DOI:
10.1039/C4RA01459F
(Paper)
RSC Adv., 2014,
4, 17179-17195
DNA/protein binding and cytotoxicity studies of copper(II) complexes containing N,N′,N′′-trisubstituted guanidine ligands†
Received
19th February 2014
, Accepted 26th March 2014
First published on 27th March 2014
Abstract
A series of N,N′,N′′-trisubstituted guanidine ligands (L1–L5) and their copper(II) complexes (1–5) [Cu(II){C4H3SCONC(NHR)NC6H5}2] [where R = p-tolyl (1), phenyl (2) benzyl (3), butyl (4) and cyclohexyl (5)] were synthesized and characterized by elemental analyses and UV-visible, FT-IR, 1H & 13C NMR/EPR and mass spectroscopic techniques. The molecular structure of L1–L5, 3, and 5 was confirmed by single crystal X-ray crystallography. The single crystal X-ray structure of the complexes reveals the square planar geometry. The interaction of calf thymus DNA (CT-DNA) and bovine serum albumin (BSA) with the copper(II) complexes was investigated using UV-visible and fluorescence spectrophotometric methods. Spectral evidence shows the intercalative mode of DNA binding (in the order of 104 M−1) with the complexes. The Stern–Volmer quenching constant (Kq) values were found from competitive binding studies and found to be in the range of 1.07–1.30 × 105 M−1 for the complexes. Spectral evidence also shows good binding properties of the complexes with the protein. Complexes 3 and 4 showed significant cytotoxicity against human breast (MCF7) and lung cancer (A549) cell lines.
1. Introduction
There is a widespread interest in the identification and development of transition metal based compounds for biological applications. It is well known that DNA is an important cellular receptor. The interaction of metal complexes with DNA has recently gained much attention because it indicates that the complexes may have potential biological activity and their activity depends on the mode and affinity of the binding with DNA.1–3 Even though many transition metal complexes were reported as antitumour agents and some of them were under clinical trials, developing copper-based metallodrug is of special importance because of its biocompatibility. Copper complexes have proved to be the best candidates towards the search of the metal complexes of biological importance.4–6 Synthetic copper(II) complexes have been reported as potential anticancer and cancer inhibiting agents7,8 and number of copper complexes9,10 have been found to be active both in vitro and in vivo. Similarly, protein was also established as one of the main molecular targets in the action of anticancer agents.11 The interaction between protein and drugs provides useful information on the structural features that determine the therapeutic effectiveness of drugs and also to study the pharmacological response of drugs.12,13 Nowadays interaction of the proteins with the metal complexes become important in the search of new drug molecules.
The guanidine molecule, (NH2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH, is an important ingredient of both organic and inorganic chemistry. It is used to synthesize a number of biologically and pharmaceutically relevant compounds.14–17 Guanidine derivatives are very useful pharmacophores in medicinal chemistry due to their capacity to interact with functional groups present in enzymes or receptors through hydrogen bonds and electrostatic interactions. The guanidine group also act as a inhibitor of urokinase which plays a vital role in tumor metastasis which implicated in a large number of malignancies, including breast, lungs, bladder, stomach, cervix, kidney, and brain cancers.18,19 Various guanidine compounds have been synthesized and tested for their antitumor activity.20,21 Copper complexes containing guanidine ligands are also found to have many biological applications.22 For instance, copper(II) complexes of bis(2-pyridylmethyl)amine ligand with guanidinium pendant groups had an enhanced ability to cleave plasmid DNA and it can also act as RNA mimic.23 Copper(II) complexes with guanidine ligands have been recently reported as urease inhibitors.24 These stem a great interest to develop guanidine based copper(II) complexes for biological applications. Herein we report the synthesis and characterization of copper(II) complexes containing trisubstituted guanidine ligands. The interaction of the copper(II) complexes with CT-DNA and BSA was studied using spectrometric methods. We have also tested the in vitro cytotoxicity of the copper(II) complexes against MCF7 and A549 cancer cell lines.
NH, is an important ingredient of both organic and inorganic chemistry. It is used to synthesize a number of biologically and pharmaceutically relevant compounds.14–17 Guanidine derivatives are very useful pharmacophores in medicinal chemistry due to their capacity to interact with functional groups present in enzymes or receptors through hydrogen bonds and electrostatic interactions. The guanidine group also act as a inhibitor of urokinase which plays a vital role in tumor metastasis which implicated in a large number of malignancies, including breast, lungs, bladder, stomach, cervix, kidney, and brain cancers.18,19 Various guanidine compounds have been synthesized and tested for their antitumor activity.20,21 Copper complexes containing guanidine ligands are also found to have many biological applications.22 For instance, copper(II) complexes of bis(2-pyridylmethyl)amine ligand with guanidinium pendant groups had an enhanced ability to cleave plasmid DNA and it can also act as RNA mimic.23 Copper(II) complexes with guanidine ligands have been recently reported as urease inhibitors.24 These stem a great interest to develop guanidine based copper(II) complexes for biological applications. Herein we report the synthesis and characterization of copper(II) complexes containing trisubstituted guanidine ligands. The interaction of the copper(II) complexes with CT-DNA and BSA was studied using spectrometric methods. We have also tested the in vitro cytotoxicity of the copper(II) complexes against MCF7 and A549 cancer cell lines.
2. Results and discussion
2.1. Synthesis
The guanidine ligands (L1–L5) were synthesized by a mercury-promoted guanylation reaction from N-thiophenecarbonyl-N′-phenylthiourea (Scheme 1). The copper(II) complexes were synthesized using Cu(CH3COO)2·H2O as the precursor (Scheme 2). All the ligands and their copper(II) complexes were characterized by elemental analyses and/or various spectroscopic techniques. The molecular structure of L1–L5, 3 and 5 were confirmed by single crystal X-ray diffraction studies.
 |
| | Scheme 1 Synthesis of N-phenyl-N′-(aryl/alkyl)-N′′-thiophenecarbonylguanidine. | |
 |
| | Scheme 2 Synthesis of copper(II) complexes. | |
2.2. Spectroscopy
The electronic spectra of the ligands showed two bands around 260 and 300 nm, which correspond to π–π* and n–π* transitions respectively. The spectra of the complexes exhibited three bands. Two bands appeared around 254–306 nm, which correspond to intraligand transitions. The broad band in the region 601–626 nm corresponds to d–d transition.
In the FT-IR spectra of the ligands, two bands (strong and weak) were observed for N–H group in the range of 3367–3150 cm−1. The weak band is due to the hydrogen bonded N–H. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency of the ligands was observed at 1588–1568 cm−1. This is an intermediate value between double and single bonds, which shows the resonance between all the three nitrogen atoms in the guanidine moiety. The C
N stretching frequency of the ligands was observed at 1588–1568 cm−1. This is an intermediate value between double and single bonds, which shows the resonance between all the three nitrogen atoms in the guanidine moiety. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency appeared around 1632–1608 cm−1 in the spectra of the ligands was shifted to a lower value in the complexes, showing a single bond behaviour due to bonding with the Cu(II) ion. Further, the weak N–H band was disappeared, which indicates the coordination of N atom after deprotonation. The strong N–H band was appeared in the spectra of the complexes with minor shift towards higher value.
O stretching frequency appeared around 1632–1608 cm−1 in the spectra of the ligands was shifted to a lower value in the complexes, showing a single bond behaviour due to bonding with the Cu(II) ion. Further, the weak N–H band was disappeared, which indicates the coordination of N atom after deprotonation. The strong N–H band was appeared in the spectra of the complexes with minor shift towards higher value.
In the 1H NMR spectra of the ligands (L1 and L2), signals of both the N–H protons were found in the same region (10.09–11.00 ppm) since both the N–H are attached to an aromatic ring. However, in the case of ligands L3, L4 and L5, one of the N–H protons gave signal in the up field range (4.40–5.25 ppm) and another in the down field range (11.82–11.69 ppm). Chemical shift of all other aromatic and aliphatic protons was observed in the expected regions. 13C NMR spectra of the ligands showed resonance due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the regions 171.8–173.4 and 156.4–158.3 ppm respectively.25
N in the regions 171.8–173.4 and 156.4–158.3 ppm respectively.25
X-band EPR spectra of the Cu(II) complexes were recorded at room temperature as well as at liquid nitrogen temperature in solid and solution states. The observation of quartet hyperfine structure on the parallel component is due to the interaction of unpaired electron of Cu(II) with Cu having nuclear spin I = 3/2.26,27 All the complexes showed well resolved quartet hyperfine splitting typical of square planar Cu(II) system (Fig. 1), which was confirmed by single crystal XRD technique. For all the complexes g‖ > g⊥ suggesting that the system is axial. The trend in the g value (g‖ > g⊥ > 2.00) and the value of exchange interaction term (G > 4.0) suggested that the unpaired electron of Cu(II) ion is present in the dx2 − y2 orbital (Table 1).
 |
| | Fig. 1 EPR spectrum of complex 1 in frozen DMF solution. Microwave power, 0.98 mW; microwave frequency, 9.1 GHz. | |
Table 1 EPR parameters of Cu(II) complexes
| Complex |
Medium & temp. |
g‖ |
g⊥ |
A‖(G) |
| 1 |
Solid state RT |
2.286 |
2.047 |
|
| Solution state LNT |
2.220 |
2.038 |
172 |
| 2 |
Solid state RT |
2.385 |
2.047 |
|
| Solution state LNT |
2.223 |
2.046 |
173 |
| 3 |
Solid state RT |
2.204 |
2.043 |
|
| Solution state LNT |
2.217 |
2.042 |
174 |
| 4 |
Solid state RT |
2.203 |
2.022 |
|
| Solution state LNT |
2.218 |
2.042 |
180 |
| 5 |
Solid state RT |
2.283 |
2.047 |
|
| Solution state LNT |
2.217 |
2.049 |
181 |
2.3. Single crystal X-ray crystallographic studies
Thermal ellipsoid plots of ligands (L1–L5) and complexes (3 and 5) with the atomic labelling schemes are shown in the Fig. 2–8. Crystal data and selected inter atomic bond lengths and angles are given in the Tables 2–5. The crystal structures of the ligands showed the existence of an intra molecular hydrogen bond between N–H and carbonyl oxygen. The thiophene ring was oriented in two opposite directions in the structures of L3–L5. Slightly elongated thermal parameters of the thiophene groups (S1, C1–C4) indicated a possible disorder, which was successfully modelled with a ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The –C(O)N
10. The –C(O)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NH–)(NH–) core exhibited a large amount of delocalization due to the Y-aromaticity, as can be observed by the C–N bond lengths [1.334–1.355 Å (L1), 1.332–1.353 Å (L2), 1.333–1.351 Å (L3), 1.329–1.350 Å (L4) and 1.329–1.356 Å (L5)].
