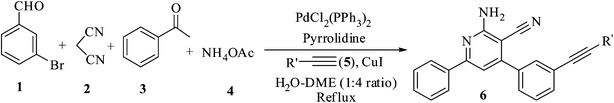
Synthesis of alkynyl/alkenyl-substituted pyridine derivatives via heterocyclization and Pd-mediated Sonogashira/Heck coupling process in one-pot: a new MCR strategy†
Mohan Reddy Bodireddya,
N. C. Gangi Reddy*a and
Sangita D. Kumarb
aDepartment of Chemistry, School of Physical Sciences, Yogi Vemana University, Kadapa 516 003, A.P., India. E-mail: ncgreddy@yogivemanauniversity.ac.in
bAnalytical Chemistry Division, Bhabha Atomic Research Centre (BARC), Trombay-400 085, Mumbai, India. Fax: +91-8562-225419; Tel: +91-8562-225410
First published on 28th March 2014
Abstract
A new class of 2-amino-4-(3/2-(alkynyl)/3-(alkenyl)phenyl)-6-phenylnicotinonitriles (6, 7 & 9) has been synthesized with good to excellent isolated yields by the multi-component reaction (MCR) of bromobenzaldehyde (1), malononitrile (2), acetophenone (3), NH4OAc (4) and a series of terminal alkynes (5)/alkenes (8) in the presence of pyrrolidine and Pd-catalyst in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) under reflux conditions in a single step. The Heck-type coupling with terminal olefins takes place stereoselectively with exclusive formation of E-isomers. This new MCR strategy opens new avenues in the development of (i) a diversity-oriented new cyanopyridine based compound library and (ii) new chemical entities other than the present reported molecules.
4 ratio) under reflux conditions in a single step. The Heck-type coupling with terminal olefins takes place stereoselectively with exclusive formation of E-isomers. This new MCR strategy opens new avenues in the development of (i) a diversity-oriented new cyanopyridine based compound library and (ii) new chemical entities other than the present reported molecules.
1. Introduction
The pyridine ring system is an ‘important structural motif’ in a large number of natural products and also in clinically useful molecules. Among this plethora of compounds, cyanopyridine derivatives have attracted great attention due to their wide range of biological activities such as antimicrobial,1,2 antipyretic,3 anti-hyperglycemic properties,4 Ca2+-channel blockers,5 vasodilator,6 antitumor,7,8 anti-diabetic agents.9 In addition, they have been evaluated as IKK-β inhibitors,10 protein kinase B (PKB/Akt) inhibitors,11 protein kinase Cθ (PKCθ) inhibitors,12 melanin-concentrating hormone receptor 1 (MCH-R1) antagonists,13 A2A adenosine receptor antagonists,14 carbonic anhydrase inhibitors,15 dipeptidyl peptidase-IV (DPP-IV) inhibitors with anti-hyperglycemic activity,16 analgesic agents,17 monoamine oxidases (MAO), cholinesterase inhibitors for the treatment of Alzheimer's disease,18 metabotropic glutamate receptor5 (mGluR5) negative allosteric modulators,19 C-Jun NH2 terminal kinase (JNKs) inhibitors,20 prion disease therapeutics21 and RET tyrosine kinase inhibitors22 and also useful in the development of non-linear optical (NLO) materials23 and nondoped organic light-emitting devices (OLEDs) too.24 As a result, cyanopyridine derivatives are regarded as “significant structural motif” and a huge number of strategies are developed for their synthesis.25,26 In addition, the development of multi-component reactions (MCRs) strategies have attracted great attention from organic chemists27–34 to prepare these useful pyridine frameworks. Multi-component reactions (MCRs) are powerful strategies for the quick synthesis of diverse and complex organic molecules of potential interest particularly in the area of material science and drug discovery.35–39 MCRs have attracted much attention owing to their excellent synthetic efficiency, intrinsic atom economy, high selectivity, procedural simplicity and environmental friendliness.40–44 Therefore, the development of new multi-component reaction (MCR) strategies for the preparation of diversity-oriented complex molecules have recently gained considerable interest from the organic chemists.45–49 In particular, structural elaboration on heterocyclic compounds with terminal alkynyl/alkenyl functionalities using Sonogashira and Heck coupling reactions is quite interesting.50–56 However, attempts are not made to investigate the structural elaboration of cyanopyridine derivatives using Pd-mediated Sonogashira and Heck coupling reactions via a one-pot multicomponent reaction (MCR).Herein, we report a new MCR strategy for the synthesis of 2-amino-4-(3/2-(alkynyl)/3-(alkenyl)phenyl)-6-phenylnicotinenitriles (6, 7 & 9) with 80–90% isolated yields via heterocyclization from bromobenzaldehyde (1), malononitrile (2), acetophenone (3) and NH4OAc (4) in the presence of pyrrolidine followed by Pd-mediated Sonogashira and Heck coupling reactions using a variety of terminal alkynes (5)/alkenes (8) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) under reflux conditions as shown in Scheme 1. The present communication addresses several challenging issues e.g. (i) MCR based synthesis of alkynyl/alkenyl substituted-heterocyclic molecules via heterocyclization followed by C–C coupling in one-pot, (ii) the optimal base, catalyst system and reaction conditions and (iii) synthesis in view to develop alkynyl/alkenyl substituted heterocyclic compounds based synthetic precursors suitable for further functional group transformations in the development of diversity oriented new compound library.
