–1426 CrossRef CAS
.
- S. Ko and C. Yao, Tetrahedron Lett., 2006, 47, 8827–8829 CrossRef CAS
.
- J. Mondal, M. Nandi, A. Modak and A. Bhaumik, J. Mol. Catal. A: Chem., 2012, 363–364, 254–264 CrossRef CAS
.
- A. Pendashteh, M. S. Rahmanifar and M. F. Mousavi, Ultrason. Sonochem., 2014, 21, 643–652 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07620f |
| This journal is © The Royal Society of Chemistry 2014 |