C(NH–)(NH–) core exhibited a large amount of delocalization due to the Y-aromaticity, as can be observed by the C–N bond lengths [1.334–1.355 Å (L1), 1.332–1.353 Å (L2), 1.333–1.351 Å (L3), 1.329–1.350 Å (L4) and 1.329–1.356 Å (L5)].
 |
| | Fig. 2 Thermal ellipsoid plot of L1. | |
 |
| | Fig. 3 Thermal ellipsoid plot of L2. | |
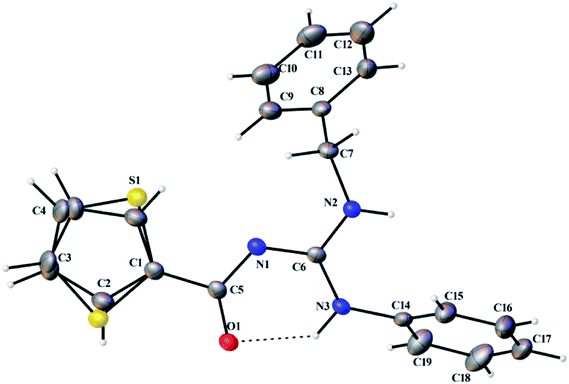 |
| | Fig. 4 Thermal ellipsoid plot of L3. | |
 |
| | Fig. 5 Thermal ellipsoid plot of L4. | |
 |
| | Fig. 6 Thermal ellipsoid plot of L5. | |
 |
| | Fig. 7 Thermal ellipsoid plot of 3. | |
 |
| | Fig. 8 Thermal ellipsoid plot of 5. | |
Table 2 Crystal data and structure refinement for ligands (L1–L5)
| |
L1 |
L2 |
L3 |
L4 |
L5 |
| Empirical formula |
C19H17N3OS |
C18H15N3OS |
C19H17N3OS |
C16H19N3OS |
C18H21N3OS |
| Formula weight |
335.42 |
321.39 |
335.42 |
301.40 |
327.44 |
| Temperature (K) |
110(2) |
110(2) |
110(2) |
110(2) |
110(2) |
| Wavelength (Å) |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
| Crystal system |
Tetragonal |
Triclinic |
Monoclinic |
Monoclinic |
Orthorhombic |
| Space group |
P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P2(1)/n |
P2(1) |
Pna2(1) |
| Unit cell dimensions |
| a (Å) |
19.258(5) |
6.4059(9) |
17.941(4) |
7.9816(19) |
11.0663(16) |
| b (Å) |
19.258(5) |
10.8648(16) |
9.5618(19) |
9.606(2) |
10.4930(15) |
| c (Å) |
9.374(2) |
12.1262(17) |
20.779(4) |
10.048(2) |
14.541(2) |
| α (°) |
90 |
79.907(2) |
90 |
90 |
90 |
| β (°) |
90 |
82.063(2) |
107.292(2) |
94.281(3) |
90 |
| γ (°) |
90 |
73.721(2) |
90 |
90 |
90 |
| Volume (Å3) |
3476.6(14) |
794.1(2) |
3403.6(12) |
768.2(3) |
1688.5(4) |
| Z |
8 |
2 |
8 |
2 |
4 |
| Density (calculated) Mg m−3 |
1.282 |
1.344 |
1.309 |
1.303 |
1.288 |
| Absorption coefficient (mm−1) |
0.196 |
0.211 |
0.200 |
0.213 |
0.200 |
| F(000) |
1408 |
336 |
1408 |
320 |
696 |
| Crystal size (mm3) |
0.60 × 0.50 × 0.20 |
0.45 × 0.44 × 0.38 |
0.42 × 0.37 × 0.27 |
0.37 × 0.27 × 0.03 |
0.56 × 0.33 × 0.32 |
| Theta range for data collection (°) |
1.06 to 27.50 |
2.42 to 27.44 |
2.05 to 25.00 |
2.03 to 25.00 |
2.39 to 25.00 |
| Index ranges |
−24 ≤ h ≤ 24, −24 ≤ k ≤ 24, −12 ≤ l ≤ 12 |
−8 ≤ h ≤ 8, −14 ≤ k ≤ 13, −15 ≤ l ≤ 15 |
−21 ≤ h ≤ 21, −11 ≤ k ≤ 11, −24 ≤ l ≤ 24 |
−9 ≤ h ≤ 9, −11 ≤ k ≤ 11, −10 ≤ l ≤ 11 |
−13 ≤ h ≤ 13, −12 ≤ k ≤ 12, −16 ≤ l ≤ 17 |
| Reflections collected |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 915 |
8598 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382 382 |
4488 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429 429 |
| Independent reflections [R(int)] |
7889(0.0327) |
3538(0.0141) |
5997(0.0614) |
2641(0.0568) |
2952(0.0285) |
| Completeness to theta = 27.50° |
99.5% |
97.3% |
99.9% |
99.7% |
99.7% |
| Absorption correction |
Semi-empirical from equivalents |
Semi-empirical from equivalents |
Semi-empirical from equivalents |
Semi-empirical from equivalents |
Semi-empirical from equivalents |
| Max. and min. transmission |
0.9618 and 0.8914 |
0.9240 and 0.9108 |
0.9479 and 0.9206 |
0.9936 and 0.9253 |
0.9388 and 0.8963 |
| Refinement method |
Full-matrix least-squares on F2 |
Full-matrix least-squares on F2 |
Full-matrix least-squares on F2 |
Full-matrix least-squares on F2 |
Full-matrix least-squares on F2 |
| Data/restraints/parameters |
7889/26/467 |
3538/0/208 |
5997/20/459 |
2641/11/205 |
2952/13/215 |
| Goodness-of-fit on F2 |
1.092 |
1.063 |
1.027 |
1.024 |
1.053 |
| Final R indices [I > 2σ(I)] |
R1 = 0.0403, wR2 = 0.0917 |
R1 = 0.0354, wR2 = 0.0882 |
R1 = 0.0357, wR2 = 0.0885 |
R1 = 0.0553, wR2 = 0.1435 |
R1 = 0.0250, wR2 = 0.0627 |
| R indices (all data) |
R1 = 0.0436, wR2 = 0.0939 |
R1 = 0.0381, wR2 = 0.0898 |
R1 = 0.0402, wR2 = 0.0921 |
R1 = 0.0563, wR2 = 0.1456 |
R1 = 0.0256, wR2 = 0.0633 |
| Largest diff. peak and hole (e Å−3) |
0.232 and −0.262 |
0.385 and −0.377 |
0.404 and −0.333 |
0.481 and −0.405 |
0.123 and −0.161 |
Table 3 Crystal data and structure refinement for complexes (3 and 5)
| |
3 |
5 |
| Empirical formula |
C38H32CuN6O2S2 |
C36H40CuN6O2S2 |
| Formula weight |
732.36 |
716.40 |
| Temperature (K) |
110(2) |
110(2) |
| Wavelength (Å) |
1.54178 |
1.54178 |
| Crystal system |
Triclinic |
Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c |
| Unit cell dimensions |
| a (Å) |
8.8813(5) |
32.090(2) |
| b (Å) |
9.9783(6) |
6.3662(4) |
| c (Å) |
10.5085(6) |
18.8845(13) |
| α (°) |
65.333(4) |
90 |
| β (°) |
83.253(4) |
118.786(4) |
| γ (°) |
86.906(4) |
90 |
| Volume (Å3) |
840.41(8) |
3381.2(4) |
| Z |
1 |
4 |
| Density (calculated) Mg m−3 |
1.447 |
1.407 |
| Absorption coefficient (mm−1) |
2.438 |
2.404 |
| F(000) |
379 |
1500 |
| Crystal size (mm3) |
0.16 × 0.06 × 0.04 |
0.12 × 0.07 × 0.05 |
| Theta range for data collection (°) |
4.66 to 59.99 |
5.35 to 60.00° |
| Index ranges |
−9 ≤ h ≤ 9, −11 ≤ k ≤ 11, −11 ≤ l ≤ 11 |
−36 ≤ h ≤ 36, −6 ≤ k ≤ 6, −20 ≤ l ≤ 20 |
| Reflections collected |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 731 731 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
| Independent reflections [R(int)] |
2447(0.0467) |
2425(0.0741) |
| Completeness to theta = 27.50° |
97.9% |
96.7% |
| Absorption correction |
Semi-empirical from equivalents |
Semi-empirical from equivalents |
| Max. and min. transmission |
0.9088 and 0.6963 |
0.8893 and 0.7613 |
| Refinement method |
Full-matrix least-squares on F2 |
Full-matrix least-squares on F2 |
| Data/restraints/parameters |
2447/0/224 |
2425/10/227 |
| Goodness-of-fit on F2 |
1.092 |
1.089 |
| Final R indices [I > 2sigma(I)] |
R1 = 0.0349, wR2 = 0.0956 |
R1 = 0.0336, wR2 = 0.0869 |
| R indices (all data) |
R1 = 0.0393, wR2 = 0.0973 |
R1 = 0.0390, wR2 = 0.0887 |
| Largest diff. peak and hole (e Å−3) |
0.321 and −0.472 |
0.304 and −0.521 |
Table 4 Selected bond lengths (Å), angles (°) of ligands
| |
L1 |
L2 |
L3 |
L4 |
L5 |
| O(1)–C(5) |
1.254(2) |
1.2463(16) |
1.2614(18) |
1.248(3) |
1.2560(16) |
| N(1)–C(6) |
1.334(3) |
1.3322(17) |
1.3427(19) |
1.350(3) |
1.3413(17) |
| N(1)–C(5) |
1.354(2) |
1.3552(16) |
1.3441(19) |
1.359(4) |
1.3476(17) |
| N(2)–C(6) |
1.339(2) |
1.3532(17) |
1.3338(19) |
1.357(4) |
1.3290(18) |
| N(2)–C(7) |
1.426(3) |
1.4240(17) |
1.4705(19) |
1.421(3) |
1.4648(17) |
| N(3)–C(6) |
1.355(2) |
1.3433(16) |
1.3517(19) |
1.329(4) |
1.3566(18) |
| N(3)–C(14) |
1.413(2) |
1.4322(16) |
1.4238(19) |
1.457(3) |
1.4230(18) |
| N(2)–H(2D) |
0.8800 |
0.8800 |
0.8800 |
0.9245 |
0.8800 |
| N(3)–H(3D) |
0.8800 |
0.8800 |
0.8800 |
0.9001 |
0.8800 |
| S(1)–C(4) |
1.728(2) |
1.7069(15) |
1.719(2) |
1.716(4) |
1.740(3) |
| S(1)–C(1) |
1.712(4) |
1.7201(13) |
1.7273(15) |
1.725(3) |
1.6980(15) |
| O(1)–C(5)–N(1) |
127.87(19) |
128.29(12) |
127.58(14) |
128.9(2) |
127.67(12) |
| C(6)–N(1)–C(5) |
119.67(16) |
120.08(11) |
121.95(13) |
119.5(2) |
120.34(11) |
| N(2)–C(6)–N(1) |
123.78(18) |
117.54(12) |
117.22(13) |
118.6(3) |
123.46(12) |
| N(2)–C(6)–N(3) |
116.68(17) |
117.04 |
119.83(13) |
117.2(2) |
118.51 |
| N(1)–C(6)–N(3) |
119.68(17) |
125.41(12) |
122.95(13) |
124.2(2) |
117.99(12) |
| C(6)–N(3)–C(14) |
127.94(17) |
123.50(11) |
128.06(12) |
126.6(2) |
126.70(11) |
| C(6)–N(2)–C(7) |
127.82(17) |
125.62(11) |
122.93(13) |
130.6(2) |
128.54(11) |
Table 5 Selected bond lengths (Å), angles (°) of complexesa
| |
3 |
5 |
| Symmetry transformations used to generate equivalent atoms: #1 −x, −y, −z. |
| Cu(1)–O(1) |
1.9087(16) |
1.9069(15) |
| Cu(1)–O(1)#1 |
1.9087(16) |
1.9070(15) |
| Cu(1)–N(3) |
1.9589(19) |
1.9688(18) |
| Cu(1)–N(3)#1 |
1.9589(19) |
1.9688(18) |
| O(1)–Cu(1)–N(3) |
90.30(7) |
90.37(7) |
| O(1)#1–Cu(1)–N(3)#1 |
90.29(7) |
90.37(7) |
| O(1)#1–Cu(1)–N(3) |
89.71(7) |
89.63(7) |