4 ratio) under reflux conditions as shown in Scheme 1. The present communication addresses several challenging issues e.g. (i) MCR based synthesis of alkynyl/alkenyl substituted-heterocyclic molecules via heterocyclization followed by C–C coupling in one-pot, (ii) the optimal base, catalyst system and reaction conditions and (iii) synthesis in view to develop alkynyl/alkenyl substituted heterocyclic compounds based synthetic precursors suitable for further functional group transformations in the development of diversity oriented new compound library.
 | ||
| Scheme 1 Synthesis of 2-amino-4-(3/2-(alkynyl)/3-(alkenyl)phenyl)-6-phenylnicotinenitriles via multi-component reaction. | ||
2. Experimental
Melting points of various products obtained are determined (uncorrected). 1H and 13C NMR spectra are recorded on a Varian 400 MHz. Chemical shifts are reported in ppm with the internal TMS signal at 0.0 ppm as a standard. The data are reported as follows: chemical shift (ppm) and multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet or unresolved, br s = broad singlet), coupling constant(s) in Hz, integration assignment. High-resolution mass spectra (HRMS) and compound purity data are acquired on a Waters LCT premier XE TOF HRMS single quadrupole system equipped with electro spray ionization (ESI) source. Thin-layer chromatography is performed on 0.25 mm Merck silica gel plates and visualized with UV light. Column chromatography is performed on silica gel (200–300 mesh). Chemicals and solvents are purchased from Sigma-Aldrich and Merck. Isolated compounds are characterized by physical and spectroscopic data.Typical experimental procedure for the preparation of 2-amino-4-(3-(3-hydroxyprop-1-ynyl)phenyl)-6-phenylnicotinonitrile (6a)
A mixture of 3-bromobenzaldehyde (1) [10.0 mmol], malononitrile (2) [11.0 mmol], acetophenone (3) [11.0 mmol] and NH4OAc (4) [20.0 mmol] in presence of pyrrolidine (5.0 mmol) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) is stirred at reflux for 1.0 h. The first phase progress of the reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is cooled to RT and then, prop-2-yn-1-ol 5a [15.0 mmol], PdCl2(PPh3)2 [0.002 mmol] and CuI [0.005 mmol] are added. Again the entire reaction mixture is kept under reflux condition for 3.0 h in open air. Final stage progress is monitored by TLC. After the completion of the reaction, the whole reaction mixture is cooled to RT and the solvent is removed under reduced pressure. The obtained crude product is purified by column chromatography using silica gel and 1
4 ratio) (10 vol) is stirred at reflux for 1.0 h. The first phase progress of the reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is cooled to RT and then, prop-2-yn-1-ol 5a [15.0 mmol], PdCl2(PPh3)2 [0.002 mmol] and CuI [0.005 mmol] are added. Again the entire reaction mixture is kept under reflux condition for 3.0 h in open air. Final stage progress is monitored by TLC. After the completion of the reaction, the whole reaction mixture is cooled to RT and the solvent is removed under reduced pressure. The obtained crude product is purified by column chromatography using silica gel and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio of EtOAc–petroleum ether (PE) to obtain pure compound 6a. The isolated yield of product 6a is 91%. The same procedure is followed for the preparation of 2-amino-4-(3-(alkynyl)phenyl)-6-phenylnicotinonitrile derivatives (6b–k) listed in Table 2. All the synthesised compounds (6a–k) gave satisfactory spectroscopic data in accordance with their proposed structures.
9 ratio of EtOAc–petroleum ether (PE) to obtain pure compound 6a. The isolated yield of product 6a is 91%. The same procedure is followed for the preparation of 2-amino-4-(3-(alkynyl)phenyl)-6-phenylnicotinonitrile derivatives (6b–k) listed in Table 2. All the synthesised compounds (6a–k) gave satisfactory spectroscopic data in accordance with their proposed structures.
Typical experimental procedure for the preparation of 2-amino-4-(2-(3-hydroxyprop-1-ynyl)phenyl)-6-phenylnicotinonitrile (7a)
A mixture of 2-bromobenzaldehyde (1a) [10.0 mmol], malononitrile (2) [11.0 mmol], acetophenone (3) [11.0 mmol] and NH4OAc (4) [20.0 mmol] in the presence of pyrrolidine (5.0 mmol) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) is stirred at reflux for 1.0 h. The first phase progress of the reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is cooled to RT and then, prop-2-yn-1-ol 5a [17.0 mmol], PdCl2(PPh3)2 [0.002 mmol] and CuI [0.005 mmol] are added. Again the entire reaction mixture is kept under reflux condition for 3.5 h in open air. Final stage progress is monitored by TLC. After the completion of the reaction, the whole reaction mixture is cooled to RT and the solvent is removed under reduced pressure. The obtained crude product is purified by column chromatography using silica gel and 1
4 ratio) (10 vol) is stirred at reflux for 1.0 h. The first phase progress of the reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is cooled to RT and then, prop-2-yn-1-ol 5a [17.0 mmol], PdCl2(PPh3)2 [0.002 mmol] and CuI [0.005 mmol] are added. Again the entire reaction mixture is kept under reflux condition for 3.5 h in open air. Final stage progress is monitored by TLC. After the completion of the reaction, the whole reaction mixture is cooled to RT and the solvent is removed under reduced pressure. The obtained crude product is purified by column chromatography using silica gel and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio of EtOAc–petroleum ether (PE) to obtain pure product 7a. The isolated yield of product 7a is 80%. The same procedure is followed for the preparation of 2-amino-4-(2-(alkynyl)phenyl)-6-phenylnicotinonitrile derivatives (7b–d) listed in Table 3. Synthesized compounds (7a–d) gave satisfactory spectroscopic data in accordance with their proposed structures.
