Qualitative and quantitative analyses of goitrin–epigoitrin in Isatis indigotica using supercritical fluid chromatography-photodiode array detector-mass spectrometry
Rui Wang†
a,
Jacquelyn Runco†b,
Li Yangc,
Kate Yub,
Yiming Lia,
Rui Chenb and
Zhengtao Wang†*c
aSchool of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
bWaters Corporation, Milford, MA 01757, USA
cThe Ministry of Education (MOE) Key Laboratory for Standardization of Chinese Medicines, Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. E-mail: ztwang@shutcm.edu.cn
First published on 25th September 2014
Abstract
A novel comprehensive method using supercritical fluid chromatography-photodiode array detector-mass spectrometry (SFC-PDA-MS) was developed for the qualitative and quantitative analyses of a chiral isomer pair in Ban Lan Gen (dried root of Isatis indigotica) and the processed product. A standard goitrin–epigoitrin racemic mixture was used for method development. A total of six chiral stationary phases (CSPs) were screened and the (S,S)-Whelk-O 1 (4.6 × 250 mm, 10 μm) column was chosen as it offers baseline resolution for the enantiomeric pair with separation accomplished in 6 minutes. A single quadrupole MS and a PDA detector were used in line for the detection. The validated method was applied successfully in the analysis of different samples. Results indicated that the developed assay was fast, sensitive and reproducible. SFC should be an integral part of the overall analytical platform for TCM and natural product research, especially in the area of chiral analysis.
1 Introduction
The therapeutic use of natural products dates back thousands of years, and continues to be an integral part of basic healthcare in many countries today. For example, Traditional Chinese Medicine (TCM) makes up half of the “basic” medicines mandated by the Chinese government for public use at all levels of its healthcare system.1 The biological activities of natural products include antimicrobial, antineoplastic, central nervous system (CNS)-active, anti-inflammatory, and cardiovascular, just to name a few.2 Drug substances from pure natural products, their derivatives, and synthetic compounds from a natural product precursor represent a major part of today's pharmaceutical market.3,4Since chirality is a fundamental characteristic of nature, it is not surprising that many of the well known ancient therapeutic reagents in natural products or TCM are chiral, such as morphine in Opium, β-dichroine in Chang Shan (the dried roots of Dichroa febrifuga Lour.), and ephedrine in Ma Huang (the dried herbaceous stems of Ephedra sinica Stapf, Ephedra intermedia Schrenk et C.A.Mey. or Ephedra equisetina Bge.).2 While one isomer possesses a desired therapeutic effect, its paired enantiomer could be inactive, have antagonist effects, or even have undesirable effects. For example, unnatural (+)-morphine has extremely weak affinity for opiate receptors while (−)-morphine is entirely different.3 S-(−)-Hyoscyamine is used in medicine and it has historically been accepted that the affinity of muscarinic receptors for S-(−)-hyoscyamine is higher than that for the R-(+) enantiomer.3 For this reason, determining the pharmacological activity of specific enantiomers of chiral compounds in TCM is becoming increasingly important. As a result, there has been growing need for chiral analysis in TCM research,5,6 primarily utilizing high performance liquid chromatography (HPLC) on chiral stationary phases (CSPs). While supercritical fluid chromatography (SFC) has become increasingly popular for chiral analysis and purification in western pharmaceutical research,7–9 the adoption of SFC in TCM research is still scarce.10
Ban Lan Gen (the dried roots of Isatis indigotica Fort) is one of the TCMs listed in the Chinese National Category of the Basic Medicines for treating fever and removing toxic heat.1 There has been considerable research effort in understanding its chemical constituents and associated pharmacological activities.11–23 Pharmacokinetic studies indicate that the R-goitrin (epigoitrin) is one of the main constituents accounting for the antiviral activity of Ban Lan Gen.19,20 The S-goitrin (goitrin), however, is a potential goitrogen causing an enlargement of the thyroid.6,24 It is therefore imperative to enantiomerically resolve R- and S-goitrin to better understand their respective pharmacological dose–response relationship and toxicity for a safe and effective use of the medicine, and to better assess the quality of the raw plants before manufacturing.
Herein, we report our investigation on employing SFC-PDA-MS for the qualitative and quantitative analyses of R/S-goitrin in Isatis indigotica Fort extract and different Ban Lan Gen powder formulations.