| O(1)–Cu(1)–N(3)#1 |
89.70(7) |
89.63(7) |
| O(1)#1–Cu(1)–O(1) |
180.00 |
180.00 |
| N(3)–Cu(1)–N(3)#1 |
180.00(10) |
180.00 |
| N(1)–C(6)–N(3) |
126.82(2) |
126.1(2) |
| N(1)–C(6)–N(2) |
113.2(2) |
113.16(19) |
| N(3)–C(6)–N(2) |
120.0(2) |
120.6(2) |
Structures of 3 and 5 confirmed the square planar geometry of the complexes. Complex 3 crystallized in triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with Z of 1 and complex 5 crystallized in monoclinic C2/c space group with Z of 4. The Cu–N bonds are longer than the Cu–O bonds [Cu(1)–O(1) 1.9087 Å, Cu(1)–N(1) 1.9589 Å (3) and Cu(1)–O(1) 1.9069 Å Cu(1)–N(1) 1.9688 Å (5)], which are in the expected range for guanidine complexes.28–31 Two guanidine ligands are coordinated to Cu(II) ion in a trans fashion. There is an increase in the C–O bond length and decrease in the C–N bond (involved in coordination) length in 3 and 5 compared to L3 and L5, respectively.
space group with Z of 1 and complex 5 crystallized in monoclinic C2/c space group with Z of 4. The Cu–N bonds are longer than the Cu–O bonds [Cu(1)–O(1) 1.9087 Å, Cu(1)–N(1) 1.9589 Å (3) and Cu(1)–O(1) 1.9069 Å Cu(1)–N(1) 1.9688 Å (5)], which are in the expected range for guanidine complexes.28–31 Two guanidine ligands are coordinated to Cu(II) ion in a trans fashion. There is an increase in the C–O bond length and decrease in the C–N bond (involved in coordination) length in 3 and 5 compared to L3 and L5, respectively.
2.4. DNA binding studies
2.4.1. Electronic absorption titration. The Cu(II) complexes (1–5) showed absorption band at 291–298 nm, which was assigned to π–π* transition. Upon the incremental addition of CT DNA to the complexes, the intensity of absorption decreases resulting in hypochromism (Δε, 15–28%) with a small red shift. Intercalative mode of binding due to the strong stacking interaction between an aromatic chromophore and the base pairs of DNA usually results in hypochromism along with or without a small red or blue shift.32 The extent of shift and hypochromism are normally found to correlate with the intercalative binding strength. The magnitude of hypochromism is in the order of 5 > 3 > 2 > 1 > 4, which reflects the DNA binding affinities of the complexes. The absorption spectra of the complexes (1–5) in the presence and absence of CT DNA are shown in Fig. 9.
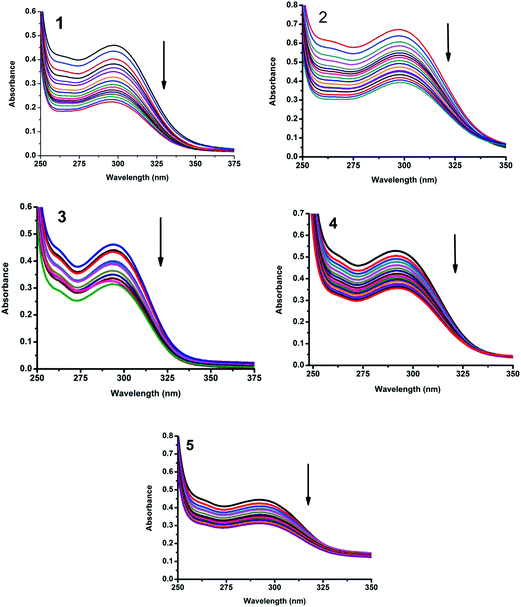 |
| | Fig. 9 Absorption spectra of complexes (1–5) in Tris–HCl buffer upon addition of CT DNA. [Complex] = 1.5 × 10−5 M, [DNA] = 0–40 μM. Arrow shows that the absorption intensities decrease upon increasing DNA concentration. | |
 |
| | Fig. 10 Fluorescence quenching curves of EB bound to DNA in the presence of 1–5. [DNA] = 5 μM, [EB] = 5 μM and [complex] = 0–25 μM. | |
The binding constant of the complexes with CT DNA (Kb) was obtained from the ratio of slope to intercept in plots [DNA]/(εa − εf) versus [DNA] according to the equation33
| [DNA]/(εa − εf) = [DNA]/(εb − εf) + 1/Kb(εb − εf) |
where [DNA] is the concentration of DNA in base pairs,
εa is the apparent extinction coefficient value found by calculating
A(observed)/[complex],
εf is the extinction coefficient for the free compound, and
εb is the extinction coefficient for the compound in the fully bound form. Each set of data, when fitted into the above equation, gave a straight line with a slope of 1/(
εb −
εf) and an
y-intercept of 1/
Kb(
εb −
εf) and the value of
Kb was determined from the ratio of slope to intercept (
Fig. 11). The magnitudes of intrinsic binding constants (
Kb) are given in
Table 6. The observed values of
Kb revealed that the Cu(
II) complexes bind to DNA
via intercalative mode.
34 The
Kb values were found to be in the range of 1.20–2.41 × 10
4 M
−1. Complex
5 showed better DNA binding affinity compared to the other complexes. The
Kb value of
1,
2,
3 and
4 differs only by a small value. In complex
5, the cyclohexyl ring is in the molecular plane, which might be the reason for its enhanced DNA binding ability compared to other complexes (
1–4).
 |
| | Fig. 11 Plot of [DNA]/(εa − εf) versus [DNA] for the titration of the complexes with CT DNA. | |
Table 6 DNA binding constant (Kb), Stern–Volmer constant (Kq) and the apparent binding constant (Kapp) for complexes 1–5
| Complex |
Kb (M−1) |
Kq (M−1) |
Kapp (M−1) |
| 1 |
1.30 × 104 |
1.11 × 105 |
5.55 × 106 |
| 2 |
1.39 × 104 |
1.07 × 105 |
5.35 × 106 |
| 3 |
1.69 × 104 |
1.14 × 105 |
5.70 × 106 |
| 4 |
1.20 × 104 |
1.07 × 105 |
5.35 × 106 |
| 5 |
2.41 × 104 |
1.30 × 105 |
6.50 × 106 |
2.4.2. Fluorescence spectroscopic studies. Fluorescence property has not been observed for the complexes at room temperature in solution or in the presence of CT DNA. So the binding of the complexes with DNA could not be directly predicted through the emission spectra. Hence, competitive binding study was done to understand the mode of DNA interaction with the complexes.35–37 Ethidium bromide (EB) emits intense fluorescence in the presence of CT DNA because of strong intercalation of the planar EB phenanthridine ring between adjacent base pairs in the double helix; therefore, EB has been considered as a typical indicator of intercalation.38 If another molecule which can bind to DNA more strongly than EB was added, the molecule will replace the bound EB and there was a quenching in the DNA induced EB emission. The extent of quenching of CT DNA–EB reflects the extent of interaction with the added molecule. On adding Cu(II) complexes (0–25 μM) to CT DNA–EB, the quenching in the emission of DNA bound EB takes place (Fig. 10). Fluorescence quenching is explained by the Stern–Volmer equation39
where Fo and F are the fluorescence intensities in the absence and presence of complex respectively, Kq is a linear Stern–Volmer quenching constant, and [Q] is the concentration of complex. The slope of the plot of Fo/F versus [Q] gave Kq (Fig. 12). The apparent DNA binding constant (Kapp) values were calculated by using the equation
where [complex] is the complex concentration at 50% reduction in the fluorescence intensity of EB, KEB = 1.0 × 107 M−1 and [EB] = 5 μM. The quenching constant Kq and Kapp values are listed in Table 6.
 |
| | Fig. 12 Stern–Volmer plot of fluorescence titrations of the complexes with CT DNA. | |
2.5. Protein binding studies
2.5.1. Absorbance and fluorescence studies. Fig. 13 shows the fluorescence emission spectra of BSA after the addition of complexes (1–5). When increasing amount of complex solution was added to a fixed quantity of BSA, there observed a decrease in the fluorescence intensity of BSA at 345 nm, upto 86.2, 88.5, 78.3, 74.6 and 83.5% for complexes 1–5 respectively, with bathochromic shift of 4, 3 and 1 nm for complexes 1, 2 and 3 respectively. There was no appreciable shift in the case of complexes 4 and 5. The observed hypochromicity has revealed that the complexes interact hydrophobically with the BSA protein.40
 |
| | Fig. 13 Fluorescence quenching curves of BSA in the absence and presence of 1–5. [BSA] = 1 μM and [complex] = 0–50 μM. | |
UV-visible absorption titration of BSA with complexes (1–5) was done to predict the type of quenching process. Addition of the complex to BSA lead to an increase in BSA absorption intensity without affecting the position of absorption band. This indicates that the type of interaction between Cu(II) complexes and BSA was mainly a static quenching process.41 The representative absorption titration spectrum is shown in Fig. 14. The fluorescence quenching is described by the Stern–Volmer relation
where
Fo and
F demonstrate the fluorescence intensities in the absence and presence of quencher, respectively.