9 ratio of EtOAc–petroleum ether (PE) to obtain pure product 7a. The isolated yield of product 7a is 80%. The same procedure is followed for the preparation of 2-amino-4-(2-(alkynyl)phenyl)-6-phenylnicotinonitrile derivatives (7b–d) listed in Table 3. Synthesized compounds (7a–d) gave satisfactory spectroscopic data in accordance with their proposed structures.
Typical experimental procedure for the preparation of (E)-methyl 3-(3-(2-amino-3-cyano-6-phenylpyridin-4-yl)phenyl)acrylate (9a)
A mixture of 3-bromobenzaldehyde (1) [10.0 mmol], malononitrile (2) [11.0 mmol], acetophenone (3) [11.0 mmol] and NH4OAc (4) [20.0 mmol] in presence of pyrrolidine (5.0 mmol) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) is stirred at reflux for 1.0 h. The first phase progress of the reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is cooled to RT and then, methyl acrylate (8a) [16.0 mmol] and PdCl2(PPh3)2 [0.002 mmol] are added. Again, the entire reaction mixture is kept under reflux condition for 3.0 h in open air. Final stage progress is monitored by TLC. After the completion of the reaction, the whole reaction mixture is cooled to RT and the solvent is removed under reduced pressure. The obtained crude product is purified by column chromatography using silica gel and 1
4 ratio) (10 vol) is stirred at reflux for 1.0 h. The first phase progress of the reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is cooled to RT and then, methyl acrylate (8a) [16.0 mmol] and PdCl2(PPh3)2 [0.002 mmol] are added. Again, the entire reaction mixture is kept under reflux condition for 3.0 h in open air. Final stage progress is monitored by TLC. After the completion of the reaction, the whole reaction mixture is cooled to RT and the solvent is removed under reduced pressure. The obtained crude product is purified by column chromatography using silica gel and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio of EtOAc–petroleum ether (PE) to obtain pure compound (9a). The isolated yield of product 9a is 88%. The same procedure is followed for the preparation of 2-amino-4-(3-(alkenyl)phenyl)-6-phenylnicotinonitrile derivatives (9b–f) listed in Table 4. Synthesized compounds (9a–f) gave satisfactory spectroscopic data in accordance with their proposed structures. Based on 1H NMR data, all the prepared alkenes (9a–f) are confirmed as ‘E’ isomers (J = 16.0–16.5 Hz).
9 ratio of EtOAc–petroleum ether (PE) to obtain pure compound (9a). The isolated yield of product 9a is 88%. The same procedure is followed for the preparation of 2-amino-4-(3-(alkenyl)phenyl)-6-phenylnicotinonitrile derivatives (9b–f) listed in Table 4. Synthesized compounds (9a–f) gave satisfactory spectroscopic data in accordance with their proposed structures. Based on 1H NMR data, all the prepared alkenes (9a–f) are confirmed as ‘E’ isomers (J = 16.0–16.5 Hz).