2 Experimental
2.1 Chemicals
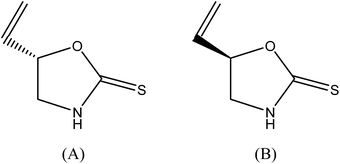
SFC grade CO2 was from Air Gas (Salem, NH, USA). HPLC grade water and diethyl ether were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade methanol and goitrin racemate (Fig. 1) were purchased from Thermo Fisher (Allentown, PA, USA). R-Goitrin, S-goitrin, ground dried roots of Isatis indigotica Fort, and ground dried roots of Baphicacanthus cusia (Nees) Bremek were gifts from Prof. Zhengtao Wang at Shanghai University of Traditional Chinese Medicine (Shanghai, China). And they were all authenticated by Prof. Zhengtao Wang. Three different Ban Lan Gen powder formulations were purchased from Beijing Tongrentang pharmaceutical factory (Beijing, China).2.2 Instrumentation and experimental conditions
All experiments were performed on a Waters Resolution SFC MS System (Waters, Milford, MA, USA) controlled by MassLynx® software. The system consists of a Fluid Delivery Module (FDM), an Alias Autosampler, a 10-port analytical-to-prep™ oven, a Waters 2998 Photodiode Array Detector (PDA), and a Waters 3100 MS detector.SFC experiments were conducted using methanol in CO2 at 3 mL min−1 flow rate. Columns were kept at 40 °C in the column oven. Column back pressure was held at 120 bar. An aliquot of 10.0 μL of each sample solution was injected into the SFC system for analysis. The flowing modifier gradient conditions were employed: starting modifier 20% (v/v), holding for 2 min; ramping to 40% (v/v) in 0.5 min, holding for 3 min; return to 20% (v/v) in 0.5 min; total run time, 6 min. The wavelength was set at 244 nm, and the on-line UV spectra was recorded in the range of 200 nm to 320 nm. MS analysis was performed in the positive ion model of atmospheric pressure chemical ionization (APCI), under the following conditions: corona current, 10 μA; source temperature, 150 °C; probe temperature, 450 °C. Full-scan spectra were recorded from m/z 100–400, mass spectrometer was also employed in selected ion recording (SIR) model to monitor m/z 130.
2.3 Sample preparation
For dried roots of Isatis indigotica Fort and Baphicacanthus cusia (Nees) Bremek, and Ban Lan Gen powder formulations, 100 mg of the solid was sonicated in 5 mL of water for 1 h and allowed to sit for 1 h. The sample was then centrifuged and the supernatant was filtered through a 0.45 μm filter. A liquid–liquid extraction was performed on the supernatant three times with 5 mL of diethyl ether. The combined diethyl ether extract (a total of 15 mL) was dried down and reconstituted in 5 mL of methanol.2.4 Precision and repeatability
Intra- and inter-day variations were evaluated to determine the precision and repeatability. To evaluate the precision, a goitrin racemate standard solution of 0.005 mg mL−1 was prepared in methanol. To evaluate the repeatability, six different solutions made from the same sample were analyzed. Six replicates were performed for the intra-day variability studies. Inter-day variability was done in three days. Each day, six replicates were performed and the average peak area was used as one data point.2.5 Linearity
A goitrin stock solution of 0.1 mg mL−1 was prepared in methanol. The stock solution was serially diluted. For each data point, triplicates were performed and the peak areas were averaged.2.6 Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ, which were expressed by 3- and 10-fold of the signal-to-noise ratio (S/N), were determined at the concentration of 0.005 mg mL−1.2.7 Recovery
The recovery test was performed by the standard addition method. Low, medium, and large high amounts of the standards were added to the sample with known goitrin content. The mixture then underwent the same procedure as listed in “Sample Preparation” before analysis. The mean recovery was calculated according to the following formula: recovery (%) = (amount found − original amount)/amount spiked × 100%, and RSD (%) = (SD/mean) × 100%.3. Results and discussion
3.1 Assay development
Chiral method development often starts with a screening of multiple CSPs and co-solvents. An ideal CSP selection for screening should include minimal number of columns with complimentary selectivity to maximize the success rate. Fig. 2 shows the SFC UV chromatograms of R/S-goitrin standard using 6 different CSPs and methanol as the co-solvent. A generic gradient was used for the screening: starting modifier 5% (v/v); ramping to 40% (v/v) in 10 min, holding for 2 min; return to 5% (v/v) in 2 min, holding for 2 min. The chiral columns, namely, CHIRALPAK® AD-H, AS-H and IC, and CHIRALCEL® OD-H and OJ-H (4.6 mm × 250 mm, 5 μm), (S,S)-Whelk-O 1 (4.6 × 250 mm, 10 μm) were investigated and compared. The AD-H, OD-H and (S,S)-Whelk-O 1 columns were all capable of separating the enantiomers. Among which, the (S,S)-Whelk-O 1 demonstrated the highest resolution. It was therefore chosen for all ensuing experiments. | ||
| Fig. 2 SFC UV chromatograms of R/S-goitrin standard using 6 different CSPs and methanol as the co-solvent. | ||
Based on the maximum absorption and full-scan experiment of the marker components in the UV spectra of the three-dimensional chromatograms obtained by PDA detection, the detection wavelength was set at 244 nm.