Kq is a linear Stern–Volmer quenching constant, and [Q] is the quencher concentration. The quenching constant (
Kq) can be calculated using the plot of log(
Fo/
F)
versus log[Q] (
Fig. 15). When small molecules bind independently to a set of equivalent site, on a macromolecule, the equilibrium between free and bound molecules is represented by the Scatchard equation
42,43log[(Fo − F)/F] = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kb + n Kb + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Q] log[Q] |
where
Kb is the binding constant of the complex with BSA and
n is the number of binding sites. From the plot of log[(
Fo −
F)/
F]
versus log[Q] (
Fig. 16), the number of binding sites (
n) and the binding constant (
Kb) values have been obtained. The quenching constant (
Kq), binding constant (
Kb) and number of binding sites (
n) for the interaction of the Cu(
II) complexes with BSA are shown in
Table 7. In all the complexes, only one binding site is available to interact with BSA. Results showed that complexes
1 and
2 interact strongly with BSA compared to
3,
4 and
5.
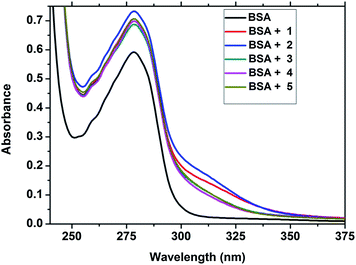 |
| | Fig. 14 The absorption spectra of BSA (10 μM) and BSA with 1–5 (4 μM). | |
 |
| | Fig. 15 Stern–Volmer plot of the fluorescence titrations of the complexes with BSA. | |
 |
| | Fig. 16 Scatchard plot of the fluorescence titrations of the complexes with BSA. | |
Table 7 Protein binding constant (Kb), quenching constant (Kq) and number of binding sites (n) for complexes 1–5
| Complex |
Kb (M−1) |
Kq (M−1) |
n |
| 1 |
2.00 × 107 |
2.31 × 105 |
1.39 |
| 2 |
2.72 × 107 |
3.35 × 105 |
1.38 |
| 3 |
3.18 × 105 |
1.32 × 105 |
1.02 |
| 4 |
1.52 × 106 |
2.20 × 105 |
1.22 |
| 5 |
8.00 × 106 |
2.09 × 105 |
1.32 |
2.5.2. Characteristics of synchronous fluorescence spectra. Synchronous fluorescence spectroscopy provides information about the molecular microenvironment, particularly in the vicinity of the fluorophore functional groups.44 Tyrosine, tryptophan and phenylalanine residues are responsible for the fluorescence property of BSA. The difference between the excitation and emission wavelength (Δλ) reflects the nature of the chromophore.45 The large Δλ value, such as 60 nm, is characteristic of tryptophan residue and a small Δλ value, such as 15 nm, is characteristic of tyrosine. The synchronous fluorescence spectra of BSA with various concentrations of Cu(II) complexes (1–5) were recorded at Δλ = 15 nm and Δλ = 60 nm. On addition of the complexes, the fluorescence intensity of tryptophan residue at 340 nm decreased in the magnitude of 82.6, 88.9, 79.2, 71.0 and 80.3% for complexes 1, 2, 3, 4 and 5 respectively (Fig. 17). Similarly, there was also decrease in the intensity of tyrosine residue at 300 nm. The magnitude of decrease was 86.6, 88.0, 80.0, 74.0 and 85.1% for complexes 1–5 respectively (Fig. 18). The synchronous fluorescence spectral studies clearly suggested that the fluorescence intensities of both the tryptophan and tyrosine were affected with increasing concentration of the complexes. The results indicate that the interaction of complexes with BSA affects the conformation of both tryptophan and tyrosine micro-region.46
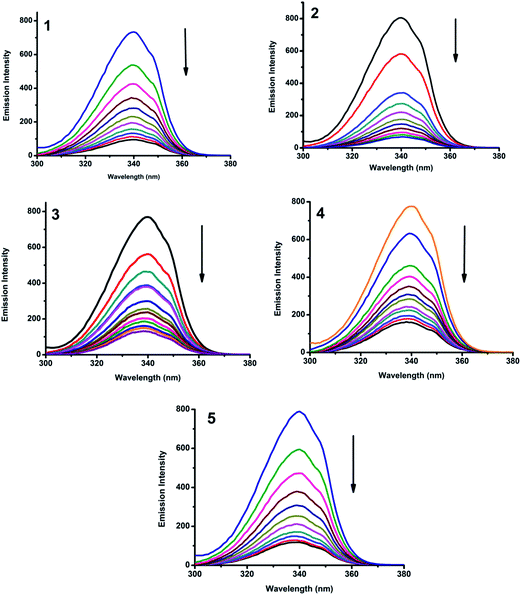 |
| | Fig. 17 Synchronous spectra of BSA (1 μM) as a function of concentration of 1–5 (0–50 μM) with Δλ = 60 nm. | |
 |
| | Fig. 18 Synchronous spectra of BSA (1 μM) as a function of concentration of 1–5 (0–50 μM) with Δλ = 15 nm. | |
2.6. Cytotoxicity assay
The cytotoxicity of the Cu(II) complexes (1–5) toward MCF7 (human breast cancer cells) and A549 (human lung cancer cells) cells has been examined by using MTT assay and compared with cyclophosphamide [IC50 = 6.58 μM (MCF7) and 22.36 μM (A549)] under identical conditions.47 Fig. 19 and 20 show the cytotoxicity of the compounds (1–5) after 24 h incubation on MCF7 and A549 cancer cell lines, respectively. Complexes 3 and 4 exhibited cytotoxicity with IC50 values of 76.05 and 61.08 μM, respectively, against MCF7 cell line. The same complexes showed IC50 values of 91.68 and 68.8 μM against A549 cell line. Complexes 1 and 5 possess a moderate cytotoxicity. The IC50 values of 1 and 5 were found to be 128.67 and 145.0 μM, respectively, against MCF7. The same complexes showed IC50 values of 241.58 and 164.01 against A549. Complex 2 exhibited least activity than the other complexes. The IC50 values of all the complexes are listed in Table 8.
 |
| | Fig. 19 Cytotoxicity of complexes 1–5 after 24 h incubation on MCF7 cell lines. | |
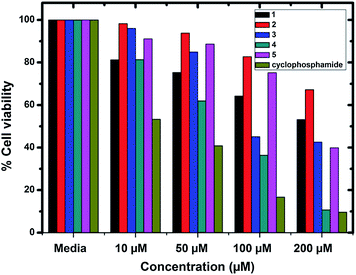 |
| | Fig. 20 Cytotoxicity of complexes 1–5 after 24 h incubation on A549 cell lines. | |
Table 8 In vitro cytotoxic studies of Cu(II) complexes against MCF7 and A549 cancer cell lines
| Complex |
IC50 |
| MCF7 (μM) |
A549 (μM) |
| 1 |
128.67 |
241.58 |
| 2 |
300.57 |
370.21 |
| 3 |
76.05 |
91.68 |
| 4 |
61.08 |
68.84 |
| 5 |
145.40 |
164.01 |
| Cyclophosphamide |
6.58 |
22.36 |
3. Conclusion
Five Cu(II) complexes with trisubstituted guanidine ligands have been synthesized. Single crystal X-ray diffraction studies revealed that the Cu(II) complexes have square planar geometry. The DNA binding of the complexes was investigated using absorption and fluorescence spectrometric techniques. The results supported the interaction of the complexes with CT DNA through non-covalent intercalation. The good protein binding ability of the complexes was revealed from fluorescence measurement. In vitro cytotoxicity results showed that the complexes have moderate activity against MCF7 and A549 cancer cell lines. The result of DNA binding does not correlate with that of the in vitro cytotoxic studies. This clearly states that the mechanism involved in cytotoxic activity of the complexes is different. Further studies are needed to study the relation between the DNA binding and cytotoxic activity of the complexes.
4. Experimental
4.1. Materials and methods
All the chemicals were purchased from Sigma Aldrich/Merck and used as received. Solvents were purified according to standard procedures. The melting points were determined on Lab India instrument and are uncorrected. The elemental analyses were performed using a Vario EL-III CHNS analyzer. FT-IR spectra were obtained as KBr pellets using a Nicolet-iS5 spectrophotometer. UV-visible spectra were recorded using a Shimadzu-2600 spectrophotometer. Emission spectra were measured on a Jasco V-630 spectrophotometer using 5% DMF in buffer as the solvent. NMR spectra were recorded in CDCl3 by using TMS as an internal standard on a Bruker 400 MHz spectrometer. EPR spectra were recorded on a JEOL EPR spectrometer at room temperature and liquid nitrogen temperature, operating at X-band frequency (9.1 GHz).
4.2. Synthesis of N,N′,N′′-trisubstituted guanidines
The guanidine ligands were synthesized from N-thiophenecarbonyl-N′-phenylthiourea by a guanylation method.48 The thiourea was mixed with the desired substituted amine in DMF in an equimolar ratio with two equivalents of triethylamine. The temperature was maintained below 5 °C using an ice bath and one equivalent of mercuric chloride was added to the reaction mixture with vigorous stirring. The ice bath was removed after 30 minutes, while the stirring continued overnight. The progress of the reaction was monitored using TLC until all the thiourea was consumed. 20 mL of chloroform was added to the reaction mixture and the suspension was filtered through a sintered glass funnel to remove the HgS residue. The solvents were evaporated under reduced pressure and the solid residue was dissolved in 20 mL of CH2Cl2, then washed with water and the organic phase was dried over anhydrous Na2SO4. The residue obtained after evaporation of the solvent was recrystallized from ethanol to get crystals of the title compounds.