3. Results and discussion
In the present study, our initial objective is to identify well-suited reaction conditions for the heterocyclization along with Pd-mediated C–C coupling process in a single step operation. Accordingly, we have studied the effect of bases, solvents, reaction temperature and Pd-catalysts as well as effect of concentration of base for the construction of pyridine frame work followed by Sonogashira/Heck coupling in one-pot. Therefore, in an initial experiment, 3-bromobenzaldehyde (1), malononitrile (2), acetophenone (3) and NH4OAc (4) in presence of K2CO3 in H2O is stirred at reflux for 10.0 h. After the completion of the reaction, the reaction mixture is cooled to RT and then, prop-2-yn-1-ol [5a], PdCl2(PPh3)2 and CuI are added. After workup and purification, the desired product 2-amino-4-(3-(3-hydroxyprop-1-ynyl)phenyl)-6-phenylnicotinonitrile (6a) is isolated in 5.0% yield (entry 1, Table 1). The obtained yield of the product (6a) is not impressive; this may be due to insolubility of the reactants. To improve the solubility of the reactants and thereby the yield of the product (6a), H2O is replaced by organic solvents such as THF, dioxane, DME, mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio), DMF and DMSO. The obtained yields are 48%, 56%, 64%, 71% and 62%, respectively (entries 2–7, Table 1). Initial effect of solvent study revealed that mixture of H2O–DME (1
4 ratio), DMF and DMSO. The obtained yields are 48%, 56%, 64%, 71% and 62%, respectively (entries 2–7, Table 1). Initial effect of solvent study revealed that mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) provided acceptable yield (71%) of the product 6a (entry 5). To increase the yield of the product (6a) further, various bases such as Na2CO3 (entry 8), Et3N (entry 9) and pyrrolidine (entry 10) are employed which resulted a yield of 56%, 78% and 91%, respectively in a mixture of H2O–DME (1
4 ratio) provided acceptable yield (71%) of the product 6a (entry 5). To increase the yield of the product (6a) further, various bases such as Na2CO3 (entry 8), Et3N (entry 9) and pyrrolidine (entry 10) are employed which resulted a yield of 56%, 78% and 91%, respectively in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio). The evaluation of suitable base revealed that pyrrolidine is more efficient in producing the maximum yield (91%) of product 6a (entry 10) in a mixture of H2O–DME (1
4 ratio). The evaluation of suitable base revealed that pyrrolidine is more efficient in producing the maximum yield (91%) of product 6a (entry 10) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio). Subsequently, the effects of temperature, Pd-catalyst and concentration of base and terminal alkyne, prop-2-yn-1-ol (5a) are investigated on the course of MCR. Lower yields (25–72%) are obtained when the same reaction is conducted at less than 80 °C in a mixture of H2O–DME (1
4 ratio). Subsequently, the effects of temperature, Pd-catalyst and concentration of base and terminal alkyne, prop-2-yn-1-ol (5a) are investigated on the course of MCR. Lower yields (25–72%) are obtained when the same reaction is conducted at less than 80 °C in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio). In this case, it is observed that some portions of the reactants are still remaining and resulting lower yields of the desired product (6a). Then, the same reaction has been conducted at reflux temperature and the obtained yield of the product (6a) has dramatically increased to 91%. In order to study the effect of other Pd-catalysts on the course of the C–C coupling reaction, various Pd-catalysts such as Pd(PPh3)4, Pd(dppf)Cl2 and 20% Pd(OH)2 have been employed which afforded 72%, 68% and 75% yields, respectively (entries 11–14). The results show the superiority of the PdCl2(PPh3)2 catalyst to obtain maximum yield (91%) of the product (6a) within 4.0 h. The effect of pyrrolidine base load is studied on the course of MCR reaction; for example 2.0, 5.0, 10.0, 15.0 and 20.0 mmol of pyrrolidine provided 72%, 91%, 78%, 71% and 65% yields of product (6a), respectively. From the results, it is concluded that 5.0 mmol of pyrrolidine offered best yield (91%) of product (6a). Variation of terminal alkyne concentration also effected the yields significantly; 10.0, 15.0, 20.0 and 25.0 mmol of prop-2-yn-1-ol 5a resulted 68%, 91%, 84% and 78% yields and the optimum concentration of terminal alkyne concentration is 15.0 mmol for maximum yield.
4 ratio). In this case, it is observed that some portions of the reactants are still remaining and resulting lower yields of the desired product (6a). Then, the same reaction has been conducted at reflux temperature and the obtained yield of the product (6a) has dramatically increased to 91%. In order to study the effect of other Pd-catalysts on the course of the C–C coupling reaction, various Pd-catalysts such as Pd(PPh3)4, Pd(dppf)Cl2 and 20% Pd(OH)2 have been employed which afforded 72%, 68% and 75% yields, respectively (entries 11–14). The results show the superiority of the PdCl2(PPh3)2 catalyst to obtain maximum yield (91%) of the product (6a) within 4.0 h. The effect of pyrrolidine base load is studied on the course of MCR reaction; for example 2.0, 5.0, 10.0, 15.0 and 20.0 mmol of pyrrolidine provided 72%, 91%, 78%, 71% and 65% yields of product (6a), respectively. From the results, it is concluded that 5.0 mmol of pyrrolidine offered best yield (91%) of product (6a). Variation of terminal alkyne concentration also effected the yields significantly; 10.0, 15.0, 20.0 and 25.0 mmol of prop-2-yn-1-ol 5a resulted 68%, 91%, 84% and 78% yields and the optimum concentration of terminal alkyne concentration is 15.0 mmol for maximum yield.