Next, we optimized the method for the analysis of Isatis indigotica Fort extract, primarily focusing on shortening the analysis time. The resulting chromatogram was shown in Fig. 3(A). Under the optimized condition, the R- and S-goitrin were separated from the sample matrix while maintaining the enantiomeric resolution between the R- and S-goitrin. The total analysis time was 6 min. This represents a nearly eight-fold increase in speed compared to the reported normal phase-HPLC (NP-HPLC) method.6 In SFC, a combination of supercritical CO2 and polar organic solvent(s), most commonly alcohol, are used as the mobile phase. Due to the inherent higher diffusivity and lower viscosity of supercritical fluid, it is not unusual that SFC provides a three- to eight-fold faster separation than NP-HPLC.7 Fig. 3(B) shows the SFC UV and MS chromatograms of the goitrin standards under the optimized condition. The peaks were identified by running an R-enantiomer standard.
 | ||
| Fig. 3 SFC chromatograms of Isatis indigotica Fort extract (A) and R/S-goitrin standard (B) under optimized condition. | ||
A validation study was performed to estimate the performance for quantitative analyses of goitrin standards. With PDA detection and MS detection, the resolutions of R- and S-goitrin were 2.85 and 2.81; the peak ratios of R- and S-goitrin were 1.01 and 1.00, respectively. The detailed information regarding the precision, calibration curves, linear ranges, LODs and LOQs of the R- and S-goitrin were summarized in Table 1.
| PDA detection | MS detection | |||
|---|---|---|---|---|
| S-goitrin | R-goitrin | S-goitrin | R-goitrin | |
| a RSD (n = 6) (%) of repeatability.b RSD (n = 18) (%) of inter-day repeatability (3 days). | ||||
| Intra-day Precisiona | 0.60 | 0.73 | 0.86 | 0.53 |
| Inter-day Precisionb | 0.68 | 0.73 | 3.08 | 2.23 |
| Regression equation (weighting index: 1/x) | Y = 294![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 847.41x − 2.20 847.41x − 2.20 |
Y = 299![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518.28x − 14.33 518.28x − 14.33 |
Y = 233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724 724![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 352.21x + 138.89 352.21x + 138.89 |
Y = 231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 405 405![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 962.98x + 3340.65 962.98x + 3340.65 |
| Correlation (R2) | 1.0000 | 0.9999 | 0.9999 | 0.9997 |
| Linearity range (mg mL−1) | 0.0005–0.05 | 0.0005–0.05 | 0.00001–0.01 | 0.00001–0.01 |
| LOD (ng mL−1) | 100 | 100 | 2.0 | 2.0 |
| LOQ (ng mL−1) | 200 | 200 | 10 | 10 |
Precision with PDA detection were below 1% and similar for both intra-day and inter-day experiments. With MS detection, inter-day variation was slightly higher than for intra-day.