4.2.1. N-Phenyl-N′-(4-methylphenyl)-N′′-thiophenecarbonylguanidine (L1). Yield: 79%. Colourless solid. M.p.: 130 °C. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 265 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 301 (26
300), 301 (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). FT-IR (KBr, ν cm−1): 3279, 3203 (N–H), 1611 (C
900). FT-IR (KBr, ν cm−1): 3279, 3203 (N–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1568 (C
O), 1568 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, DMSO-d6): δ, ppm 2.26 (s, 3H), 7.44–7.011 (m, 9H), 7.65 (d, J = 3.6 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.08 (t, J = 3.6 Hz, 1H), 10.46 (s, 1H), 10.09 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ, ppm 21.0 (aliphatic CH3), 123.9, 124.2, 125.1, 128.4, 129.9, 130.8, 132.1, 134.7. 135.1, 138.1, 144.4 (aromatic C), 156.4 (C
N). 1H NMR (400 MHz, DMSO-d6): δ, ppm 2.26 (s, 3H), 7.44–7.011 (m, 9H), 7.65 (d, J = 3.6 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.08 (t, J = 3.6 Hz, 1H), 10.46 (s, 1H), 10.09 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ, ppm 21.0 (aliphatic CH3), 123.9, 124.2, 125.1, 128.4, 129.9, 130.8, 132.1, 134.7. 135.1, 138.1, 144.4 (aromatic C), 156.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 171.8 (C
N), 171.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4197.
O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4197.
4.2.2. N,N′-Diphenyl-N′′-thiophenecarbonylguanidine (L2). Yield: 78%. Colourless solid. M.p.: 120 °C. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 266 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 302 (16
500), 302 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800). FT-IR (KBr): ν, cm−1 3394, 3165 (N–H), 1602 (C
800). FT-IR (KBr): ν, cm−1 3394, 3165 (N–H), 1602 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1571 (C
O), 1571 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3) δ, ppm: 7.09 (t, J = 4 Hz, 1H), 7.07–7.46 (m, 11H), 7.79 (d, J = 3.6 Hz, 1H), 10.30 (s, 2H). 13C NMR (100 MHz, CDCl3): δ, ppm 124.1, 126.1, 127.7, 129.5, 130.9, 131.1, 136.3, 144.4 (aromatic C), 156.1 (C
N). 1H NMR (400 MHz, CDCl3) δ, ppm: 7.09 (t, J = 4 Hz, 1H), 7.07–7.46 (m, 11H), 7.79 (d, J = 3.6 Hz, 1H), 10.30 (s, 2H). 13C NMR (100 MHz, CDCl3): δ, ppm 124.1, 126.1, 127.7, 129.5, 130.9, 131.1, 136.3, 144.4 (aromatic C), 156.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 173.3 (C
N), 173.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS calcd for C18H15N3OS: 321.3962 found: 321.4001.
O). HRMS calcd for C18H15N3OS: 321.3962 found: 321.4001.
4.2.3. N-Phenyl-N′-benzyl-N′′-thiophenecarbonylguanidine (L3). Yield: 82%. Colourless solid. M.p.: 100 °C. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 262 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 296 (28
000), 296 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500). FT-IR (KBr): ν, cm−1 3267, 3202 (N–H), 1611 (C
500). FT-IR (KBr): ν, cm−1 3267, 3202 (N–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1569 (C
O), 1569 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3): δ, ppm 4.72 (s, 2H), 5.25 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.43–7.23 (m, 11H), 7.80 (d, J = 2.4 Hz, 1H), 11.82 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 45.2 (aliphatic, CH2), 125.7, 127.2, 127.7, 127.8, 128.8, 130.2, 130.5, 130.7, 135.8, 144.8 (aromatic C), 158.2 (C
N). 1H NMR (400 MHz, CDCl3): δ, ppm 4.72 (s, 2H), 5.25 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.43–7.23 (m, 11H), 7.80 (d, J = 2.4 Hz, 1H), 11.82 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 45.2 (aliphatic, CH2), 125.7, 127.2, 127.7, 127.8, 128.8, 130.2, 130.5, 130.7, 135.8, 144.8 (aromatic C), 158.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 173.4 (C
N), 173.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4194.
O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4194.
4.2.4. N-Phenyl-N′-butyl-N′′-thiophenecarbonylguanidine (L4). Yield: 74%. Colourless solid. M.p.: 80 °C. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 264 (9400), 296 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). FT-IR (KBr): ν, cm−1 3341, 3230 (N–H), 1607 (C
300). FT-IR (KBr): ν, cm−1 3341, 3230 (N–H), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556 (C
O), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3): δ, ppm 0.92–0.95 (t, J = 5.6 Hz, 3H), 1.34–1.38 (q, J = 5.6 Hz, 2H), 1.56–1.55 (t, J = 5.6 Hz, 2H), 3.46 (s, 2H), 4.96 (s, 1H), 7.68–7.05 (m, 8H), 11.69 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 13.9, 20.1, 31.9, 41.2 (aliphatic), 125.6, 127.1, 130.2, 130.3, 130.5, 135.9, 140.5 (aromatic C), 158.3 (C
N). 1H NMR (400 MHz, CDCl3): δ, ppm 0.92–0.95 (t, J = 5.6 Hz, 3H), 1.34–1.38 (q, J = 5.6 Hz, 2H), 1.56–1.55 (t, J = 5.6 Hz, 2H), 3.46 (s, 2H), 4.96 (s, 1H), 7.68–7.05 (m, 8H), 11.69 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 13.9, 20.1, 31.9, 41.2 (aliphatic), 125.6, 127.1, 130.2, 130.3, 130.5, 135.9, 140.5 (aromatic C), 158.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 172.8 (C
N), 172.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); HRMS calcd for C16H19N3OS: 301.4066 found: 301.4001.
O); HRMS calcd for C16H19N3OS: 301.4066 found: 301.4001.
4.2.5. N-Phenyl-N′-cyclohexyl-N′′-thiophenecarbonylguanidine (L5). Yield: 71%. Colourless solid. M.p.: 131 °C. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 263 (7700), 296 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). FT-IR (KBr): ν, cm−1 3307, 3207 (N–H), 1608 (C
300). FT-IR (KBr): ν, cm−1 3307, 3207 (N–H), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1553 (C
O), 1553 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3): δ, ppm 2.05–1.15 (m, 11H), 4.80 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.41–7.23 (m, 6H), 7.77 (s, 1H), 11.71 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 24.9, 25.6, 33.1, 50.4 (aliphatic C), 125.3, 126.8, 127.1, 127.7, 130.2, 136.1, 145.1 (aromatic C), 157.3 (C
N). 1H NMR (400 MHz, CDCl3): δ, ppm 2.05–1.15 (m, 11H), 4.80 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.41–7.23 (m, 6H), 7.77 (s, 1H), 11.71 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 24.9, 25.6, 33.1, 50.4 (aliphatic C), 125.3, 126.8, 127.1, 127.7, 130.2, 136.1, 145.1 (aromatic C), 157.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 172.7 (C
N), 172.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); HRMS calcd for C18H21N3OS: 327.4438 found: 327.4397.
O); HRMS calcd for C18H21N3OS: 327.4438 found: 327.4397.
4.3. Synthesis of copper(II) complexes (1–5)
The methanolic solution of Cu(CH3COO)2·H2O (1 mmol) was added into the solution of an appropriate guanidine (2 mmol) in methanol at room temperature. The reaction mixture was stirred for 6 h under an inert atmosphere, and then the precipitate formed was filtered and washed with methanol. The suitable crystals of 3 and 5 for X-ray diffraction were grown from CHCl3–n-hexane mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
4.3.1. Bis(N-phenyl-N′-(4-methylphenyl)-N′′-thiophenecarbonylguanidinato)copper(II) (1). Yield: 74%. Light blue solid. M.p.: 212 °C. Anal. calcd for C38H32CuN6O2S2 (732.38): C, 62.32; H, 4.40; N, 11.48; S, 8.76. Found: C, 61.93; H, 3.91; N, 11.50; S, 6.73. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 267 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 298 (30
300), 298 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 604 (147). FT-IR (KBr): ν, cm−1 3394 (N–H), 1594 (C
000), 604 (147). FT-IR (KBr): ν, cm−1 3394 (N–H), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1560 (C
O), 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K): ‘g’ values 2.286, 2.047. EPR (LNT): ‘g’ values 2.220, 2.038.
N). EPR (300 K): ‘g’ values 2.286, 2.047. EPR (LNT): ‘g’ values 2.220, 2.038.
4.3.2. Bis(N,N′-diphenyl-N′′-thiophenecarbonylguanidinato)copper(II) (2). Yield: 85%. Light blue solid. M.p.: 215 °C. Anal. calcd for C36H28CuN6O2S2 (704.32): C, 61.39; H, 4.01; N, 11.93; S, 9.1. Found: C, 61.17; H, 3.45; N, 12.10; S, 8.61. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 269 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 306 (25
500), 306 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), 601 (88). FT-IR (KBr): ν, cm−1 3402 (N–H), 1593 (C
600), 601 (88). FT-IR (KBr): ν, cm−1 3402 (N–H), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556 (C
O), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.385, 2.047 EPR (LNT, ‘g’ value): 2.223, 2.046.
N). EPR (300 K, ‘g’ value): 2.385, 2.047 EPR (LNT, ‘g’ value): 2.223, 2.046.
4.3.3. Bis(N-phenyl-N′-benzyl-N′′-thiophenecarbonylguanidinato)copper(II) (3). Yield: 83%. Blue solid. M.p.: 202 °C. Anal. calcd for C38H32CuN6O2S2 (732.38): C, 62.32; H, 4.40; N, 11.48; S, 8.76. Found: C, 62.66; H, 3.72; N, 11.80; S, 7.97. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 254 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), 298 (29
100), 298 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 603 (154). FT-IR (KBr): ν, cm−1 3421 (N–H), 1557 (C
000), 603 (154). FT-IR (KBr): ν, cm−1 3421 (N–H), 1557 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1533 (C
O), 1533 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.204, 2.043 EPR (LNT, ‘g’ value): 2.217, 2.042.
N). EPR (300 K, ‘g’ value): 2.204, 2.043 EPR (LNT, ‘g’ value): 2.217, 2.042.
4.3.4. Bis(N-phenyl-N′-butyl-N′′-thiophenecarbonylguanidinato)copper(II) (4). Yield: 71%. Light blue solid. M.p: 172 °C. Anal. calcd for C32H36CuN6O2S2 (664.34): C, 57.85; H, 5.46; N, 12.65; S, 9.65. Found: C, 57.50; H, 5.44; N, 12.48; S, 9.88. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 254 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 298 (28
900), 298 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 626 (298). FT-IR (KBr): ν, cm−1 3418 (N–H), 1560 (C
900), 626 (298). FT-IR (KBr): ν, cm−1 3418 (N–H), 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1537 (C
O), 1537 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.203, 2.022 EPR (LNT, ‘g’ value): 2.218, 2.042.
N). EPR (300 K, ‘g’ value): 2.203, 2.022 EPR (LNT, ‘g’ value): 2.218, 2.042.
4.3.5. Bis(N-phenyl-N′-cyclohexyl-N′′-thiophenecarbonylguanidinato)copper(II) (5). Yield: 73%. Light blue solid. M.p.: 210 °C. Anal. calcd for C36H40CuN6O2S2 (716.42): C, 60.35; H, 5.63; N, 11.73; S, 8.95. Found: C, 59.91; H, 5.05; N, 11.69; S, 8.63. UV-vis (5% CHCl3): λmax, nm (ε, dm3 mol−1 cm−1) 254 (4600), 299 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), 608 (66). FT-IR (KBr): ν, cm−1 3418 (N–H), 1566 (C
600), 608 (66). FT-IR (KBr): ν, cm−1 3418 (N–H), 1566 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1532 (C
O), 1532 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.283, 2.047 EPR (LNT, ‘g’ value): 2.217, 2.049.
N). EPR (300 K, ‘g’ value): 2.283, 2.047 EPR (LNT, ‘g’ value): 2.217, 2.049.
4.4. Single crystal X-ray diffraction studies
A Bruker APEX2 X-ray (three-circle) diffractometer was employed for crystal screening, unit cell determination, and data collection. The X-ray radiation employed was generated from a Mo sealed X-ray tube (Kα = 0.70173 Å with a potential of 40 kV, 40 mA) fitted with a graphite monochromator in the parallel mode (175 mm collimator with 0.5 mm pinholes). Sixty data frames were taken at widths of 0.5°. These reflections were used in the auto-indexing procedure to determine the unit cell. A suitable cell was found and refined by nonlinear least squares and Bravais lattice procedures. The unit cell was verified by examination of the hkl overlays on several frames of data by comparing with both the orientation matrices. No super-cell or erroneous reflections were observed. After careful examination of the unit cell, a standard data collection procedure was initiated using omega scans. Integrated intensity information for each reflection was obtained by reduction of the data frames with the program APEX2.49 The integration method employed a three dimensional profiling algorithm and all data were corrected for Lorentz and polarization factors, as well as for crystal decay effects. Finally, the data were merged and scaled to produce a suitable data set. The absorption correction program SADABS50 was employed to correct the data for absorption effects. Systematic reflection conditions and statistical tests of the data suggested the space group. Solution was obtained readily using SHELXTL (XS).51 Hydrogen atoms were placed in idealized positions and were set riding on the respective parent atoms. All non-hydrogen atoms were refined with anisotropic thermal parameters. The structure was refined (weighted least squares refinement on F2) to convergence.51,52 Olex2 was employed for the final data presentation and structure plots.52
4.5. DNA binding studies
The interaction of metal complexes with CT DNA was carried out in Tris–HCl/NaCl buffer (pH 7.2). The bulk solution of CT DNA was prepared by diluting the CT DNA using Tris–HCl/NaCl buffer followed by stirring at 4 °C for three days, and kept at 4 °C for not more than a week. The stock solution of CT DNA gave a ratio of UV absorbance at 260 and 280 nm (A260/A280) of 1.89, indicating that the DNA was sufficiently free of proteins. The bulk DNA solution was further diluted to 10 folds to show maximum absorbance at 260 nm. The absorption coefficient of CT DNA was 6600 cm−1 M−1 per nucleotide.53 Cu(II) complexes of required concentration were prepared by dissolving the calculated amount of the complexes in 5% DMF/Tris–HCl/NaCl. Complex solution of concentration 15 μM was taken in cuvette and CT DNA of equivalent concentration (5–40 μM) was added each time and the significant absorbance change was noted.
The competitive binding of each complex with EB has been investigated by fluorescence spectroscopic technique in order to examine whether the complex can displace EB from its CT DNA–EB complex. Ethidium bromide solution was prepared using Tris–HCl/NaCl buffer (pH 7.2). The test solution was added in aliquots of 2.5 μM concentration to DNA–EB and the change in fluorescence intensities at 596 nm (450 nm excitation) was noted down.
4.6. Protein binding studies
The binding of copper(II) complexes (1–5) with BSA was studied using fluorescence spectra recorded at a fixed excitation wavelength corresponding to BSA at 280 nm and monitoring the emission at 335 nm. The excitation and emission slit widths and scan rates were constantly maintained for all the experiments. Stock solution of BSA was prepared in 50 mM phosphate buffer (pH = 7.2) and stored in the dark at 4 °C for further use. Concentrated stock solutions of each test compound were prepared by dissolving them in DMF–phosphate buffer (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) and diluted with phosphate buffer to get required concentrations. 2.5 mL of BSA solution was titrated by successive additions of a 10−6 M stock solution of the complexes using a micropipette. For synchronous fluorescence spectra measurements, the same concentration of BSA and the complexes were used and the spectra were measured at two different Δλ (difference between the excitation and emission wavelengths of BSA) values of 15 and 60 nm.
95) and diluted with phosphate buffer to get required concentrations. 2.5 mL of BSA solution was titrated by successive additions of a 10−6 M stock solution of the complexes using a micropipette. For synchronous fluorescence spectra measurements, the same concentration of BSA and the complexes were used and the spectra were measured at two different Δλ (difference between the excitation and emission wavelengths of BSA) values of 15 and 60 nm.
4.7. Cytotoxic studies
Cytotoxicity studies of the copper complexes were carried out on human breast (MCF7) and lung (A549) cancer cell lines. Cell viability was carried out using the MTT assay method.54 The non-small lung adenocarcinoma cells (A549 cells) and human breast cancer cells (MCF-7) were plated separately in 96 well plates at a concentration of 1 × 105 cells per well. Complexes (1–5) of concentration ranging from 10–200 μM dissolved in DMSO were seeded to the wells. DMSO was used as the control. It is important to mention here that complexes (1–5) are stable in DMSO. After 24 h, the wells were treated with 20 μL MTT [5 mg mL−1 phosphate buffered saline (PBS)] and incubated at 37 °C for 4 h. The purple formazan crystals formed were dissolved in 200 μL DMSO. The absorbance of the solution was measured at a wavelength of 570 nm using a Beckmann Coulter Elisa plate. Triplicate samples were analyzed for each experiment. The percentage inhibition was calculated using the formula.
Acknowledgements
K. J. thanks DST for fellowship under DST INSPIRE programme. We thank Sophisticated Analytical Instrumentation facility (SAIF), Indian Institute of Technology – Madras and Indian Institute of Technology – Bombay for NMR and EPR analyses respectively. R. K. gratefully acknowledges DST for the financial support under FIST programme.
References
- Y. B. Zeng, N. Yang, W. S. Liu and N. Tang, J. Inorg. Biochem., 2003, 97, 258–264 CrossRef CAS.
- R. Nagane, T. Koshigoe and M. Chikira, J. Inorg. Biochem., 2003, 93, 204–212 CrossRef CAS.
- A. Silvestri, G. Barone, G. Ruisi, M. Giudice and S. Tumminello, J. Inorg. Biochem., 2004, 98, 589–594 CrossRef CAS PubMed.
- A. T. Chaviara, P. C. Christidis, A. Papageorgiou, E. Chrysogelou, D. J. Hadjipavlou-Litina and C. A. Bolos, J. Inorg. Biochem., 2005, 99, 2102–2109 CrossRef CAS PubMed.
- T. Fujimori, S. Yamada, H. Yasui, H. Sakurai, Y. In and T. J. Ishida, J. Biol. Inorg. Chem., 2005, 10, 831–841 CrossRef CAS PubMed.
- F. L. Yin, J. Shen, J. J. Zou and R. C. Li, Acta Chim. Sin., 2003, 61, 556–561 CAS.
- P. M. May and D. R. Williams, in Metal Ions in Biological Systems, ed. H. Sigel, Marcel Dekker, New York, 1981, pp. 283–317 Search PubMed.
- T. Miura, A. Hori-i, H. Mototani and H. Takeuchi, Biochemistry, 1999, 38, 11560–11569 CrossRef CAS PubMed.
- C. Fernandes, G. L. Parrilha, J. A. Lessa, L. J. M. Santiago, M. M. Kanashiro, F. S. Boniolo, A. J. Bortoluzzi, N. V. Vugman, M. H. Herbst and A. Horn Jr, Inorg. Chim. Acta, 2006, 359, 3167–3176 CrossRef CAS PubMed.
- B. C. Bales, T. Kodama, Y. N. Weledji, M. Pitie, B. Meunier and M. M. Greenberg, Nucleic Acids Res., 2005, 33, 5371–5379 CrossRef CAS PubMed.
- P. K. M. Siu, D. L. Ma and C. M. Che, Chem. Commun., 2005, 1025–1027 Search PubMed.
- J. N. Tian, J. Q. Liu, X. Tian, Z. D. Hu and X. G. Chen, J. Mol. Struct., 2004, 691, 197–202 CrossRef CAS PubMed.
- J. Seetharamappa and B. P. Kamat, Chem. Pharm. Bull., 2004, 52, 1053–1057 CrossRef CAS.
- G. Durant, Chem. Soc. Rev., 1985, 14, 375–398 RSC.
- R. G. S. Berlinck, Nat. Prod. Rep., 1996, 13, 377–409 RSC.
- R. G. S. Berlinck, Nat. Prod. Rep., 1999, 16, 339–365 RSC.
- L. Heys, C. G. Moore and P. J. Murphy, Chem. Soc. Rev., 2000, 29, 57–67 RSC.
- P. J. Hajduk, S. Boyd, D. Nettesheim, V. Nienaber, J. Severin, R. Smith, D. Davidson, T. Rockway and S. W. Fesik, J. Med. Chem., 2000, 43, 3862–3866 CrossRef CAS PubMed.
- D. M. Evens, K. Sloanstakleff, M. Arvan and D. P. Guyton, Clin. Exp. Metastasis, 1998, 16, 353–357 CrossRef.
- J. Chern, Y. Leu, S. Wang, R. Jou, S. Hsu, Y. Liaw and H. Lin, J. Med. Chem., 1997, 40, 2276–2286 CrossRef CAS PubMed.
- Z. Brzozowski, F. Saczewski and M. Gdaniec, Eur. J. Med. Chem., 2002, 37, 285–293 CrossRef CAS.
- H. Fu, Y. H. Zhou, W. L. Chen, Z. G. Deqing, M. L. Tong, L. N. Ji and Z. W. Mao, J. Am. Chem. Soc., 2006, 128, 4924–4925 CrossRef CAS PubMed.
- M. J. Belousoff, L. Tjioe, B. Graham and L. Spiccia, Inorg. Chem., 2008, 47, 8641–8651 CrossRef CAS PubMed.
- G. Murtaza, A. Badshah, M. Said, H. Khan, A. Khan, S. Khan, S. Siddiq, M. I. Choudhary, J. Boudreauc and F. G. Fontainec, Dalton Trans., 2011, 40, 9202–9211 RSC.
- S. Cunha, M. T. Rodrigues, C. C. de Silva, H. B. Napolitano, I. Vencato and C. Laricci, Tetrahedron, 2005, 61, 10536–10540 CrossRef CAS PubMed.
- S. S. Batsanov, J. Mol. Struct., 2011, 990, 63–66 CrossRef CAS PubMed.
- B. Cordero, V. Gomez, A. E. Platero-Prats, M. Reves, J. Echeverria, E. Cremades, F. Barragan and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC.
- U. Schroder, L. Beyer, R. Richter, J. Angulo-Cornejo, M. Castillo-Montoya and M. Lino-Pacheco, Inorg. Chim. Acta, 2003, 353, 59–67 CrossRef CAS.
- L. Beyer, R. Richter, R. Wolf, J. Zaumseil, M. Lino-Pacheco and J. Angulo-Cornejo, Inorg. Chem. Commun., 1999, 2, 184–187 CrossRef CAS.
- M. J. Begley, P. Hubberstey and C. H. M. Moore, J. Chem. Res., Synop., 1986, 5, 172–173 Search PubMed.
- A. Tomas, B. Viossat, M. F. Charlot, J. J. Girerd and D. N. Huy, Inorg. Chim. Acta, 2005, 358, 3253–3258 CrossRef CAS PubMed.
- J. S. Guerrero, P. C. Sanchez, E. R. Perez, F. V. Garcia, M. E. B. Gomez and L. R. Azuara, Toxicol. in Vitro, 2011, 25, 1376–1384 CrossRef PubMed.
- A. M. Pyle, J. P. Rehmann, R. Meshoyrer, C. V. Kumar, N. J. Turro and J. K. Barton, J. Am. Chem. Soc., 1989, 111, 3051–3058 CrossRef CAS.
- A. Wolf, G. H. Shimer and T. Meehan, Biochemistry, 1987, 26, 6392–6396 CrossRef.
- N. Chitrapriya, T. Sathiya Kamatchi, M. Zeller, H. Lee and K. Natarajan, Spectrochim. Acta, Part A, 2011, 81, 128–134 CrossRef CAS PubMed.
- J. R. Lakowicz and G. Webber, Biochemistry, 1973, 12, 4161–4170 CrossRef CAS.
- B. C. Baguley and M. Lebret, Biochemistry, 1984, 23, 937–943 CrossRef CAS.
- W. D. Wilson, L. Ratmeyer, M. Zhao, L. Strekowski and D. Boykin, Biochemistry, 1993, 32, 4098–4104 CrossRef CAS.
- K. S. Ghosh, B. K. Sahoo, D. Jana and S. Dasgupta, J. Inorg. Biochem., 2008, 102, 1711–1718 CrossRef CAS PubMed.
- D. Senthil Raja, N. S. P. Bhuvanesh and K. Natarajan, Eur. J. Med. Chem., 2011, 46, 4584–4594 CrossRef PubMed.
- D. Senthil Raja, G. Paramaguru, N. S. P. Bhuvanesh, J. H. Reibenspies, R. Renganathan and K. Natarajan, Dalton Trans., 2011, 40, 4548–4559 RSC.
- J. R. Lakowicz, Fluorescence Quenching: Theory and Applications. Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, New York, 1999, pp. 53–127 Search PubMed.
- X. Z. Feng, Z. Yang, L. J. Wang and C. Bai, Talanta, 1998, 47, 1223–1229 CrossRef CAS.
- G. Z. Chen, X. Z. Huang, Z. Z. Zheng, J. G. Xu and Z. B. Wang, Analysis Method of Fluorescence, Science Press, Beijing, 3rd edn, 1990 Search PubMed.
- J. N. Miller, Proc. Anal. Div. Chem. Soc., 1979, 16, 203–208 CAS.
- P. Krishnamoorthy, P. Sathyadevi, A. H. Cowley, R. R. Butorac and N. Dharmaraj, Eur. J. Med. Chem., 2011, 46, 3376–3387 CrossRef CAS PubMed.
- T. K. Goswami, B. V. Chakravarthi, M. Roy, A. A. Karande and A. R. Chakravarty, Inorg. Chem., 2011, 50, 8452–8464 CrossRef CAS PubMed.
- S. Cunha, M. B. Costa, H. B. Napolitano, C. Lauriucci and I. Vecanto, Tetrahedron, 2001, 57, 1671–1675 CrossRef CAS.
- APEX2 “Program for Data Collection on Area Detectors”, BRUKER AXS Inc., 5465 East Cheryl Parkway, Madison, WI 53711-5373, USA.
- SADABS, G. M. Sheldrick, “Program for Absorption Correction of Area Detector Frames”, BRUKER AXS Inc., 5465 East Cheryl Parkway, Madison, WI 53711-5373, USA.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- M. E. Reichmann, S. A. Rice and P. Thomas, J. Am. Chem. Soc., 1954, 76, 3047–3053 CrossRef CAS.
- T. Mossman, J. Immunol. Methods, 1983, 65, 55–63 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH, is an important ingredient of both organic and inorganic chemistry. It is used to synthesize a number of biologically and pharmaceutically relevant compounds.14–17 Guanidine derivatives are very useful pharmacophores in medicinal chemistry due to their capacity to interact with functional groups present in enzymes or receptors through hydrogen bonds and electrostatic interactions. The guanidine group also act as a inhibitor of urokinase which plays a vital role in tumor metastasis which implicated in a large number of malignancies, including breast, lungs, bladder, stomach, cervix, kidney, and brain cancers.18,19 Various guanidine compounds have been synthesized and tested for their antitumor activity.20,21 Copper complexes containing guanidine ligands are also found to have many biological applications.22 For instance, copper(II) complexes of bis(2-pyridylmethyl)amine ligand with guanidinium pendant groups had an enhanced ability to cleave plasmid DNA and it can also act as RNA mimic.23 Copper(II) complexes with guanidine ligands have been recently reported as urease inhibitors.24 These stem a great interest to develop guanidine based copper(II) complexes for biological applications. Herein we report the synthesis and characterization of copper(II) complexes containing trisubstituted guanidine ligands. The interaction of the copper(II) complexes with CT-DNA and BSA was studied using spectrometric methods. We have also tested the in vitro cytotoxicity of the copper(II) complexes against MCF7 and A549 cancer cell lines.
NH, is an important ingredient of both organic and inorganic chemistry. It is used to synthesize a number of biologically and pharmaceutically relevant compounds.14–17 Guanidine derivatives are very useful pharmacophores in medicinal chemistry due to their capacity to interact with functional groups present in enzymes or receptors through hydrogen bonds and electrostatic interactions. The guanidine group also act as a inhibitor of urokinase which plays a vital role in tumor metastasis which implicated in a large number of malignancies, including breast, lungs, bladder, stomach, cervix, kidney, and brain cancers.18,19 Various guanidine compounds have been synthesized and tested for their antitumor activity.20,21 Copper complexes containing guanidine ligands are also found to have many biological applications.22 For instance, copper(II) complexes of bis(2-pyridylmethyl)amine ligand with guanidinium pendant groups had an enhanced ability to cleave plasmid DNA and it can also act as RNA mimic.23 Copper(II) complexes with guanidine ligands have been recently reported as urease inhibitors.24 These stem a great interest to develop guanidine based copper(II) complexes for biological applications. Herein we report the synthesis and characterization of copper(II) complexes containing trisubstituted guanidine ligands. The interaction of the copper(II) complexes with CT-DNA and BSA was studied using spectrometric methods. We have also tested the in vitro cytotoxicity of the copper(II) complexes against MCF7 and A549 cancer cell lines.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency of the ligands was observed at 1588–1568 cm−1. This is an intermediate value between double and single bonds, which shows the resonance between all the three nitrogen atoms in the guanidine moiety. The C
N stretching frequency of the ligands was observed at 1588–1568 cm−1. This is an intermediate value between double and single bonds, which shows the resonance between all the three nitrogen atoms in the guanidine moiety. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency appeared around 1632–1608 cm−1 in the spectra of the ligands was shifted to a lower value in the complexes, showing a single bond behaviour due to bonding with the Cu(II) ion. Further, the weak N–H band was disappeared, which indicates the coordination of N atom after deprotonation. The strong N–H band was appeared in the spectra of the complexes with minor shift towards higher value.
O stretching frequency appeared around 1632–1608 cm−1 in the spectra of the ligands was shifted to a lower value in the complexes, showing a single bond behaviour due to bonding with the Cu(II) ion. Further, the weak N–H band was disappeared, which indicates the coordination of N atom after deprotonation. The strong N–H band was appeared in the spectra of the complexes with minor shift towards higher value.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the regions 171.8–173.4 and 156.4–158.3 ppm respectively.25
N in the regions 171.8–173.4 and 156.4–158.3 ppm respectively.25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The –C(O)N
10. The –C(O)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NH–)(NH–) core exhibited a large amount of delocalization due to the Y-aromaticity, as can be observed by the C–N bond lengths [1.334–1.355 Å (L1), 1.332–1.353 Å (L2), 1.333–1.351 Å (L3), 1.329–1.350 Å (L4) and 1.329–1.356 Å (L5)].
C(NH–)(NH–) core exhibited a large amount of delocalization due to the Y-aromaticity, as can be observed by the C–N bond lengths [1.334–1.355 Å (L1), 1.332–1.353 Å (L2), 1.333–1.351 Å (L3), 1.329–1.350 Å (L4) and 1.329–1.356 Å (L5)].
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915
915![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382
382![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429
429![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 731
731![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427
427![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with Z of 1 and complex 5 crystallized in monoclinic C2/c space group with Z of 4. The Cu–N bonds are longer than the Cu–O bonds [Cu(1)–O(1) 1.9087 Å, Cu(1)–N(1) 1.9589 Å (3) and Cu(1)–O(1) 1.9069 Å Cu(1)–N(1) 1.9688 Å (5)], which are in the expected range for guanidine complexes.28–31 Two guanidine ligands are coordinated to Cu(II) ion in a trans fashion. There is an increase in the C–O bond length and decrease in the C–N bond (involved in coordination) length in 3 and 5 compared to L3 and L5, respectively.
space group with Z of 1 and complex 5 crystallized in monoclinic C2/c space group with Z of 4. The Cu–N bonds are longer than the Cu–O bonds [Cu(1)–O(1) 1.9087 Å, Cu(1)–N(1) 1.9589 Å (3) and Cu(1)–O(1) 1.9069 Å Cu(1)–N(1) 1.9688 Å (5)], which are in the expected range for guanidine complexes.28–31 Two guanidine ligands are coordinated to Cu(II) ion in a trans fashion. There is an increase in the C–O bond length and decrease in the C–N bond (involved in coordination) length in 3 and 5 compared to L3 and L5, respectively.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kb + n
Kb + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Q]
log[Q]
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 301 (26
300), 301 (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). FT-IR (KBr, ν cm−1): 3279, 3203 (N–H), 1611 (C
900). FT-IR (KBr, ν cm−1): 3279, 3203 (N–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1568 (C
O), 1568 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, DMSO-d6): δ, ppm 2.26 (s, 3H), 7.44–7.011 (m, 9H), 7.65 (d, J = 3.6 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.08 (t, J = 3.6 Hz, 1H), 10.46 (s, 1H), 10.09 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ, ppm 21.0 (aliphatic CH3), 123.9, 124.2, 125.1, 128.4, 129.9, 130.8, 132.1, 134.7. 135.1, 138.1, 144.4 (aromatic C), 156.4 (C
N). 1H NMR (400 MHz, DMSO-d6): δ, ppm 2.26 (s, 3H), 7.44–7.011 (m, 9H), 7.65 (d, J = 3.6 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.08 (t, J = 3.6 Hz, 1H), 10.46 (s, 1H), 10.09 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ, ppm 21.0 (aliphatic CH3), 123.9, 124.2, 125.1, 128.4, 129.9, 130.8, 132.1, 134.7. 135.1, 138.1, 144.4 (aromatic C), 156.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 171.8 (C
N), 171.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4197.
O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4197.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 302 (16
500), 302 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800). FT-IR (KBr): ν, cm−1 3394, 3165 (N–H), 1602 (C
800). FT-IR (KBr): ν, cm−1 3394, 3165 (N–H), 1602 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1571 (C
O), 1571 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3) δ, ppm: 7.09 (t, J = 4 Hz, 1H), 7.07–7.46 (m, 11H), 7.79 (d, J = 3.6 Hz, 1H), 10.30 (s, 2H). 13C NMR (100 MHz, CDCl3): δ, ppm 124.1, 126.1, 127.7, 129.5, 130.9, 131.1, 136.3, 144.4 (aromatic C), 156.1 (C
N). 1H NMR (400 MHz, CDCl3) δ, ppm: 7.09 (t, J = 4 Hz, 1H), 7.07–7.46 (m, 11H), 7.79 (d, J = 3.6 Hz, 1H), 10.30 (s, 2H). 13C NMR (100 MHz, CDCl3): δ, ppm 124.1, 126.1, 127.7, 129.5, 130.9, 131.1, 136.3, 144.4 (aromatic C), 156.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 173.3 (C
N), 173.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS calcd for C18H15N3OS: 321.3962 found: 321.4001.
O). HRMS calcd for C18H15N3OS: 321.3962 found: 321.4001.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 296 (28
000), 296 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500). FT-IR (KBr): ν, cm−1 3267, 3202 (N–H), 1611 (C
500). FT-IR (KBr): ν, cm−1 3267, 3202 (N–H), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1569 (C
O), 1569 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3): δ, ppm 4.72 (s, 2H), 5.25 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.43–7.23 (m, 11H), 7.80 (d, J = 2.4 Hz, 1H), 11.82 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 45.2 (aliphatic, CH2), 125.7, 127.2, 127.7, 127.8, 128.8, 130.2, 130.5, 130.7, 135.8, 144.8 (aromatic C), 158.2 (C
N). 1H NMR (400 MHz, CDCl3): δ, ppm 4.72 (s, 2H), 5.25 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.43–7.23 (m, 11H), 7.80 (d, J = 2.4 Hz, 1H), 11.82 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 45.2 (aliphatic, CH2), 125.7, 127.2, 127.7, 127.8, 128.8, 130.2, 130.5, 130.7, 135.8, 144.8 (aromatic C), 158.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 173.4 (C
N), 173.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4194.
O). HRMS calcd for C19H17N3OS: 335.4228 found: 335.4194.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). FT-IR (KBr): ν, cm−1 3341, 3230 (N–H), 1607 (C
300). FT-IR (KBr): ν, cm−1 3341, 3230 (N–H), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556 (C
O), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3): δ, ppm 0.92–0.95 (t, J = 5.6 Hz, 3H), 1.34–1.38 (q, J = 5.6 Hz, 2H), 1.56–1.55 (t, J = 5.6 Hz, 2H), 3.46 (s, 2H), 4.96 (s, 1H), 7.68–7.05 (m, 8H), 11.69 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 13.9, 20.1, 31.9, 41.2 (aliphatic), 125.6, 127.1, 130.2, 130.3, 130.5, 135.9, 140.5 (aromatic C), 158.3 (C
N). 1H NMR (400 MHz, CDCl3): δ, ppm 0.92–0.95 (t, J = 5.6 Hz, 3H), 1.34–1.38 (q, J = 5.6 Hz, 2H), 1.56–1.55 (t, J = 5.6 Hz, 2H), 3.46 (s, 2H), 4.96 (s, 1H), 7.68–7.05 (m, 8H), 11.69 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 13.9, 20.1, 31.9, 41.2 (aliphatic), 125.6, 127.1, 130.2, 130.3, 130.5, 135.9, 140.5 (aromatic C), 158.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 172.8 (C
N), 172.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); HRMS calcd for C16H19N3OS: 301.4066 found: 301.4001.
O); HRMS calcd for C16H19N3OS: 301.4066 found: 301.4001.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). FT-IR (KBr): ν, cm−1 3307, 3207 (N–H), 1608 (C
300). FT-IR (KBr): ν, cm−1 3307, 3207 (N–H), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1553 (C
O), 1553 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, CDCl3): δ, ppm 2.05–1.15 (m, 11H), 4.80 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.41–7.23 (m, 6H), 7.77 (s, 1H), 11.71 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 24.9, 25.6, 33.1, 50.4 (aliphatic C), 125.3, 126.8, 127.1, 127.7, 130.2, 136.1, 145.1 (aromatic C), 157.3 (C
N). 1H NMR (400 MHz, CDCl3): δ, ppm 2.05–1.15 (m, 11H), 4.80 (s, 1H), 7.07 (t, J = 3.6 Hz, 1H), 7.41–7.23 (m, 6H), 7.77 (s, 1H), 11.71 (s, 1H). 13C NMR (100 MHz, CDCl3): δ, ppm 24.9, 25.6, 33.1, 50.4 (aliphatic C), 125.3, 126.8, 127.1, 127.7, 130.2, 136.1, 145.1 (aromatic C), 157.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 172.7 (C
N), 172.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); HRMS calcd for C18H21N3OS: 327.4438 found: 327.4397.
O); HRMS calcd for C18H21N3OS: 327.4438 found: 327.4397.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 298 (30
300), 298 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 604 (147). FT-IR (KBr): ν, cm−1 3394 (N–H), 1594 (C
000), 604 (147). FT-IR (KBr): ν, cm−1 3394 (N–H), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1560 (C
O), 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K): ‘g’ values 2.286, 2.047. EPR (LNT): ‘g’ values 2.220, 2.038.
N). EPR (300 K): ‘g’ values 2.286, 2.047. EPR (LNT): ‘g’ values 2.220, 2.038.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 306 (25
500), 306 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), 601 (88). FT-IR (KBr): ν, cm−1 3402 (N–H), 1593 (C
600), 601 (88). FT-IR (KBr): ν, cm−1 3402 (N–H), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556 (C
O), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.385, 2.047 EPR (LNT, ‘g’ value): 2.223, 2.046.
N). EPR (300 K, ‘g’ value): 2.385, 2.047 EPR (LNT, ‘g’ value): 2.223, 2.046.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), 298 (29
100), 298 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 603 (154). FT-IR (KBr): ν, cm−1 3421 (N–H), 1557 (C
000), 603 (154). FT-IR (KBr): ν, cm−1 3421 (N–H), 1557 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1533 (C
O), 1533 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.204, 2.043 EPR (LNT, ‘g’ value): 2.217, 2.042.
N). EPR (300 K, ‘g’ value): 2.204, 2.043 EPR (LNT, ‘g’ value): 2.217, 2.042.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 298 (28
900), 298 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 626 (298). FT-IR (KBr): ν, cm−1 3418 (N–H), 1560 (C
900), 626 (298). FT-IR (KBr): ν, cm−1 3418 (N–H), 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1537 (C
O), 1537 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.203, 2.022 EPR (LNT, ‘g’ value): 2.218, 2.042.
N). EPR (300 K, ‘g’ value): 2.203, 2.022 EPR (LNT, ‘g’ value): 2.218, 2.042.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), 608 (66). FT-IR (KBr): ν, cm−1 3418 (N–H), 1566 (C
600), 608 (66). FT-IR (KBr): ν, cm−1 3418 (N–H), 1566 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1532 (C
O), 1532 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EPR (300 K, ‘g’ value): 2.283, 2.047 EPR (LNT, ‘g’ value): 2.217, 2.049.
N). EPR (300 K, ‘g’ value): 2.283, 2.047 EPR (LNT, ‘g’ value): 2.217, 2.049.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) and diluted with phosphate buffer to get required concentrations. 2.5 mL of BSA solution was titrated by successive additions of a 10−6 M stock solution of the complexes using a micropipette. For synchronous fluorescence spectra measurements, the same concentration of BSA and the complexes were used and the spectra were measured at two different Δλ (difference between the excitation and emission wavelengths of BSA) values of 15 and 60 nm.
95) and diluted with phosphate buffer to get required concentrations. 2.5 mL of BSA solution was titrated by successive additions of a 10−6 M stock solution of the complexes using a micropipette. For synchronous fluorescence spectra measurements, the same concentration of BSA and the complexes were used and the spectra were measured at two different Δλ (difference between the excitation and emission wavelengths of BSA) values of 15 and 60 nm.



