| Entry | Catalyst | Solvent | Base | Time (h)/temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: 3-bromobenzaldehyde 1 (10.0 mmol), malononitrile 2 (11.0 mmol), acetophenone 3 (11.0 mmol), NH4OAc 4 (20.0 mmol), base (5.0 mmol); prop-2-yn-1-ol 5a (15.0 mmol), PdCl2(PPh3)2 (0.002 mmol) and CuI (0.005 mmol) in a solvent or mixture of solvents (10 vol) at specified temperature.b Isolated yield.c Act as catalyst cum base. | |||||
| 1 | PdCl2(PPh3)2 | H2O | K2CO3 | 24.0; reflux | 5 |
| 2 | PdCl2(PPh3)2 | THF | K2CO3 | 16.0; reflux | 48 |
| 3 | PdCl2(PPh3)2 | Dioxane | K2CO3 | 12.0; reflux | 56 |
| 4 | PdCl2(PPh3)2 | DME | K2CO3 | 9.0; reflux | 64 |
| 5 | PdCl2(PPh3)2 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
K2CO3 | 7.0; reflux | 71 |
| 6 | PdCl2(PPh3)2 | DMF | K2CO3 | 8.0; 90–100 | 62 |
| 7 | PdCl2(PPh3)2 | DMSO | K2CO3 | 6.0; 90–100 | 65 |
| 8 | PdCl2(PPh3)2 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
Na2CO3 | 9.0; reflux | 56 |
| 9 | PdCl2(PPh3)2 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
Et3N | 6.0; reflux | 78 |
| 10 | PdCl2(PPh3)2 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
Pyrrolidine | 4.0; reflux | 91 |
| 11 | Pd(PPh3)4 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
Pyrrolidine | 10; reflux | 68 |
| 12 | Pd(PPh3)4 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
Pyrrolidine | 8.0; reflux | 72 |
| 13 | Pd(dppf)Cl2 | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
Pyrrolidine | 7.0; reflux | 68 |
| 14 | 20%Pd(OH)2c | H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) 4 ratio) |
— | 6.0; reflux | 75 |
Having prepared 2-amino-4-(3-(3-hydroxyprop-1-ynyl)phenyl)-6-phenylnicotinonitrile (6a) through heterocyclization followed by Sonogashira reaction efficiently under optimized reaction conditions, it is further investigate the further substrate scope and generality of this process using various terminal alkynes i.e. 6a–k and the obtained results are presented in Table 2. From this study, it is found that various functional groups of terminal alkyne substrates (6a–k) are well tolerated to the reaction conditions and provided good yields of the desired products (6a–k).
| Entry | R′ of terminal alkyne (5) substrate | Product (6) | Time (h) | Yieldb (%) | Rfc |
|---|---|---|---|---|---|
a Reagents and conditions: 3-bromobenzaldehyde 1 (10.0 mmol), malononitrile 2 (11.0 mmol), acetophenone 3 (11.0 mmol), NH4OAc 4 (20.0 mmol), pyrrolidine (5.0 mmol); terminal alkynes 5a–k (15.0 mmol), PdCl2(PPh3)2 (0.002 mmol) and CuI (0.005 mmol) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) at reflux.b Isolated yield.c Retention factor: 40% EtOAc in PE. 4 ratio) (10 vol) at reflux.b Isolated yield.c Retention factor: 40% EtOAc in PE. |
|||||
| 1 | CH2OH (5a) |  |
4.0 | 91 | 0.4 |
| 2 | CH2CH2OH (5b) |  |
4.0 | 89 | 0.45 |
| 3 | CH2CH2CH2OH (5c) |  |
3.5 | 85 | 0.5 |
| 4 | CH2CH2CH2Cl (5d) |  |
3.5 | 87 | 0.7 |
| 5 | CH2CH(OH)CH3 (5e) |  |
4.0 | 89 | 0.4 |
| 6 | (OH)C(CH3)2 (5f) | 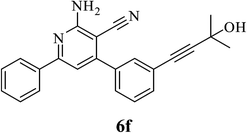 |
5.0 | 85 | 0.4 |
| 7 | CH2(CH2)2CH3 (5g) | 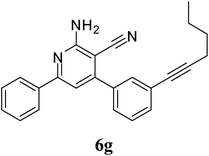 |
4.5 | 88 | 0.75 |
| 8 | CH2(CH2)3CH3 (5h) |  |
4.5 | 86 | 0.8 |
| 9 | CH2(CH2)4CH3 (5i) |  |
4.5 | 88 | 0.85 |
| 10 | CH2(CH2)6CH3 (5j) |  |
4.5 | 87 | 0.85 |
| 11 | 1-Hydroxy-1-cyclohexyl (5k) |  |
5.0 | 85 | 0.45 |
The results obtained reveal that 3-bromobezaldehyde (1) can act as an efficient key starting material in the formation of 2-amino-4-(3-(alkynyl)phenyl)-6-phenylnicotinonitriles (6a–k) via heterocyclization followed by Sonogashira reaction (Table 2); further it is found that the scope of the same reaction with 2-bromobenzandehyde (1a) as a key starting material resulted in the production of desired products (7a–d) presented in Table 3. The study reveal that lower yields of final products (7a–d) are obtained due to steric hindrance in the course of the C–C coupling process. It is found that lower yields of products (7a–d) are offered in all cases (entries 1–4, Table 3) irrespective of the nature of substituents present on terminal alkyne substrates (5a, 5b, 5f and 5i) when 2-bromobenzandehyde (1a) is used instead of 3-bromobenzandehyde (1).