The LOD and LOQ with PDA detection are one order of magnitude lower than those reported using an NP-HPLC method.6 In our experiments, reference wavelength compensation was used in data acquisition. Reference wavelength compensation collects wide-band absorbance data in a region where the analytes have minimal or no absorption. The detector calculates the compensation value by averaging the absorbance values within the selected range of wavelengths. The averaged value is then subtracted from the absorbance value. Since the main absorbance (220–320 nm in our experiments) includes the reference bands (270–320 nm), noises from common sources, such as mechanical and thermal noise, can be effectively reduced; hence, increasing S/N.25
With MS detection, the LOD and LOQ were 2 and 10 ng mL−1, respectively. At LOD, with a 10 μl injection, as little as 100 pg of each goitrin enantiomer was detected. This represents a three to four orders of magnitude improvement in detection sensitivity over the reported NP-HPLC method with UV detection.6 This improvement, of course, arises from a more sensitive MS detection. However, in NP-HPLC, hexane, heptanes, dichloromethane (DCM), isopropanol and their mixtures are often used as the mobile phase. These solvents are not ideal, if not prohibitive, for MS detection. In SFC, on the other hand, CO2 combined with MS friendly alcohols, most commonly methanol, is used as the mobile phase. This, in turn, enables the incorporation of a sensitive MS detection in SFC, which is often necessary for quantitative chiral analysis. SFC hyphenated with MS has indeed become a viable analytical tool in pharmaceutical research.26 Furthermore, the use of alcohol in SFC is more cost effective and environmentally sustainable compared to the use of hexane, heptanes, and halogenated solvents in NP-HPLC.
Calibration curves for both UV and MS were constructed by analyzing the serially diluted goitrin standards in triplicates. All calibration curves exhibited excellent linearity with the square of correlation coefficient (R2) above 0.999. There are also superb agreements between the R- and S-goitrin with both UV and MS detection.
3.2 Analyses of Isatis indigotica Fort extract
With PDA detection and MS detection, the peak ratios of R- and S-goitrin were 1.99 and 2.03, the ratios calculate from amount of R- and S-goitrin were 1.96 and 2.05, respectively. Table 2 summarizes the results from the analyses of Isatis indigotica Fort extracts. Compared to the results from the goitrin standards (Table 1), there are slightly higher variations with both UV and MS detection. It is also noted that batch-to-batch variation was consistently between 5–6%. We speculate that this increased variation resulted from the sample preparation procedure. Epiprogoitrin ((2S)-2-hydroxy-3-butenyl glucosinolate) is a secondary metabolite abundant in many plants. Through a myrosinase-catalyzed hydrolysis in the presence of water, R-goitrin can be formed by the cleavage of the D-glucose group from epiprogoitrin.27 Our initial step in sample preparation involved soaking the dried roots of Isatis indigotica Fort in water. As a result, there were possible epiprogoitrin–epigoitrin transformations until the enzymatic activity of myrosinase was quenched. Therefore, caution should be exercised to ensure a precise timing control in sample preparation to minimize this variability.| PDA detection | MS detection | |||
|---|---|---|---|---|
| S-goitrin | R-goitrin | S-goitrin | R-goitrin | |
| a RSD (n = 6) (%) of repeatability.b RSD (n = 18) (%) of inter-day repeatability (3 days).c RSD (n = 3) (%) of batch-to-batch repeatability.d The values are mean (n = 6).e The values are mean (n = 3). | ||||
| Repeatabilitya | 0.52 | 1.40 | 0.62 | 1.70 |
| Inter-day repeatabilityb | 2.00 | 1.69 | 3.21 | 3.87 |
| Batch-to-batch repeatabilityc | 5.69 | 5.59 | 6.29 | 5.07 |
| Recoveryd | 99.2 | 100.4 | 98.9 | 105.0 |
| Amount (mg/100 mg sample)e | 0.0368 | 0.0723 | 0.0416 | 0.0853 |
3.3 Using goitrin enantiomer pairs as markers to authenticate Ban Lan Gen
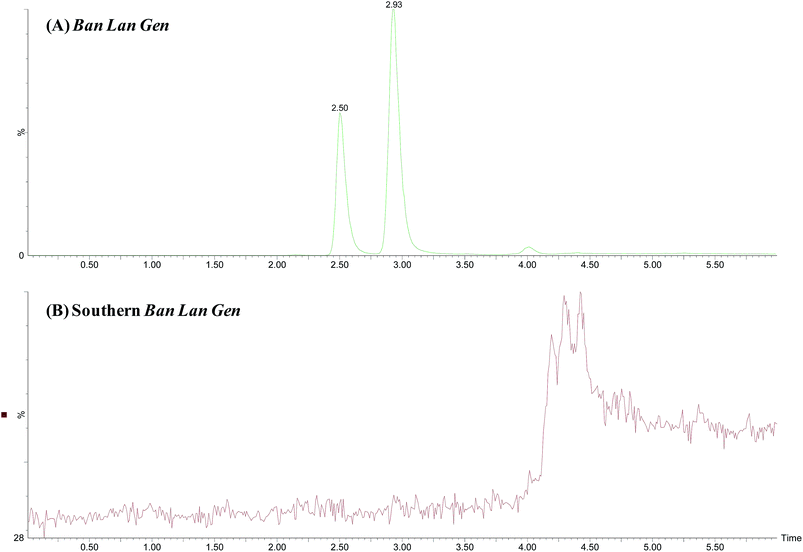
