One-pot synthesis of thiol- and amine-bifunctionalized mesoporous silica and applications in uptake and speciation of arsenic
Peng Li,
Xiao-qin Zhang,
Yi-jun Chen,
Tian-yi Bai,
Hong-zhen Lian* and
Xin Hu*
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry & Chemical Engineering and Center of Materials Analysis, Nanjing University, 22 Hankou Road, Nanjing, 210093, China. E-mail: hzlian@nju.edu.cn; huxin@nju.edu.cn; Fax: +86 25 83325180; Fax: +86 25 83325180; Tel: +86 25 83686075 Tel: +86 25 83592247
First published on 19th September 2014
Abstract
A series of thiol- and amine-bifunctionalized mesoporous silicas were synthesized via one-pot co-condensation of tetraethylorthosilicate, 3-mercaptopropyltrimethoxysilane and N-(2-aminoethyl)-3-aminopropyltriethoxysilane. The mesoporous materials were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, nitrogen gas adsorption, infrared spectroscopy, thermogravimetric analysis and elemental analysis. As(V) and As(III) were effectively adsorbed by amine and thiol on the functionalized silica, respectively, through electrostatic interaction and chelation. Adsorption isotherms and kinetic uptake profiles of As(V) and As(III) onto these adsorbents were investigated by batch adsorption experiments. Moreover, this material was employed for the speciation analysis of arsenic using a home-made syringe-based solid phase extraction device. As(V) and As(III) were effectively separated and pre-concentrated in a single run through a sequential elution strategy, in which 0.1 M HNO3 was first used to selectively elute As(V), and then 1 M HNO3 with 0.01 M KIO3 was used to elute As(III). The merits of easy preparation, low cost, high adsorption capacity and selective desorption make the bifunctional mesoporous silica an ideal solid material for the removal and speciation analysis of arsenic in environmental waters.
1. Introduction
In recent decades, heavy metal contamination of natural waters has been of great concern because of the toxicity of heavy metals in relatively low concentrations, and their tendency for bioaccumulation in plants, animals and human beings.1,2 Thus It is necessary to control the harmful effects of heavy metal ions through daily monitoring and removal technologies. Solid materials such as adsorbent that are used for the adsorption of heavy metal ions has increasingly received considerable attention in recent years because they are simple, cost-effective and easy to be automated.3 Moreover, they can be easily incorporated into automated solid phase extraction (SPE) procedures for the determination of trace metal ions and their species.4 A variety of adsorbents, such as biomass,5 carbon,6 zeolites,7 and functionalized inorganic supports8 have been applied for the study of trace elements with satisfactory results. In contrast to some other solid materials, significant attention has been paid to mesoporous silica due to its large specific surface area, good dispersibility and controllable morphology.9,10 Recently, Wang et al.11 prepared an L-cysteine-functionalized SBA-15 for Hg(II) sorption, which shows a large adsorption capacity. Awual et al.12 synthesized 6-((2-(2-hydroxy-1-naphthoyl)hydrazono)methyl)benzoic acid (HMBA) functionalized material as optical mesoporous adsorbent for the selective recognition and removal of ultra-trace Cu(II) and Pd(II) ions.To date, a series of functionalized mesoporous silicas, generally synthesized by the post modification of pure mesoporous silica gel or direct co-condensation of organosilane reagents with tetra-alkoxysilanes (either tetraethoxysilane (TEOS) or tetramethoxysilane (TMOS)), are currently available to adsorb heavy metal ions. Three main types of functional groups (amine, thiol and carboxylic) have been demonstrated to be the specific ligands for most of the metal ions.13–15 However, there are certain differences for some elements, e.g., arsenic, selenium, antimony and tellurium, because they exist as different species in environmental water, and the properties of each species are considerably different.16 For example, arsenic is an omnipresent toxic trace element, and it is mainly found in environmental waters in two oxidation states, As(III) and As(V). Because of the different properties of these two species, it is hard to simultaneously adsorb them on mono-functionalized mesoporous silica with thiol or amine functional groups.8,17–19 In addition, it is worth noting that most of the adsorbents, including mesoporous silica, are facing a similar problem; thus, a pre-oxidation or pre-reduction operation is indispensable for the uptake of both arsenic species. For example, pre-oxidization of As(III) to As(V) is usually performed using oxidizing agents or photocatalytic oxidation to enhance As(III) removal.20,21
The studies have shown that the toxic effects of arsenic in an environmental or biological system critically depend on its chemical form, and As(III) is the most toxic form of the water-soluble species, while As(V) is also considerably toxic. Thus, it is not only important to remove As(III) and As(V), but also necessary for the quantitative determination of each arsenic species in daily monitoring.22–29 Conventionally, the speciation of inorganic arsenic without employing chromatographic separation can be achieved by the selective adsorption of one species onto adsorbents, and the other species calculated by subtraction from the total inorganic arsenic. To obtain the total inorganic arsenic content, an additional extraction procedure is usually needed after the pre-oxidization/pre-reduction of arsenic in the sample,24–27 or adjustment of the sample pH,28,29 which makes the sample pretreatment slightly time-consuming.
For the removal or speciation analysis of arsenic, the conversion of species from one form to another may not only cause operational complexity, but also introduce interferences. In this paper, we report the first attempt to synthesize a thiol and amine-bifunctionalized mesoporous silica by a one-step method for the simultaneous uptake of As(III) and As(V). The novel materials synthesized from different reactant proportions of organosilane reagents and TEOS were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), N2 adsorption, Fourier-transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA) and elemental analysis (EA). The batch adsorption experiments were performed to investigate their adsorption behaviors and capacities for As(III) and As(V). Then, using a facilely homemade syringe-based SPE device, a sequential elution strategy for arsenic speciation analysis was proposed, and the simultaneous separation and pre-concentration of As(III) and As(V) was achieved in a single run without changing any sample conditions.
2. Experimental
2.1 Reagents and materials
Cetyltrimethylammonium bromide (CTAB) was purchased from TCI (Tokyo, Japan). Tetraethylorthosilicate (TEOS) and 3-mercaptopropyltrimethoxysilane (MPTMS) were purchased from Alfa Aesar (Tianjing, China), and N-(2-aminoethyl)-3-aminopropyltriethoxysilane (AAPTES) was purchased from Ourchem (Shanghai, China). HNO3 was of guaranteed reagent grade and obtained from Merck (Zurich, Switzerland). All other chemicals were at least of analytical grade, and used without further purification. Deionized water (DIW, 18.25 MΩ cm) obtained from a Milli-Q water system (Millipore, Bedford, MA, USA) was used throughout the experiment.Stock standard solutions of As(III) and As(V), 1000.0 mg L−1, were prepared by respectively dissolving appropriate amounts of Na3AsO3 and As2O5 (both of analytical grade, purchased from Johnson Matthey, UK) in DIW. Lower concentration standard solutions were prepared daily by appropriate dilutions from their stock solutions.
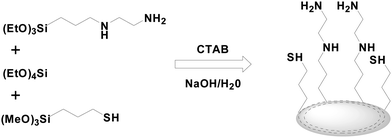
2.2 Synthesis of bifunctional mesoporous silica
Bifunctional mesoporous silica was synthesized via co-condensation of TEOS, MPTMS and AAPTES with surfactant (CTAB) as the template in alkaline solution (Scheme 1). Typically, 120 mL DIW and 3.5 mL NaOH (1 M) were added to 0.5 g CTAB and stirred at 80 °C for 30 min. Then, a certain amount of TEOS, MPTMS and AAPTES were mixed and added to the above clear basic surfactant solution. Following the injection, a white precipitate was observed within 4 min. The reaction mixture was allowed to stir at 80 °C for 2 h under the atmosphere of nitrogen, and then the resulting slurry was filtered and rinsed with excess of H2O, followed by ethanol and naturally dried overnight. Subsequently, the dried solid was extracted with a mixed solution of 0.5 mL HCl and 150 mL ethanol by stirring at 50 °C for 3 h. The extraction was repeated twice to accomplish the complete removal of CTAB. Then, the solid was filtered and washed with copious amounts of water until neutral, and subsequently repeatedly rinsed with ethanol. The resulting mesoporous silica was finally dried under vacuum for 24 h.The molar composition of the reaction mixture was (1 − 2X)TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X MPTMS
X MPTMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X AAPTES
X AAPTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 CTAB
0.11 CTAB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.28 NaOH
0.28 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 H2O, where the reaction molar proportion between MPTMS/AAPTES and total Si is represented by X. Four different molar proportions, X = 0, 0.025, 0.075 and 0.15 were taken, and the bifunctionalized mesoporous silica materials thus obtained are hereafter noted as MP-AAP-0%, MP-AAP-2.5%, MP-AAP-7.5% and MP-AAP-15%, respectively.
532 H2O, where the reaction molar proportion between MPTMS/AAPTES and total Si is represented by X. Four different molar proportions, X = 0, 0.025, 0.075 and 0.15 were taken, and the bifunctionalized mesoporous silica materials thus obtained are hereafter noted as MP-AAP-0%, MP-AAP-2.5%, MP-AAP-7.5% and MP-AAP-15%, respectively.
2.3 Characterization of bifunctional mesoporous silica
XRD, SEM, TEM, N2 adsorption, FT-IR, TGA and EA were employed for the characterization of the synthesized bifunctional mesoporous silica. Powder XRD experiment was performed on the Philip X'Pert Pro using a Cu Kα radiation source. Low angle diffraction with a 2-theta range of 1 to 10° was used to investigate the long-range order of the materials. Particle morphology of these materials was obtained by SEM using a Hitachi S-3400N II with 10 kV accelerating voltage and 0.005 nA of beam current for imaging. The microstructure was characterized by high resolution transmission electron microscopy (HRTEM) on a JEM-200CX microscope operating at a 200 kV accelerating voltage. N2 adsorption measurement was carried out on a Micromeritics ASAP 2020 BET surface analyzer system at liquid N2 temperature (−196 °C). Before measurement, the sample was outgassed at 90 °C for 1 h and then at 150 °C for 4 h. The data were evaluated using the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods to calculate the surface area and pore volumes/pore size distributions, respectively. FT-IR spectra were recorded on a Nicolet-6700 spectrometer in the range of 4000–450 cm−1. TGA of the materials was performed on a Perkin-Elmer Pyris 1 DSC from 303 to 973 K with a heating rate of 10 K min−1. EA of the particles was carried out on an Elementar Vario EL III using oxygen as the combustion gas.2.4 Batch adsorption experiments
The adsorption of As(III) and As(V) on the bifunctional mesoporous silica was measured in batch experiments by adding a fixed amount of adsorbent (0.010 g) into a definite volume (10 mL) of either As(III) or As(V) solution. The initial pH of the solutions was adjusted with diluted HNO3 or NH3·H2O using a pH meter. The mixture was placed in a rotary mixer and shaken (100 rpm) at room temperature for 30 min. After centrifugation, the remaining arsenic in the supernatant liquid was quantified using an inductively coupled plasma atomic emission spectrometer (ICP-AES, Perkin-Elmer, Model Optima 5300DV) by measuring the signal intensity at the emission wavelength of As, 189.042 nm. The amount of arsenic adsorbed was calculated using the following equation:| Qe = [(C0 − Ce)V]/m |
2.5 Speciation analysis procedure
A syringe filter tip (0.45 μm) of 13 mm diameter was used to form a simple SPE device. Briefly, 200 mg mesoporous silica were dispersed into 10 mL DIW, the suspension was then sequentially and rapidly injected evenly into ten syringe filter tips using a 10 mL syringe cylinder. In this case, DIW was passed through the filter membrane while the silica particles were collected on the surface of the filter membrane. As a result, a layer of 20 mg silica particles was immobilized in each syringe filter tip.In the extraction step, 10 mL As(III) or As(V) solution was allowed to flow through the filter tip at a flow rate of 1 mL min−1 for the preconcentration of As(III) and As(V). The flow rate was precisely controlled by a pump (Baoding Longer, Model BT100) or roughly controlled by pushing the syringe by hand. The effluent was collected and 75As was monitored by an inductively coupled plasma mass spectrometer (ICP-MS, Perkin-Elmer, Model Elan-9000) to calculate the adsorption percentage. In the elution step, 1.5 mL of 0.1 mol L−1 HNO3 was injected into the filter to firstly desorb As(V) from the silica and the eluent was collected. Then, 1.5 mL of 1 M HNO3 with 0.01 M KIO3 was injected into the filter to desorb As(III) and the eluent was also collected. Finally, two portions of the eluent were directly introduced into ICP-MS for the determination of As(V) and As(III), respectively.
3. Results and discussion
3.1 Structure, property and function of the bifunctional silica
Small-angle XRD patterns of the final materials with different initial reactant ratios are shown in Fig. 1. The well-resolved diffraction patterns characteristic of hexagonal MCM-41 silica, including (1 0 0), (1 1 0) and (2 0 0) peaks, were observed in MP-AAP-0%, MP-AAP-2.5% and MP-AAP-7.5%, while MP-AAP-15% appeared to be disordered. The peak intensity of the samples weakens as the organosilane dosage increases, implying that the organosilane in the synthesis mixture would perturb the self-assembly of surfactant micelles and the silica precursors. Moreover, a systematic shift of the diffraction peaks to larger 2θ angles with increasing organosilane contents suggests that slight lattice contractions occur as a result of the incorporation of the organosilane groups within the mesostructure synthesis (Table 1).
| Adsorbent | Initial X | d spacing (nm) | SBET (m2 g−1) | Pore size (nm) | Pore volume (cm3 g−1) | EA (μmol g−1) | Qm (μmol g−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sulfur | Nitrogen | As(III) | As(V) | ||||||
| MP-AAP-0% | 0 | 4.0 | 992 | 3.5 | 0.87 | 0 | 0 | 0 | 0 |
| MP-AAP-2.5% | 0.025 | 3.9 | 940 | 3.2 | 0.73 | 199 | 536 | 33 | 49 |
| MP-AAP-7.5% | 0.075 | 3.8 | 706 | 2.0 | 0.35 | 739 | 807 | 139 | 156 |
| MP-AAP-15% | 0.15 | 3.4 | 547 | <2.0 | 0.34 | 1912 | 1500 | 192 | 417 |
The particle morphology and mesoporous structure of the resulting materials were analyzed by SEM and TEM. The SEM micrographs in Fig. 2A show a dramatic transformation of the particle morphology, with ellipsoidal particles being observed at the low concentrations of organosilane reagents (MP-AAP-0% and 2.5%), and elongated rods being observed at a higher concentration of organosilane reagents (MP-AAP-7.5%). The TEM micrographs of Fig. 2B revealed that the mesopores are uniformly aligned along the long axes of MP-AAP-0%, MP-AAP-2.5% and MP-AAP-7.5%. However, there were no ordered pores observed on the irregular particles for MP-AAP-15%, which is consistent with the result from XRD observation shown in Fig. 1.
The N2 adsorption analyses were performed on the synthesized materials. As shown in Fig. 3, MP-AAP-0%, 2.5% and 7.5% display a type IV isotherm, while the isotherm of MP-AAP-15% is different probably due to its out-of-order structure. The surface area, pore volume and pore size distribution of the bifunctional materials are listed in Table 1. It was found that the overall trend is a decrease in these parameters with the increase of the initial organosilanes (X).
TGA (Fig. 5) shows that the weight loss of MP-AAP-X materials in the temperature range between 473 and 923 K was 6.1%, 10.5%, 13.8% and 22.2% for MP-AAP-0%, MP-AAP-2.5%, MP-AAP-7.5% and MP-AAP-15%, respectively, which is attributed to the decomposition of the organic functional groups. The results were in accordance with the FT-IR and EA.
3.2 Batch adsorption behaviors of the bifunctional mesoporous silica
Fig. 7 displays the As(V) and As(III) uptake curves for the two bifunctional materials, MP-AAP-7.5% and MP-AAP-15%. It can be seen that two adsorbents exhibit similar performance in the equilibrium time. The adsorption for As(V) was completed in 30 s, while for As(III) in 5 min. As mentioned in the previous section of the characterization of the adsorbents, the difference between MP-AAP-7.5% and MP-AAP-15% is the degree of mesoporous structure order and surface area. However, despite the high surface areas and uniform mesoporosities of the adsorbents, the results do not demonstrate any remarkably rapid arsenic uptake by the mesostructure.
| Adsorbent | Adsorption capacity (mg g−1) | Ref. | |
|---|---|---|---|
| As(V) | As(III) | ||
| MP-AAP-2.5% | 3.7 | 2.5 | This work |
| MP-AAP-7.5% | 11.7 | 10.4 | |
| MP-AAP-15% | 31.3 | 14.4 | |
| Mercapto-functionalized mesoporous silica | — | 19.4 | 18 |
| AAAPTS modified silica gel | 13.9 | — | 19 |
| AAPTS modified mesoporous silica | 10.3 | — | 26 |
| (NH2 + SH) modified silica gel | 0.29 | 2.7 | 29 |
3.3 Speciation analysis of arsenic species
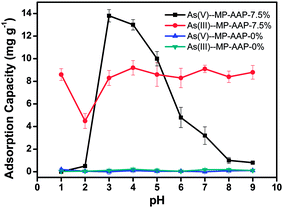
As described in the previous section, As(III) can be adsorbed on the bifunctional silica at both the pH 1.0 and 4.0, suggesting 0.1 M HNO3 (pH 1.0) does not affect the adsorption of As(III) on the adsorbent. In the case of As(V), the adsorption was considerably affected by pH, in particular the adsorption percentage, which decreased to 0% at pH 1.0. Therefore, a sequential elution strategy for As(III) and As(V) was proposed, in which 0.1 M HNO3 was employed to first selectively elute As(V), and then 1 M HNO3 with 0.01 M KIO3 was employed to elute As(III). The probable elution mechanism can be explained as follows: As(V) is transformed from anion to uncharged species (H3AsO4) in 0.1 M HNO3 and loses electrostatic interaction with the adsorbent, and then mercapto groups are oxidized by KIO3 and the chelation between mercapto and As(III) is destroyed. Using individual As(V) or As(III) solution, it was found that As(V) can be quantitatively eluted by 1.5 mL 0.1 M HNO3, and no As(III) was observed in this process, and then, As(III) can be quantitatively eluted by 1.5 mL 1 M HNO3 with 0.01 M KIO3. These results confirmed the feasibility of the sequential elution strategy.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039.4x + 507.1 (R2 = 0.999) and y = 13
039.4x + 507.1 (R2 = 0.999) and y = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 841.8x + 1494.0 (R2 = 0.998), respectively. The precisions (relative standard deviations, RSDs) for six replicate determinations of 10 μg L−1 As(V) and As(III) were 5.6% and 4.5%, respectively. Adsorbents (MP-AAP-7.5%) prepared within batch (n = 4) and between different batches (n = 3) were examined by measuring the recoveries of the same solution containing 10 μg L−1 As(V) and As(III) under the optimized conditions. It was found that the RSDs of eluted As(V) and As(III) were 4.0% and 6.9% for intra-batch, and they were 4.3% and 4.7% for inter-batch, respectively.
841.8x + 1494.0 (R2 = 0.998), respectively. The precisions (relative standard deviations, RSDs) for six replicate determinations of 10 μg L−1 As(V) and As(III) were 5.6% and 4.5%, respectively. Adsorbents (MP-AAP-7.5%) prepared within batch (n = 4) and between different batches (n = 3) were examined by measuring the recoveries of the same solution containing 10 μg L−1 As(V) and As(III) under the optimized conditions. It was found that the RSDs of eluted As(V) and As(III) were 4.0% and 6.9% for intra-batch, and they were 4.3% and 4.7% for inter-batch, respectively.The accuracy of the proposed separation scheme was evaluated by analyzing standard solutions and two certified reference materials, namely GSBZ 50004-88 (standard environmental water sample) and GSB 080230 (standard seawater sample). The results of these analyses are summarized in Table 3. As can be seen, the concentrations of As(V), As(III) and As(Total) were in good agreement with the standard or certified values. These results indicated that As(III), As(V) and As(Total) in these water samples can be successfully determined based on the simultaneous retention of As(Total) on the SPE tip, and sequential elution with appropriate eluent.
| Sample | Certified (μg L−1) | Found (μg L−1) | ||||
|---|---|---|---|---|---|---|
| As(V) | As(III) | As(Total) | As(V) | As(III) | As(Total) | |
| ST1 | 10 | 10 | 20 | 10.8 ± 0.7 | 11.0 ± 0.9 | 21.8 ± 1.6 |
| ST2 | 100 | 100 | 200 | 98.6 ± 7.8 | 107.5 ± 8.6 | 216.1 ± 16.4 |
| 50004-88 | — | — | 124 ± 8 | 2.1 ± 0.8 | 128.5 ± 9.6 | 130.6 ± 10.5 |
| 080230 | — | — | 1000 ± 40 | 70.4 ± 16.6 | 945.3 ± 73.4 | 1015.7 ± 80.0 |
In addition, the levels of monomethylarsenic acid (MMA) and dimethylarsenic acid (DMA) were also considered, a tandem SPE method that combined the MP-AAP-7.5% with strong cation-exchange resin (SCX 732, Sinopharm Chemical Reagent Co., Ltd) was investigated (Fig. 8B). The first SPE unit was filled with SCX, and the second SPE unit was filled with MP-AAP-7.5%. After loading the sample solution, the tandem syringe filter tips were separated. The first one was eluted by 1 M HNO3 for the retained DMA, and the second one was orderly eluted by 50 mM acetic acid (HAc) for MMA, 0.1 M HNO3 for As(V) and 1 M HNO3 with 0.01 M KIO3 for As(III). The recoveries in ranges of 97–104%, 83–92%, 92–101% and 96–108% were obtained for 10 mL of 10 μg L−1 DMA, MMA, As(V) and As(III), respectively.
4. Conclusions
Thiol- and amine-bifunctionalized mesoporous silica was synthesized for the first time by one-pot co-condensation of TEOS, MPTMS and AAPTES under basic CTAB solution. The amounts of MPTMS and AAPTES in the initial synthetic mixture were varied, and their effects on the structural and chemical properties of these porous materials were systematically investigated. Due to the different interaction mechanisms between arsenic with bifunctional groups, As(V) and As(III) can be simultaneously adsorbed by the adsorbents in a wide pH range, and their separation can be realized by selective and sequential elution of As(V) with diluted 0.1 M HNO3 and then As(III) with 1 M HNO3 with 0.01 M KIO3. The homemade syringe-based SPE device possessing the characteristics such as portability and simplicity provides a promising application for field sampling and pretreatment. These advantages make the bifunctional mesoporous silica an attractive and desirable adsorbent not only for arsenic removal from contaminated water, but also for arsenic speciation analysis.Further investigations on the adsorption of other heavy metal ions on the bifunctionalized mesoporous silica have been conducted to fully evaluate the properties of this adsorbent, which would be reported elsewhere.
Acknowledgements
This work was supported by National Natural Science Foundation of China (21275069, 90913012), National Basic Research Program of China (973 program, 2009CB421601, 2011CB911003), National Science Funds for Creative Research Groups (21121091), and Analysis & Test Fund of Nanjing University.References
- S. Babel and T. A. Kurniawan, J. Hazard. Mater., 2003, 97, 219–243 CrossRef CAS.
- F. L. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- T. M. Mututuvari and C. D. Tran, J. Hazard. Mater., 2014, 264, 449–459 CrossRef CAS PubMed.
- B. Hu, F. Zheng, M. He and N. Zhang, Anal. Chim. Acta, 2009, 650, 23–32 CrossRef CAS PubMed.
- X. W. Chen, A. M. Zou, M. L. Chen, J. H. Wang and P. K. Dasgupta, Anal. Chem., 2009, 81, 1291–1296 CrossRef CAS PubMed.
- M. L. Chen, Y. M. Lin, C. B. Gu and J. H. Wang, Talanta, 2013, 104, 53–57 CrossRef CAS PubMed.
- W. Qiu and Y. Zheng, J. Hazard. Mater., 2007, 148, 721–726 CrossRef CAS PubMed.
- S. Mandal, T. Padhi and R. K. Patel, J. Hazard. Mater., 2011, 192, 899–908 CrossRef CAS PubMed.
- J. Aguado, J. M. Arsuaga, A. Arencibia, M. Lindo and V. Gascón, J. Hazard. Mater., 2009, 163, 213–221 CrossRef CAS PubMed.
- B. G. Trewyn, I. I. Slowing, S. Giri, H. T. Chen and V. S. Lin, Acc. Chem. Res., 2007, 40, 846–853 CrossRef CAS PubMed.
- Q. Li, Z. Wang, D. M. Fang, H. Y. Qu, Y. Zhu, H. J. Zou, Y. R. Chen, Y. P. Du and H. L. Hu, New J. Chem., 2014, 38, 248–254 RSC.
- M. R. Awual, I. M. Rahman, T. Yaita, M. A. Khaleque and M. Ferdows, Chem. Eng. J., 2014, 236, 100–109 CrossRef CAS PubMed.
- D. Rahmi, Y. Takasaki, Y. Zhu, H. Kobayashi, S. Konagaya, H. Haraguchi and T. Umemura, Talanta, 2010, 81, 1438–1445 CrossRef CAS PubMed.
- L. Zhang, W. Zhang, J. Shi, Z. Hua, Y. Li and J. Yan, Chem. Commun., 2003, 210–211 RSC.
- Y. L. Wang, L. J. Song, L. Zhu, B. L. Guo, S. W. Chen and W. S. Wu, Dalton Trans., 2014, 43, 3739–3749 RSC.
- Z. Mester, R. Sturgeon and J. Pawliszyn, Spectrochim. Acta, Part B, 2001, 56, 233–260 CrossRef.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed.
- E. McKimmy, J. Dulebohn, J. Shah and T. J. Pinnavaia, Chem. Commun., 2005, 3697–3699 RSC.
- H. T. Fan, T. Sun, H. B. Xu, Y. J. Yang, Q. Tang and Y. Sun, Desalination, 2011, 278, 238–243 CrossRef CAS PubMed.
- T. Nakajima, Y. H. Xu, Y. Mori, M. Kishita, H. Takanashi, S. Maeda and A. Ohki, J. Hazard. Mater., 2005, 120, 75–80 CrossRef CAS PubMed.
- A. H. Fostier, M. d. S. S. Pereira, S. Rath and J. R. Guimarães, Chemosphere, 2008, 72, 319–324 CrossRef CAS PubMed.
- X. C. Le, X. Lu and X. F. Li, Anal. Chem., 2004, 76, 26 A–33 A CrossRef CAS.
- T. Llorente-Mirandes, J. Calderón, F. Centrich, R. Rubio and J. F. López-Sánchez, Food Chem., 2014, 147, 377–385 CrossRef CAS PubMed.
- X. P. Yan, X. B. Yin, X. W. He and Y. Jiang, Anal. Chem., 2002, 74, 2162–2166 CrossRef CAS.
- X. P. Yan, R. Kerrich and M. J. Hendry, Anal. Chem., 1998, 70, 4736–4742 CrossRef CAS.
- D. H. Chen, C. Z. Huang, M. He and B. Hu, J. Hazard. Mater., 2009, 164, 1146–1151 CrossRef CAS PubMed.
- M. L. Chen, C. B. Gu, T. Yang, Y. Sun and J. H. Wang, Talanta, 2013, 116, 688–694 CrossRef CAS PubMed.
- C. Z. Huang, B. Hu and Z. C. Jiang, Spectrochim. Acta, Part B, 2007, 62, 454–460 CrossRef PubMed.
- E. Boyacı, A. Çağır, T. Shahwan and A. E. Eroğlu, Talanta, 2011, 85, 1517–1525 CrossRef PubMed.
- A. Howard, M. Volkan and D. Y. Ataman, Analyst, 1987, 112, 159–162 RSC.
| This journal is © The Royal Society of Chemistry 2014 |