| Entry | R′ of terminal alkyne (5) substrate | Product (7) | Time (h) | Yieldb (%) | Rfc |
|---|---|---|---|---|---|
a Reagents and conditions: 2-bromobenzaldehyde 1a (10.0 mmol), malononitrile 2 (11.0 mmol), acetophenone 3 (11.0 mmol), NH4OAc 4 (20.0 mmol), pyrrolidine (5.0 mmol); terminal alkynes 5a/b/f/i (15.0 mmol), PdCl2(PPh3)2 (0.002 mmol) and CuI (0.005 mmol) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) at reflux.b Isolated yield.c Retention factor: 40% EtOAc in PE. 4 ratio) (10 vol) at reflux.b Isolated yield.c Retention factor: 40% EtOAc in PE. |
|||||
| 1 | CH2OH (5a) |  |
4.5 | 81 | 0.35 |
| 2 | CH2CH2OH (5b) | 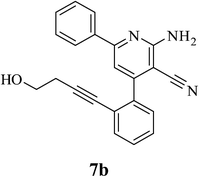 |
4.5 | 78 | 0.4 |
| 3 | (OH)C(CH3)2 (5f) |  |
5.5 | 76 | 0.4 |
| 4 | CH2(CH2)4CH3 (5i) | 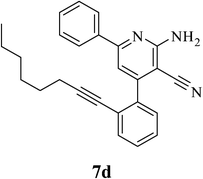 |
5.0 | 79 | 0.85 |
Further substrate scope is investigated to assess the generality of the proposed MCR under Heck reaction conditions as shown in Table 4. Towards this direction, a mixture of 3-bromobenzaldehyde (1) malononitrile (2), acetophenone (3), NH4OAc (4) and methyl acrylate (8a) in presence of PdCl2(PPh3)2 and pyrrolidine in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) are stirred for 5.0 h at reflux temperature to obtain product 9a. The yield (88%) of product 9a is good (entry 1, Table 4). Further, a series of terminal alkenes i.e. 8b–f are tested for the preparation of desired alkenyl-substituted pyridine derivatives (9b–f) and found that various sensitive functional groups of terminal alkenes (8a–f) are well tolerated to the reaction conditions and provided good yields of the desired products (9b–f) (entries 2–6, Table 4). Based on the obtained 1H NMR data, all the prepared 2-amino-4-(3-(alkenyl)phenyl)-6-phenylnicotinonitrile derivatives (9a–f) are confirmed as ‘E’ isomers (J = 16.0–16.5 Hz).
4 ratio) (10 vol) are stirred for 5.0 h at reflux temperature to obtain product 9a. The yield (88%) of product 9a is good (entry 1, Table 4). Further, a series of terminal alkenes i.e. 8b–f are tested for the preparation of desired alkenyl-substituted pyridine derivatives (9b–f) and found that various sensitive functional groups of terminal alkenes (8a–f) are well tolerated to the reaction conditions and provided good yields of the desired products (9b–f) (entries 2–6, Table 4). Based on the obtained 1H NMR data, all the prepared 2-amino-4-(3-(alkenyl)phenyl)-6-phenylnicotinonitrile derivatives (9a–f) are confirmed as ‘E’ isomers (J = 16.0–16.5 Hz).
| Entry | R′′ of terminal alkene (8) substrate | Product (9) | Time (h) | Yieldb (%) | Rfc |
|---|---|---|---|---|---|
a Reagents and conditions: 3-bromobenzaldehyde 1 (10.0 mmol), malononitrile 2 (11.0 mmol), acetophenone 3 (11.0 mmol), NH4OAc 4 (20.0 mmol), pyrrolidine (5.0 mmol) and terminal alkenes 8a–f (16.0 mmol), PdCl2(PPh3)2 (0.002 mmol) in a mixture of H2O–DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) (10 vol) at reflux.b Isolated yield.c Retention factor: 30% EtOAc in PE. 4 ratio) (10 vol) at reflux.b Isolated yield.c Retention factor: 30% EtOAc in PE. |
|||||
| 1 | COOCH3 (8a) |  |
5.0 | 88 | 0.55 |
| 2 | COOCH2CH3 (8b) | 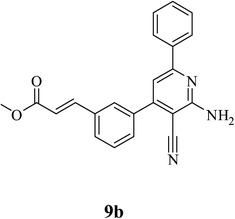 |
5.5 | 82 | 0.55 |
| 3 | COOC(CH3)3 (8c) |  |
5.0 | 83 | 0.6 |
| 4 | COCH3 (8d) |  |
5.5 | 86 | 0.4 |
| 5 | CONH2 (8e) | 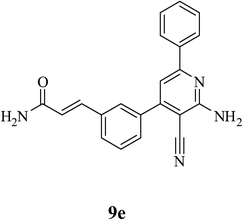 |
5.5 | 87 | 0.1 |
| 6 | CN (8f) |  |
4.5 | 85 | 0.3 |
4. Conclusions
In summary, general syntheses of a new library of structurally diverse alkynyl/alkenyl-substituted pyridine derivatives (6, 7 & 9) have been accomplished via heterocyclization and subsequent Pd-mediated structural elaboration under Sonogashira and Heck coupling reactions in single step. Interestingly, the Heck coupling with terminal alkenes takes place stereoselectively with exclusive formation of the E-isomers. This MCR strategy offers advantages like easy isolation of products, simple construction of diversity oriented library of highly substituted pyridine derivatives and wide applications in the synthesis of valuable heterocyclic synthetic precursors bearing carbon–carbon triple and double bond functionalities other than the present reported molecules. The prepared synthetic precursors will be suitable for further functional group transformations in the development of new and novel heterocyclic molecules of potential pharmacological interest.Acknowledgements
We gratefully acknowledge for the financial support from Council of Scientific and Industrial Research (CSIR), New Delhi, Govt of India through a major research project no. 01 (2391)/10/EMR-IIand Department of Atomic Energy-Board of Research in Nuclear Sciences (DAE-BRNS), Mumbai, Govt of India through a major research project no. 2011/37C/52/BRNS/2264.References
- A. Bogdanowicz, H. Foks, K. Gobis, A. Kędzia, E. Kwapisz, A. Olczak and M. L. Główka, J. Heterocycl. Chem., 2013, 50, 544–550 CrossRef CAS PubMed.
- P. Patel, S. Koregaokar, M. Shah and H. Parekh, Farmaco, 1996, 51, 59–63 CAS.
- F. Manna, F. Chimenti, A. Bolasco, A. Filippelli, A. Palla, W. Filippelli, E. Lampa and R. Mercantini, Eur. J. Med. Chem., 1992, 27, 627–632 CrossRef CAS.
- K. R. Kim, S. D. Rhee, H. Y. Kim, W. H. Jung, S. D. Yang, S. S. Kim, J. H. Ahn and H. G. Cheon, Eur. J. Pharmacol., 2005, 518, 63–70 CrossRef CAS PubMed.
- T. Godfraind, R. Miller and M. Wibo, Pharmacol. Rev., 1986, 38, 321–416 CAS.
- J. J. Baldwin, US Pat., no. 4000282, December 28, 1976.
- H. A. El-Sayed, A. H. Moustafa, E. Z. Haikal, R. A. El-Halawa and E. S. H. El Ashry, Eur. J. Med. Chem., 2011, 46, 2948–2954 CrossRef CAS PubMed.
- Z. Pinghu, Y. Xiaohui, Z. Luyong, Z. Yonghong and W. Zhimin, China Patent no. CN102875462A, 16th January 2013.
- J. H. Ahn, H. M. Kim, S. H. Jung, S. K. Kang, K. R. Kim, S. D. Rhee, S. D. Yang, H. G. Cheon and S. S. Kim, Bioorg. Med. Chem. Lett., 2004, 14, 4461–4465 CrossRef CAS PubMed.
- T. Murata, M. Shimada, S. Sakakibara and T. Yoshino, et al., Bioorg. Med. Chem. Lett., 2004, 14, 4019–4022 CrossRef CAS PubMed.
- M. T. Bilodeau, M. E. Duggan, J. C. Hartnett, C. W. Lindsley, Z. Wu and Z. Zhao, US Pat., no. 7579355 B2, August 25, 2009.
- L. N. Tumey, N. Bhagirath, A. Brennan, N. Brooijmans, J. Lee, X. Yang and D. H. Boschelli, Bioorg. Med. Chem., 2009, 17, 7933–7948 CrossRef CAS PubMed.
- D. Meibom, A. Vakalopoulos, B. Albrecht-Kiipper, K. Zimmermann, P. Nell and F. SiiBmeier, US Patent no. 8,426,602 B2, 23rd April 2013.
- M. Mantri, O. De Graaf, J. Van Veldhoven, A. Göblyös, J. K. Von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. Link, H. De Vries, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2008, 51, 4449–4455 CrossRef CAS PubMed.
- S. Ayvaz, M. Çankaya, A. Atasever and A. Altuntas, J. Enzyme Inhib. Med. Chem., 2013, 28, 305–310 CrossRef PubMed.
- K. R. Kim, S. D. Rhee, H. Y. Kim, W. H. Jung, S. D. Yang, S. S. Kim, J. H. Ahn and H. G. Cheon, Eur. J. Pharmacol., 2005, 518, 63–70 CrossRef CAS PubMed.
- J. Ji, W. H. Bunnelle, D. J. Anderson, C. Faltynek, T. Dyhring, P. K. Ahring, L. E. Rueter, P. Curzon, M. J. Buckley, K. C. Marsh, A. Kempf-Grote and M. D. Meyer, Biochem. Pharmacol., 2007, 74, 1253–1262 CrossRef CAS PubMed.
- A. Samadi, M. Chioua, I. Bolea, C. de los Ríos, I. Iriepa, I. Moraleda, A. Bastida, G. Esteban, M. Unzeta, E. Gálvez and J. Marco-Contelles, Eur. J. Med. Chem., 2011, 46, 4665–4668 CrossRef CAS PubMed.
- T. M. Keck, M. F. Zou, P. Zhang, R. P. Rutledge and A. H. Newman, ACS Med. Chem. Lett., 2012, 3, 544–549 CrossRef CAS PubMed.
- H. Zhao, M. D. Serby, Z. Xin, B. G. Szczepankiewicz, M. Liu, C. Kosogof, B. Liu, L. T. J. Nelson, E. F. Johnson, S. Wang, T. Pederson, R. J. Gum, J. E. Clampit, D. L. Haasch, C. Abad-Zapatero, E. H. Fry, C. Rondinone, J. M. Trevillyan, H. L. Sham and G. Liu, J. Med. Chem., 2006, 49, 4455–4458 CrossRef CAS PubMed.
- T. R. K. Reddy, R. Mutter, W. Heal, K. Guo, V. J. Gillet, S. Pratt and B. Chen, J. Med. Chem., 2006, 49, 607–615 CrossRef CAS PubMed.
- W. Brandt, L. Mologni, L. Preu, T. Lemcke, C. Gambacorti-Passerini and C. Kunick, Eur. J. Med. Chem., 2010, 45, 2919–2927 CrossRef CAS PubMed.
- V. Raghukumar, D. Thirumalai, V. T. Ramakrishnan, V. Karunakaran and P. Ramamurthy, Tetrahedron, 2003, 59, 3761–3768 CrossRef CAS.
- N. Li, S. L. Lai, P. Wang, F. Teng, Z. Liu, C. Lee and S. T. Lee, Appl. Phys. Lett., 2009, 95, 133301 CrossRef PubMed.
- D. Spitzner, Pyridines, in Science of Synthesis, ed. D. Black, Georg Thieme Verlag, Stuttgart, 2004, vol. 15, p. 11 Search PubMed.
- Md. N. Khan, S. Pal, S. Karamthulla and L. H. Choudhury, RSC Adv., 2014, 4, 3732–3741 RSC.
- B. Jiang, X. Wang, F. Shi, S.-J. Tu and G. Li, Org. Biomol. Chem., 2011, 9, 4025–4028 CAS.
- F. Liéby-Muller, C. Allais, T. Constantieux and J. Rodriguez, Chem. Commun., 2008, 4207–4209 RSC.
- L. Shen, S. Cao, J. Wu, J. Zhang, H. Li, N. Liu and X. Qian, Green Chem., 2009, 11, 1414–1420 RSC.
- C. Allais, T. Constantieux and J. Rodriguez, Chem.–Eur. J., 2009, 15, 12945–12948 CrossRef CAS PubMed.
- J. Liu, C. Wang, L. Wu, F. Liang and G. Huang, Synthesis, 2010, 24, 4228–4234 Search PubMed.
- M. Syamala, Org. Prep. Proced. Int., 2005, 37, 103–171 CrossRef CAS.
- Multicomponent Reaction, ed. T. J. Donohoe, J. A. Basutto, J. F. Bower, J. Zhu and H. Bienaymé, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
- A. Rathi, Org. Lett., 2011, 13, 1036–1039 CrossRef PubMed , and references cited therein.
- Z. Geng, Y. Ju, Q. Wang, W. Wang and Z. Li, RSC Adv., 2013, 3, 14798–14806 RSC.
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547–4592 RSC.
- U. Sharma, S. Ahmed and R. C. Boruah, Tetrahedron Lett., 2000, 41, 3493–3495 CrossRef CAS.
- S. Brauch, S. S. van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948–4962 RSC.
- K. Shekarrao, P. P. Kaishap, S. Gogoi and R. C. Boruah, RSC Adv., 2014, 4, 14013–14023 RSC.
- For review see: A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef CAS.
- For recent review, see: B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef CAS PubMed.
- H. Bienayme, C. Hulme, G. Oddon and P. Schmitt, Chem.–Eur. J., 2000, 6, 3321–3329 CrossRef CAS.
- B. M. Trost, Science, 1991, 254, 1471–1477 CAS.
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed.
- E. Yamaguchi, F. Shibahara and T. Murai, Chem.–Eur. J., 2010, 16, 12746–12753 CrossRef PubMed.
- E. Yamaguchi, F. Shibahara and T. Murai, J. Org. Chem., 2011, 76, 6146–6158 CrossRef CAS PubMed.
- D. M. D'Souza and T. J. J. Müller, Nat. Protoc., 2008, 3, 1660–1665 CrossRef PubMed.
- F. Yu, X. D. Lian and S. M. Ma, Org. Lett., 2007, 9, 1703–1706 CrossRef CAS PubMed.
- K. T. Yip, N. Y. Zhu and D. Yang, Org. Lett., 2009, 11, 1911–1914 CrossRef CAS PubMed.
- J. J. Li and G. W. Gribble, in ‘Palladium in Heterocyclic Chemistry’, Pergamon, Oxford, 2000, p. 183 Search PubMed.
- J. Namyslo and D. E. Kaufman, Synlett, 1999, 6, 804–806 CrossRef PubMed , and references cited therein.
- N. Robert, C. Hoarau, S. Célanire, P. Ribéreau, A. Godard, G. Quéguiner and F. Marsais, Tetrahedron, 2005, 61, 4569–4576 CrossRef CAS PubMed.
- P. Nussbaumer, I. Leitner, K. Mraz and A. Stuetz, J. Med. Chem., 1995, 38, 1831–1836 CrossRef CAS.
- I. N. Houpis, D. Shilds, U. Nettekoven, A. Schnyder, E. Bappert, K. Weerts, M. Canters and W. Vermuelen, Org. Process Res. Dev., 2009, 13, 598–606 CrossRef CAS.
- H. Li, Z. Xia, S. Chen, K. Koya, M. Ono and L. Sun, Org. Process Res. Dev., 2007, 11, 246–250 CrossRef CAS.
- P. Sun, X. Qu, T. Li, Y. Zhu, H. Yang, Z. Xing and J. Mao, Synlett, 2012, 23, 150–154 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01053a |
| This journal is © The Royal Society of Chemistry 2014 |