There are two types of Ban Lan Gen in China, namely, Ban Lan Gen (the dried root of Isatis indigotica Fort, also referred to as the Northern Ban Lan Gen) and Southern Ban Lan Gen (the dried root of Baphicacanthus cusia (Nees) Bremek). Despite bearing similar names, the chemical constituents and the sources of these two plants are vastly different. Since epigoitrin is the main constituent contributing to Ban Lan Gen's antiviral activity, Wang et al. proposed the use of goitrin enantiomer pairs as potential markers for Ban Lan Gen.16–18 Fig. 4 shows the SFC-MS chromatograms of the Ban Lan Gen and the Southern Ban Lan Gen. Even with 2 ng mL−1 detection limit, there is no observable epigoitrin and goitrin in the Southern Ban Lan Gen. Our results support the notion that epigoitrin and goitrin are specific for Ban Lan Gen, and can be used as markers for its authentication.3.4 Analysis of three different Ban Lan Gen powder formulations
The three different powder formulations were all marketed as “Ban Lan Gen powder” by three different manufacturers. The powders all have tan color and similar appearance. Fig. 5 shows the SFC-MS chromatograms of the three formulations. Quantitative results were summarized in Table 3. It is evident that the three powder formulations differ substantially in goitrin content. Formulation 1 only contains detectable but not quantifiable goitrin, i.e. the concentration was between 2–10 ng mL−1. Formulation 3 contains 5 times more goitrin than Formulation 2. It is also interesting to note that the R- and S-goitrin ratios are different between formulation 2 (2.13) and 3 (2.22). Currently, goitrin content is determined by reverse phase HPLC (RP-HPLC) based methodology where R- and S-goitrin are not resolved.21,22 Clearly, with varying R- and S-goitrin ratio evidenced in this study, the goitrin content cannot be accurately assessed via RP-HPLC. Our observation underscores the importance of the enantiomeric resolution of R- and S-goitrin for better quantitation of the bioactive R-goitrin, better controlled pharmacological studies such as dose–response relationship and toxicity, and better quality control in Ban Lan Gen formulation manufacturing.4 Conclusions
In this communication, the development of an SFC-UV-MS based assay for the qualitative and quantitative analyses of R- and S-goitrin is described. Under optimized conditions, the goitrin can be separated from the sample matrix while maintaining the enantiomeric resolution between the R- and S-goitrin. The total analysis time was 6 min, representing an eight-fold increase in speed compared to the NP-HPLC method. Excellent repeatability, intermediate precision and linearity were achieved with the developed assay. With UV detection, the LOD and LOQ were one order of magnitude lower than those from NP-HPLC UV. With MS detection, the LOD and LOQ were three to four orders of magnitude lower than those from NP-HPLC UV.The assay was then applied to the authentication of Ban Lan Gen. Even with the sensitive MS detection of 2 ng mL−1 LOD, there was no observable goitrin in the Southern Ban Lan Gen extract. Our results support the theory that goitrin is specific to Ban Lan Gen and can therefore be used as a potential marker. The SFC based methodology is fast, sensitive and reproducible.
Finally, different Ban Lan Gen formulations were analyzed using the developed assay. The three powder formulations differ substantially in the goitrin content. It is also noted that the R/S ratio varies from sample to sample. Our observation underscores the importance of the enantiomeric resolution of R- and S-goitrin for better quantitation of the R-goitrin, the active enantiomer contributing to the antiviral activity. SFC should be an integral part of the overall analytical platform for TCM and natural product research, especially in the area of chiral analysis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81202883 and 81222053).References
- http://www.gov.cn/gzdt/2009-08/18/content_1395524.htm.
- P. Gal, in, Chirality in Drug Research, ed. E. Francotte and W. Lindner, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006, pp. 3–9 Search PubMed.
- J. C. Leffingwell, Leffingwell Rep., 2003, 3(1), 1–27 Search PubMed.
- A. Harvey, Drug Discovery Today, 2000, 5, 294–300 CrossRef.
- D. J. Newman, G. M. Cragg and K. M. Snader, J. Nat. Prod., 2003, 10, 1022–1037 CrossRef PubMed.
- X. W. Wang and S. Zeng, Curr. Pharm. Anal., 2010, 6, 39–52 CrossRef CAS.
- L. X. Nie, G. L. Wang, Z. Dai and R. C. Lin, Chin. J. Chromatogr., 2010, 28, 1001–1004 CAS.
- Y. Zhang, D. R. Wu, D. B. Wang-Iverson and A. A. Tymiak, Drug Discovery Today, 2005, 10, 571–577 CrossRef CAS.
- T. J. Ward and B. A. Baker, Anal. Chem., 2008, 80, 4363–4372 CrossRef CAS PubMed.
- D. Mangelings and Y. V. Heyden, J. Sep. Sci., 2008, 31, 1252–1273 CrossRef CAS PubMed.
- L. Gao, J. Zhang, W. B. Zhang, Y. C. Shan, Z. Liang, L. H. Zhang, Y. S. Huo and Y. K. Zhang, J. Sep. Sci., 2010, 33, 3817–3821 CrossRef CAS PubMed.
- L. Ma, J. Y. Tang, Z. L. Li, Y. Liu, C. Jin, Y. L. Zhao and X. H. Xiao, Chin. Tradit. Herb. Drugs, 2007, 38, 1143–1146 CAS.
- L. Ma, J. Y. Tang, Z. L. Li, Y. L. Zhao, Q. W. Liao, X. H. Xiao, X. J. Zhao and C. Jin, China J. Chin. Mater. Med., 2006, 31, 804–806 CAS.
- J. Wu, D. D. Sun, X. Li, J. W. Chen, L. W. He and W. W. Dong, J. China Pharm. Univ., 2008, 19, 2354–2356 CAS.
- X. Li, A. J. Chen and C. Li, Chin. J. Exp. Tradit. Med. Formulae, 2010, 16, 64–67 CrossRef CAS.
- S. Liu, J. Yan, H. L. Li, F. R. Song, Z. Y. Liu, Z. Q. Liu and S. Y. Liu, Chem. Res. Chin. Univ., 2010, 31, 1137–1142 CAS.
- R. Wang, H. Y. Yang, Q. W. Yang, S. J. Huang and Z. T. Wang, Chin. Tradit. Herb. Drugs, 2010, 41, 478–480 CAS.
- Y. H. Shi, Z. Y. Xie, R. Wang, S. J. Huang, Y. M. Li and Z. T. Wang, Int. J. Mol. Sci., 2012, 13, 9035–9050 CrossRef CAS PubMed.
- Y. H. Shi, Z. Y. Xie, R. Wang, S. J. Huang, Y. M. Li and Z. T. Wang, J. Liq. Chromatogr. Relat. Technol., 2013, 36, 80–93 Search PubMed.
- L. H. Xu, F. Huang, T. Chen and J. Wu, Chin. J. Nat. Med., 2005, 3, 359–361 CAS.
- F. Huang, Y. T. Xiong, L. H. Xu and X. D. Liu, J. China Pharm. Univ., 2006, 37, 519–522 CAS.
- Y. Q. An, X. B. Jia, H. J. Yuan, E. Sun and Z. Z. Xu, China J. Chin. Mater. Med., 2008, 33, 2074–2076 CAS.
- Y. Q. An, X. B. Jia, Y. Chen, E. Sun and X. Y. Jin, Chin. Tradit. Herb. Drugs, 2008, 39, 1739–1741 CAS.
- E. B. Astwood, M. A. Greer and M. G. Ettlinger, J. Biol. Chem., 1949, 181, 121–130 CAS.
- L. Subbarao, J. Cole and R. Chen, The Application Notebook, LC GC, 2009, pp. 50–55 Search PubMed.
- R. Chen, Chromatography, 2009, 2, 11–19 Search PubMed.
- S. Galletti, R. Bernardi, O. Leoni, P. Rollin and S. Palmieri, J. Agric. Food Chem., 2001, 49, 471–476 CrossRef CAS PubMed.
Footnote |
| † R. Wang and J. Runco contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |