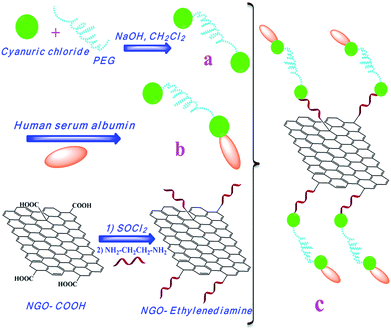
Albumin–graphene oxide conjugates; carriers for anticancer drugs
Safura Jokara,
Ali Pourjavadia and
Mohsen Adeli*ab
aPolymer Research Laboratory, Department of Chemistry, Sharif University of Technology, Tehran, Iran
bDepartment of Chemistry, Faculty of Science, Lorestan University, Khoramabad, Iran. E-mail: mohadeli@yahoo.com; m.aadeli@fu-berlin.de
First published on 9th July 2014
Abstract
In order to improve its biological properties, graphene oxide can be modified with hydrophilic polymers. Therefore, in this study, the surface of graphene oxide was modified with polyethylene glycol and albumin by covalent methods. In the subsequent step, paclitaxel which is a hydrophobic anticancer drug was loaded onto the surface of the functionalized graphene by π–π interactions. The synthesis of the nanocarrier and its interaction with paclitaxel were evaluated by FT-IR, CD, TEM, UV, AFM, DLS and fluorescence experiments. Release of the loaded drug from albumin–graphene conjugate was investigated at pH 5.4, 6.8 and 7.4.
Introduction
Recently graphene and its derivatives, in comparison with other carbon nanomaterials, have attracted much attention in biosensors, drug delivery and cancer therapies due to their unique chemical and physical properties.1 Graphene has a huge surface area (theoretically 2600 m2 g−1), which can be used in the delivery of many aromatic molecules, such as anticancer drugs.2 In order to improve the solubility and functionality of graphene sheets, they can be functionalized by hydrophilic polymers and proteins through covalent or non-covalent approaches. In the past years graphene has been functionalized with many polymers such as PEG,3 chitosan4 or dextran.5 Although these hydrophilic and biocompatible materials have been used to functionalize graphene sheets, we still need to coat graphene with other new hydrophilic materials in order to achieve new hybrid materials with desirable properties for different applications in biomedicine.Albumin is the most abundant protein in plasma which is extensively used to functionalize nanoparticles and also as a drug delivery system because of its hydrophilicity, biocompatibility, biodegradability and safety. It has 585 amino acid residues that are arranged in three domains (I, II, III), each containing two subdomains (A, B).6 It can bond to hydrophobic molecules and transport them to target tissues.7 Albumin can accumulate and stay in tumor tissues for a long time due to passive targeting by enhanced permeable reaction (EPR) effects and active targeting by gp60 receptors on the membrane of the cancer cells.8
Paclitaxel is a potent anticancer drug that can be used for a wide range of tumors. Due to its poor solubility in water and side effects, cremophor EL/ethanol is used to improve its solubility but cremophor EL is poisonous to the human body.9
The aim of this work is to conjugate albumin onto the surface of graphene by PEG linkages and then use the obtained hybrid nanomaterial to deliver paclitaxel. This system will benefit from both graphene and albumin properties in terms of high loading capacity, stability and passive targeting and at the same time transport paclitaxel to cancer cells successfully.
Experimental
Materials and methods
Natural graphite powder, potassium permanganate (KMnO4), sodium nitrate (NaNO3), sulfuric acid (H2SO4), hydrogen peroxide (H2O2 30%), cyanuric chloride, polyethylene glycol (Mw = 1000), dichloromethane (CH2Cl2), diethyl ether, tetrahydrofuran (THF), ethylenediamine, sodium hydroxide (NaOH), hydrochloric acid (HCl, 10%) and paclitaxel (PTX) were purchased from Merck Company, Germany. Human serum albumin (HAS) manufactured by CLS Behring AG Wankdorfstrasse 10 CH-3000 Bern 22 Switzerland. Dialysis bags (Mn cutoff 3500 Da and 12 kDa) were provided from Sigma Aldrich (St Louis, Missouri). Fourier-transform infrared (FT-IR) spectra were recorded by ABB Bomem MB-100 spectrometer using KBr pellets. Fluorescence spectra were taken on a Cary Eclipse EL05053669 spectrofluorimeter with quartz cells (1.0 cm). The excitation and emission were set at λ = 280 and 300 nm respectively. The UV-vis spectra were recorded on a Perkin-Elmer Lambda 25 spectrometer using quartz cells (1.00 cm) at room temperature. Far-UV CD spectra were obtained by a AVIV 215 (made in U.S.A) spectropolarimeter with a quartz cell (1.0 nm), at room temperature and the scan rate was 1.00 nm min−1. Ultrasonic bath VGT-1730 QTD 220–240 V, 100 W, Freq = 42 kHz was used to disperse nanoparticles in suitable solvent. Dynamic light scattering (DLS) diagrams were measured by Zetasizer ZS, Malvern Instruments to determine the size distribution profile of nanoparticles in solutions. Surface imaging observations were collected by atomic force microscopy (AFM) (Model: DME, Dualscope/Rasterscope) on mica or lamella surface to survey surface morphology and particles size. Transmission electron microscopy (TEM) images were recorded by a Zeiss – EM10C electron microscope with accelerating voltage of 80 kV.Preparation of graphene oxide (GO)
Graphene oxide was synthesized from nature graphite by Hummers method.10 Briefly, graphite powder (1.0 g) was stirred in the presence of sodium nitrate (0.5 g) and concentrated H2SO4 (23 ml) for 2 h at 5 °C. Then, KMnO4 (0.5 g) was slowly added to mixture over 1 h. Mixture was stirred for 2 h and then deionized water and H2O2 (10 ml) were respectively added to the solution. Obtained suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and washed by diluted HCl (10%) and deionized water several times to remove excess acid and graphene oxide was dried at 40 °C under vacuum for 72 h.
000 rpm for 5 min and washed by diluted HCl (10%) and deionized water several times to remove excess acid and graphene oxide was dried at 40 °C under vacuum for 72 h.
Synthesis of amine-functionalized GO
GO (0.001 g) was sonicated in thionyl chloride (SOCl2) (30 ml) for 1 h at room temperature. Suspension was refluxed and stirred at 120 °C for 48 h. Then it was centrifuged and the obtained powder was washed with tetrahydrofuran (THF) and dried at 40 °C under vacuum. Next step, the ethylenediamine (excess 5.00 ml) was added to acylchloride derivative of GO (0.0001 g). The mixture was stirred at room temperature for 20 h. The suspension was centrifuged and washed three times with THF to remove excess ethylenediamine and product was dried at 40 °C for 48 h.Functionalization of polyethylene glycol by cyanuric chloride (PC)
Polyethylene glycol was functionalized by cyanuric chloride according to reported procedure in literature.11 A solution of polyethylene glycol (4.0 g) and sodium hydroxide (0.32 g) in water (10 ml) was drop wise added to cyanuric chloride (3.69 g) in dichloromethane (50 ml) at 0 °C. Then, the mixture was stirred at room temperature for 1 h and finally was refluxed for 6 h. Mixture was cooled, filtered off and solvent was evaporated. The obtained product was dissolved in dichloromethane and was precipitated in diethyl ether at 0 °C for several times. Finally pure product was obtained as a white solid.Conjugation of albumin to PC
Albumin was drop wise added to a solution of PC (0.2 g) in water (5 ml) at room temperature. Mixture was gently stirred at room temperature for 2 days. Mixture was directly dialyzed by using a dialysis bag with a molecular weight cutoff of 3500 Da for 2 days at room temperature. The purified product (APC) in water was separated as a transparent solution.Attachment of APC to the amino-functionalized GO (APC-amino-GO)
Amino-functionalized GO (0.1 g) was dispersed in water (10 ml) at pH 8 for 30 min. Then, the resulting mixture was drop wise added to a solution of APC in water. The final mixture was gently stirred for 48 h at 60 °C to obtain a biomolecule functionalized graphene oxide. After centrifugation, mixture was washed three times with water.Loading of paclitaxel on APC-amino-GO (APC-amino-GO-PTX)
For physical loading of PTX onto graphene oxide surface. Due to hydrophobicity of PTX, first a saturated solution of PTX in dichloromethane (2 ml) was prepared. Then it was slowly added to a solution of APC-amino-GO (0.003 g) in water (5 ml). The resulted mixture was sonicated at about second at room temperature in interval times and was stirred for 48 h at room temperature. Then dichloromethane was evaporated. The used dichloromethane and ultrasonication had low effects on the sensitive structure of albumin and did not denature its structure. According to fluorescence spectrum, the fluorescence intensity of albumin increase after loading PTX onto nanostructure. This result confirms that albumin interacts with graphene surface mainly and upon interfering PTX its fluorescence again relive. In order to purify the prepared drug delivery system, it was transferred into a dialysis bag (with Mn cutoff of 12 kDa) and the bag was placed into a flask containing H2O (50 ml) and acetonitrile (10 ml) and was dialyzed for 2 h to remove untreated material (such as free PTX). Then product was dried at 40 °C under vacuum.Release of PTX from APC-amino-GO-PTX
In a typical process, APC-amino-GO-PTX (0.001 g) was dispersed in PBS buffer (1.00 ml) pH 7.4. Then the solution was transferred into a dialysis bag with a molecular weight cutoff of 12 kDa. Tube was placed into a flask containing buffer (12.0 ml) solution. The flask was kept in a water bath at 37 °C and outside solution was stirred at 285 rpm. At predetermined time intervals, 3 ml of the release medium was removed and replaced with fresh medium. Samples were diluted with acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and released PTX was determined through UV-vis spectra and using absorbance peak of PTX at 227 nm. This procedure was also used to determine the rate of release of PTX in other pHs (5 and 6.8). The PTX-loading efficiency was calculated by following equation:
1) and released PTX was determined through UV-vis spectra and using absorbance peak of PTX at 227 nm. This procedure was also used to determine the rate of release of PTX in other pHs (5 and 6.8). The PTX-loading efficiency was calculated by following equation:| PTX-loading efficiency = 100([PTX]feed − [PTX]free)/[PTX]feed | (1) |
Result and discussion
Synthesis of APC and conjugation to the amino-functionalized GO is shown in Scheme 1. In order to synthesize APC, PC was prepared by nucleophilic substitution reaction between cyanuric chloride and polyethylene glycol in the presence of sodium hydroxide. A chloride of the cyanuric chloride was replaced by hydroxyl groups of PEG (a). In the next step, the excess of albumin was added to the solution of PC, and it replaced second chloride of cyanuric chloride through its amino group in lysine residue or thiol group in the cysteine 37 residue (b). Then the amino groups of ethylenediamine resides onto the surface of graphene oxide were conjugated to APC by nucleophilic substitution reaction (c). The obtained APC-amino-GO can be well dispersed in aqueous solution due to high hydrophilic nature of APC compound.Fig. 1a shows the IR spectrum of PC in which absorbance bands for aliphatic C–H group, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–O are appeared at about 2877 and 2972, 1726 and 1110 cm−1 respectively. Upon attachment of albumin to PC, absorbance bands of amidic carbonyl (C
N and C–O are appeared at about 2877 and 2972, 1726 and 1110 cm−1 respectively. Upon attachment of albumin to PC, absorbance bands of amidic carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) I and II groups in albumin are appeared at 1685 and 1554 cm−1 respectively and also intensity of absorbance band of aliphatic C–H group is increased (Fig. 1c). The results indicate that albumin has been attached to the PC compound. The IR spectra graphene oxide and its amino functionalization derivative are shown in Fig. 2a and b. The absorbance band at 1621 cm−1 is associated with the vibration of C
O) I and II groups in albumin are appeared at 1685 and 1554 cm−1 respectively and also intensity of absorbance band of aliphatic C–H group is increased (Fig. 1c). The results indicate that albumin has been attached to the PC compound. The IR spectra graphene oxide and its amino functionalization derivative are shown in Fig. 2a and b. The absorbance band at 1621 cm−1 is associated with the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group of the GO. Absorbance band at 1727 cm−1 is assigned to C
C group of the GO. Absorbance band at 1727 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carboxylic acid group (Fig. 2a). Graphene oxide was treated with thionyl chloride and then ethylenediamine to synthesis amino-functionalized GO. In the IR spectrum of amino-functionalized GO, disappearance of an absorbance band at 1727 cm−1 and appearance of a new absorbance band at 1560 cm−1 confirms preparation of this compound (Fig. 2b).
O stretching of the carboxylic acid group (Fig. 2a). Graphene oxide was treated with thionyl chloride and then ethylenediamine to synthesis amino-functionalized GO. In the IR spectrum of amino-functionalized GO, disappearance of an absorbance band at 1727 cm−1 and appearance of a new absorbance band at 1560 cm−1 confirms preparation of this compound (Fig. 2b).
Fig. 2d shows the IR spectrum of APC-amino-functionalized GO. Absorbance band at 1727 cm−1 is associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the carboxylic acid groups in albumin protein.
O stretching vibration of the carboxylic acid groups in albumin protein.
UV-vis absorption is a simple and appropriate method to study the supramolecular interactions between albumin and small molecules. Fig. 3 shows the absorbance spectra of free albumin and PC, APC, GO and APC-amino-functionalized GO compounds. Normally, albumin has a strong absorption band centered on 204 nm due to the n → π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and a weak absorption band at 277 nm assigned to π → π* transitions from the phenyl rings in Trp, Tyr and phenylalanine resides (Fig. 3a). These absorption peaks are still observable in the UV-vis spectra of APC. GO shows a weak peak at 230 nm, which is a characteristic for π → π* transition in GO sheets (Fig. 3e). Paclitaxel show a maximum absorption at 227 nm (Fig. 3f). Upon loading of paclitaxel by APC-amino-GO the intensity of the adsorption band at 204 nm decreases while the intensity of peak at 277 nm increases (Fig. 3c and d) showing that structure of protein is dramatically changed and there are strong interactions between paclitaxel and albumin.12
O and a weak absorption band at 277 nm assigned to π → π* transitions from the phenyl rings in Trp, Tyr and phenylalanine resides (Fig. 3a). These absorption peaks are still observable in the UV-vis spectra of APC. GO shows a weak peak at 230 nm, which is a characteristic for π → π* transition in GO sheets (Fig. 3e). Paclitaxel show a maximum absorption at 227 nm (Fig. 3f). Upon loading of paclitaxel by APC-amino-GO the intensity of the adsorption band at 204 nm decreases while the intensity of peak at 277 nm increases (Fig. 3c and d) showing that structure of protein is dramatically changed and there are strong interactions between paclitaxel and albumin.12
 | ||
| Fig. 3 UV-vis absorption spectra of (a) human serum albumin, (b) APC, (c) APC-amino-GO, (d) APC-amino-GO-PTX, (e) GO and (f) PTX. | ||
CD spectra are a suitable method for survey the secondary structure of albumin. As it has been shown in Fig. 4a, there are two strong negative bands at 207 and 218 nm due to the π → π* and n → π* electron transitions, respectively which are characteristic of α-helical secondary structure.13 Although the intensity of these bands decrease upon conjugation to polyethylene glycol and graphene oxide, confirming that these conjugates affect the α-helical secondary structure of albumin, it is still α-helical mainly. Interestingly loading of PTX onto the APC-amino-GO disturbs this structure completely. This result shows that there are strong interactions between PTX and albumin conjugated to GO. On the other words noncovalent interactions affect the secondary structure of albumin more that covalent interactions.
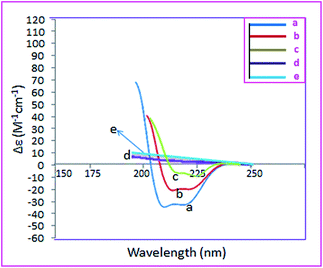 | ||
| Fig. 4 CD spectra of (a) human serum albumin, (b) APC, (c) APC-amino-GO, (d) APC-amino-GO-PTX and (e) GO. | ||
Conjugation of albumin to polyethylene glycol and GO and also noncovalent interactions between PTX and the resulted conjugates was investigated by using fluorescence spectroscopy. Albumin shows a strong emission band at 347 nm which it is due to Trp-214 residue (Fig. 5a). Interactions between other molecules, especially those having π system, with albumin affect its fluorescence intensity. Since fluorescence of albumin is assigned to Trp-214, changes in its intensity prove π–π interactions between them. The variation in the fluorescence intensity of albumin result from the changed albumin conformation or direct quenching effect by strong π–π interactions between albumin and aromatic system of the graphene oxide. Polyethylene glycol should interact with this part of albumin through its triazine end groups leading to diminish in the fluorescence intensity. Due to the huge π system of Go, this effect could be seen clearly when albumin is conjugated to its surface so that over 95% quenching of fluorescence emission was observed.14 Increasing of the fluorescence intensity of albumin after loading of PTX is due to the interfering of PTX molecules in the interactions between GO and albumin. This observation dictates that anticancer drug interact with albumin via hydrophobic region located its inside.
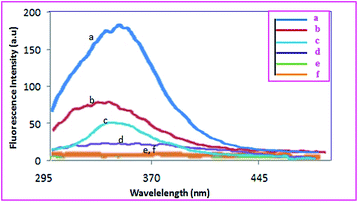 | ||
| Fig. 5 Fluorescence spectra of (a) human serum albumin, (b) APC, (c) APC-amino-GO PTX, (d) APC-amino-GO 0.002 mg ml−1 and (e) APC-amino-GO 0.0002 mg ml−1, (f) GO. | ||
Atomic force microscopy is a proper tool to establish the thickness and surface roughness and shape of the graphene-based nanomaterials. Fig. 6 shows AFM image of GO. According to this image thickness of GO is around 12 nm confirming that several layers are attacking on each other. Topographic AFM image and its profile show that APC is attached to the GO surface and they can be seen as globular objects with an average size around 180 nm (Fig. 7). These globular objects disperse and their size diminishes upon loading of PTX onto the surface of APC-amino-GO. Since it was proved that loaded PTX molecules interact with conjugated albumins, this result clearly show that PTX molecules interfere in the aggregations of conjugated albumin and disperse them. A part of PTX molecules load inside albumins probably.
 | ||
| Fig. 7 AFM images of (a) APC-amino-GO and (b) images profile of APC-amino-GO on lamella and (c) APC-amino-GO-PTX on mica surface. | ||
Fig. 8 shows TEM images of GO and APC-amino-GO. By comparison TEM images of graphene and APC-amino-GO; it can be found that the agglomerates concerning APC unites are attached onto the surface of graphene oxide (Fig. 8b). After conjugating of the polymer onto the surface of graphene oxide its morphology change from thin layer to thicker and agglomerated object.
Although DLS can be used for globular objects, in this work it has been used to compare hydrodynamic volume of different systems and changes in these systems upon conjugation together. Fig. 9 shows DLS diagrams of APC, APC-amino-GO and APC-amino-GO-PTX systems. The average sizes for APC, APC-amino-GO and APC-amino-GO-PTX are 151.2, 269.3 and 223.4 nm respectively. Big size for APC show that they are aggregate in the aqueous solutions, due to the hydrophobicity of triazine unites of PEG and also multifunctionality of albumin. Attaching of APC onto the surface of GO leads to objects with average sizes much smaller than that for GO observed in TEM. However loading of PTX reduce the size of APC-amino-GO around 46 nm.
 | ||
| Fig. 9 DLS diagrams of (a) APC, (b) APC-amino-GO and (c) APC-amino-GO-PTX systems600 dpi in TIF format)??>. | ||
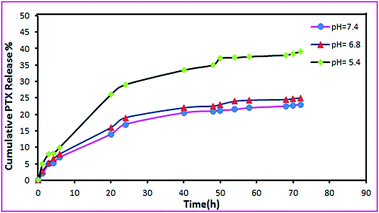
The pH value changes from 7.4 in extracellular parts in normal tissues to 6.8 in tumor tissues and to 5.0 in the endosomes and lysosomes.15 Thus, the release profiles of PTX from APC-amino-GO molecules were investigated at all mentioned pHs. Fig. 10 shows the release profiles of PTX from APC-amino-GO in different pHs. It was found that at pH 7.4 PTX release from APC-amino-GO slowly and after 72 h around 22% of the loaded drug was released. However the rate of release at pH 6.8 and 5 are faster so that at pH 5, 40% of PTX has been released in 72 h. The faster release of PTX from APC-amino-GO in lower pHs could be assigned to the reducing of the interactions between APC-amino-GO and PTX, due to the protonation of functional groups of the surface of GO and also albumin. The PTX-loading efficiency is at about 22%, and this loading is due to albumin protein and the extremely large surface area of graphene.
Conclusions
In this research, we have developed a simple and effective method to attach albumin to GO. PTX was efficiently loaded and transferred by the prepared drug delivery system. Release of drug from the graphene–albumin conjugate showed a pH-dependent behavior.Acknowledgements
Authors would like to thank Iran National Science Foundation: INSF to support this work financially.Notes and references
- (a) Y. J. Song, K. G. Qu, C. Zhao, J. S. Ren and X. G. Qu, Adv. Mater., 2010, 22, 2206 CrossRef CAS PubMed; (b) C. Shan, H. Yang, D. Han, Q. Zhang, A. Ivaska and L. Niu, Langmuir, 2009, 25, 12030 CrossRef CAS PubMed; (c) Y. Wang, Z. H. Li, D. H. Hu, C. T. Lin, J. H. Li and Y. H. Lin, J. Am. Chem. Soc., 2010, 132, 9274 CrossRef CAS PubMed; (d) B. Gulbakan, E. Yasun, M. I. Shukoor, Z. Zhu, M. You and X. Tan, et al., J. Am. Chem. Soc., 2010, 132, 17408 CrossRef CAS PubMed.
- (a) X. Y. Yang, X. Y. Zhang, Z. F. Liu, Y. F. Ma, Y. Huang and Y. Chen, J. Phys. Chem. C, 2008, 112, 17554 CrossRef CAS; (b) X. Yang, Y. Wang, X. Huang, Y. Ma, Y. Huang and R. Yang, et al., J. Mater. Chem., 2011, 21, 3448 RSC; (c) L. M. Zhang, J. G. Xia, Q. H. Zhao, L. W. Liu and Z. J. Zhang, Small, 2010, 6, 537 CrossRef CAS PubMed; (d) L. Zhang, Z. Lu, Q. Zhao, J. Huang, H. Shen and Z. Zhang, Small, 2011, 7, 460 CrossRef CAS PubMed.
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876 CrossRef CAS PubMed.
- D. Depan, J. Shah and R. D. K. Misra, Mater. Sci. Eng., C, 2011, 31, 1305 CrossRef CAS PubMed.
- S. Zhang, K. Yang, L. Feng and Z. Liu, Carbon, 2011, 49, 4040 CrossRef CAS PubMed.
- (a) M. Dockal, D. C. Carter and F. Ruker, J. Biol. Chem., 1999, 274, 29303 CrossRef CAS PubMed; (b) X. M. He and D. C. Carter, Nature, 1992, 358, 209 CrossRef CAS PubMed; (c) D. C. Carter and J. X. Ho, Adv. Protein Chem., 1994, 45, 153 CrossRef CAS.
- D. C. Carter and J. X. Ho, Adv. Protein Chem., 1994, 45, 153 CrossRef CAS.
- (a) H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271 CrossRef CAS; (b) N. Desai, V. Trieu, Z. Yao, L. Louie, S. Ci, A. Yang, C. Tao, T. De, B. Beals, D. Dykes, P. Noker, R. Yao, E. Labao, M. Hawkins and P. Soon-Shiong, Clin. Cancer Res., 2006, 12, 1317 CrossRef CAS PubMed; (c) T. A. John, S. M. Vogel, C. Tiruppathi, A. B. Malik and R. D. Minshall, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2003, 284, 187 Search PubMed.
- (a) E. K. Rowinsky and R. C. onehower, N. Engl. J. Med., 1995, 332, 1004 CrossRef CAS PubMed; (b) A. K. Singla, A. Garg and D. Aggarwal, Int. J. Pharm., 2002, 235, 179 CrossRef CAS; (c) H. Gelderblom, J. Verweij, K. Nooter and A. Sparreboom, Eur. J. Cancer., 2001, 37, 1590 CrossRef CAS.
- (a) W. S. Hummers and R. E Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS; (b) C. M. Chen, Q. H. Yang, Y. G. Yang, W. Lv, Y. F. Wen, P. X. Hou, M. Z. Wang and H. M. Cheng, Adv. Mater., 2009, 21, 3007 CrossRef CAS.
- H. Namazi and M. Adeli, Polymer, 2005, 45, 10788 CrossRef PubMed.
- Y. Y. Yue, X. G. Chen, J. Qin and X. J. Yao, Colloids Surf., B, 2009, 69, 51 CrossRef CAS PubMed.
- N. Ibrahim, H. Ibrahim, S. Kim, J. P. Nallet and F. Nepveu, Biomacromolecules, 2010, 11, 3341 CrossRef CAS PubMed.
- N. Varghese, U. Mogera, A. Govindaraj, A. Das, P. K. Maiti, A. K. Sood and C. N. R. Rao, ChemPhysChem, 2009, 10, 206 CrossRef CAS PubMed; Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876 CrossRef PubMed.
- E. S. Lee, Z. Gao and Y. H. Bae, J. Controlled Release, 2008, 132, 164 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |