High-strain shape memory polymers with movable cross-links constructed by interlocked slide-ring structure†
Yaru Wangab,
Xingjian Liab,
Yi Pana,
Zhaohui Zhenga,
Xiaobin Ding*a and
Yuxing Penga
aChengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, China. E-mail: xbding@cioc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 31st March 2014
Abstract
A novel type of shape memory polymer network with movable cross-links is designed and fabricated, which is different from the conventional chemically cross-linked or physically cross-linked networks. The unique interlocked slide-ring structure endows the material with outstanding shape memory performance even at high strain.
Shape memory polymers (SMPs) are a promising class of stimuli-responsive materials that have the ability to recover their permanent shape in a predefined way from the temporary shape, when they are responsive to external stimuli such as heat,1–3 light,4,5 electricity,6,7 magnetic field,8,9 and chemicals.10–12 In recent years, SMPs have received more and more attention because of their scientific and technological significance. SMPs have great potential application in many areas, including biomedicine,13 aerospace engineering,14 information storage,15 and intelligent textiles,16 owing to their shape memory performances and multifunctional characteristics. However, they also show some defects that limit their application, such as poor mechanical properties, unsatisfactory shape memory performance, slow recovery speed, and short cycle life. Such limitations necessitate the innovation of novel SMPs with unique structure and composition.
At the molecular level, SMPs are elastic polymer networks which consist of netpoints and switches. The netpoints determine the permanent shape of polymer network and can be of a chemical or physical nature. The switches are the major constituents and are responsible for controlling the shape fixity and recovery upon a specific and predetermined external stimulus.17 The shape memory capacity of polymers lies in the entropy-driven tendency for polymer chains to adopt a randomly coiled configuration. This entropically driven process is governed by the cooperative action of the netpoints and switching segments. The intrinsic mechanism for shape memory capacity of polymers is the freezing and activation of the long-range motion of polymer chain segments below and above the transition temperature (Ttrans), respectively.18 Therefore, it is not difficult to understand that the distribution of the interaction forces between the molecular chains plays a crucial role in shape memory capacity.
Structurally, netpoints which determine the permanent shape can either be chemical (covalent bond) or physical (non-covalent bond) cross-linking. Chemically cross-linked networks are rigid. Although could improve the strength of materials, chemically cross-linked networks restrict the movement of the molecular chains. As a result, high-strain could not be achieved. In contrast, physically cross-linked networks, in which polymer chains interact with each other through reversible cross-linkages such as hydrogen bonding, ionic interaction and hydrophobic interaction, can reversible dissociation and formation. However, their mechanical strength and stability is poor. Compared with the chemically cross-linked ones, physically cross-linked networks often possess high-strain capacities, but shape recovery at high strain is really unsatisfactory. Irreversible shape recovery caused by the slipping of molecular chains during stretching will occur. The creation of fully recoverable high-strain SMPs may facilitate further innovation into the next generation of smart devices.
Besides of the above two cross-linking mode, can we design and construct a new type of cross-linking unlike the conventional chemical or physical cross-linking, to regulate the interaction force between the molecular chains fundamentally, eventually imparting the material with new structure and excellent shape memory properties? Recently, a novel cross-linked polymer network reported by Ito has attracted our attention.19 The polymer chains are cross-linked neither by covalent bonds as in a chemical network nor by attractive interactions as in a traditional physical network, but are interlocked by movable cross-links. They are considered as an intermediate between physical and chemical cross-links. As the ring-like molecules can rotate and slide along the polymer chains, in an analogy to a pulley, the interactions between the polymer chains can be equalized, leading the network homogeneous. The materials with movable cross-links show excellent swelling capacity and mechanical property.19–22 The slide-ring material is a new cross-linking concept for the polymer network as well as a real example of a slip-link model,23 which was previously considered only theoretically.
Here we design a novel SMPs network with movable cross-links, constructed by topologically interlocked slide-ring structure based on the sliding phenomenon (Scheme 1). Thermally reversible methyl acrylate (MA) and methyl methacrylate (MMA) copolymer were used as switching segments, which were responsible for controlling the shape fixity and recovery upon heating. Modified α-cyclodextrin (α-CD) in polyrotaxane was used as a movable cross-linker, which determined the permanent shape. As the cross-links are capable of sliding and rotating along the polymer chains during the deformation process, the movement of the molecular chains in the movable cross-linked networks will not be restricted as in chemically cross-linked networks, besides, the slippage of the molecular chains will not occur as in physically cross-linked networks. These cross-links can pass along the polymer chains to equalize the tension of the polymer chains similarly to pulleys. Hence, polymer chain segments movement can achieve complete freezing below Ttrans and full activation above Ttrans. Consequently, the unique interlocked slide-ring structure will endow the material with high strain and outstanding shape memory performance even at high strain.
The preparation of polyrotaxane (PR) and modified polyrotaxane (MPR) are shown in Experimental section. The slide-ring materials were prepared by free radical polymerization using modified α-CD in PR as a cross-linker, MA and MMA as monomers and AIBN as an initiator. The amount of the cross-linker was 1 mol% based on monomer content (see ESI, Table S1†). Typical networks with chemical and physical cross-links were also prepared under identical conditions and were used as references against the slide-ring materials. EGDMA was chosen as a cross-linker of chemical cross-linked network instead of α-CD and with the same amount of the cross-linker. The notation and compositions of samples are listed in Table S1.† The samples are denominated as MC (movable cross-linking)-SMP, CC (chemical cross-linking)-SMP and PC (physical cross-linking)-SMP, respectively.
Dynamic mechanical analysis was carried out to determine the glass transition temperature (Tg) and the storage modulus of the three series of networks on a TA Q800 DMA. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and the storage modulus of the specimen as a function of temperature are shown in Fig. 1. According to viscoelastic and thermomechanical models that correlate the viscoelastic/thermal properties to the shape-memory behavior of SMPs, the ratio of glassy modulus to rubbery modulus (Eg/Er) plays a key role on shape-memory performance.24 As shown in Fig. 1, all samples exhibited similar temperature-dependent viscoelastic properties. An exciting revelation from these experiments was the observation of all samples exhibited sharp storage modulus changes of up to 3 orders of magnitude around the Tg respectively, and the storage modulus drop around the Tg was more pronounced for MC-SMP. This may be attributed to the different cross-linked networks and will endow the material with excellent shape memory performance.
δ and the storage modulus of the specimen as a function of temperature are shown in Fig. 1. According to viscoelastic and thermomechanical models that correlate the viscoelastic/thermal properties to the shape-memory behavior of SMPs, the ratio of glassy modulus to rubbery modulus (Eg/Er) plays a key role on shape-memory performance.24 As shown in Fig. 1, all samples exhibited similar temperature-dependent viscoelastic properties. An exciting revelation from these experiments was the observation of all samples exhibited sharp storage modulus changes of up to 3 orders of magnitude around the Tg respectively, and the storage modulus drop around the Tg was more pronounced for MC-SMP. This may be attributed to the different cross-linked networks and will endow the material with excellent shape memory performance.
 | ||
| Fig. 1 Storage modulus-temperature and loss angle-temperature curves of MC-SMP, CC-SMP and PC-SMP, respectively. | ||
An example of the macroscopic shape memory effect of MC-SMP is demonstrated in Fig. 2. A pentagram was changed into a compressive shape above Tg, and then cooled rapidly to room temperature (25 °C). Once it was heated to a temperature above Tg again, the specimen recovered its original shape within 30 s.
Quantitative assessment of the shape memory performance was carried out through cyclic thermomechanical experiment on DMA Q800. The temperature for stress deformation was selected to be Tg + 15 °C, respectively. And the deformation strain was 50% for all samples. As presented in Fig. 3, MC-SMP showed excellent shape memory performance compared to the other two specimens. The shape fixity ratio of three series were all above 98%, suggesting that all samples could be stably fixed at a temporary shape upon cooling, which indicated complete freezing of chain segment motions below the Tg in all networks. The shape recovery property of PC-SMP was poor, due to the instability of physical cross-links on tensile deformation. Apparently, as shown in Table S2,† MC-SMP showed excellent shape recovery with shape recovery ratio near 100%, which was better than those of the conventional CC-SMP and PC-SMP. These observations are in accordance to the result that the modulus ratio of MC-SMP was higher than that of the other two specimens. A high storage modulus ratio may imply good shape fixity on cooling and a large shape recovery upon heating.25
 | ||
| Fig. 3 Cyclic thermomechanical experiments on MC-SMP, CC-SMP and PC-SMP: (1) tensile deformation, (2) cooling, (3) unloading of tensile stress, (4) recovering. | ||
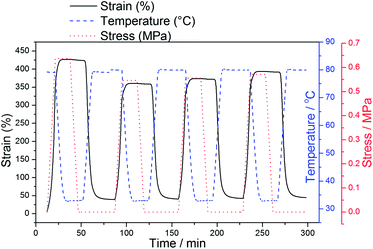
Furthermore, when MC-SMP was subjected to 4 cycles at 400% deformation strain, it still maintained excellent shape memory properties without any defects (as shown in Fig. 4). It exhibited a high shape fixity ratio (>99%) and shape recovery ratio (>96%). Although the deformation strain of PC-SMP could achieve 400%, the shape recovery capacity was poor, due to the disentanglement of cross-links or the slippage of molecular chains (Fig. S6†). The maximum deformation strain of CC-SMP could only achieve 200%. In addition, the sample cracked in the third cycle (Fig. S6†). These observations indicate the MC-SMP is capable of achieving large deformation, moreover, it shows excellent shape memory performance and recyclability, compared to PC-SMP and CC-SMP. At the same time, the results further proved that MC-SMP exhibits outstanding mechanical properties. Through tensile test (Fig. S8†), we have discovered that the elongation at break of MC-SMP was significantly better than that of CC-SMP, which may be attributed to inhomogeneties of spatial network in chemical cross-linking system. The cross-linking cannot avoid the localization of the stress due to the heterogeneous polymer chains length between fixed cross-links under external force. As a result, the polymer chains in CC-SMP are gradually broken. On the other hand, the movable cross-links in MC-SMP can rotate and slide along the polymer chains, the tension of the polymer chains can be equalized. This property may diminish the localization of the stress; eventually the elongation at break of MC-SMP is significantly better than that of CC-SMP at the same content of cross-linkers.
It was surprised to find that MC-SMP still showed excellent shape memory performance with Rf = 94.4%, Rr = 92.5%, even at 800% deformation strain in our experiment (Fig. 5, S7 and Table S3†), due to the movable netpoints effect. Whereas the shape recovery of the PC-SMP was poor, resulting in large residual strains. This is maybe because of the disentanglement of cross-links or the slippage of molecular chains during the programming process, which will not recover their initial state when the temperature is raised.
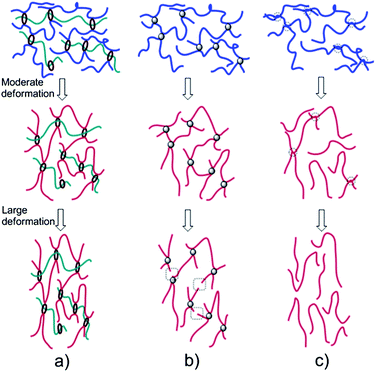
A possible mechanism for the shape memory effect of slide-ring polymer network is proposed as follows: the thermally reversible polyacrylate amorphous phase serves as a “switch”, which determines the temporary shape, and the slide-ring cross-links are responsible for determining the permanent shape. As shown in Fig. 6, under moderate tensile deformation process, the coiled segments of the chains are stretched and elongated. The disentanglement of cross-links may occur in PC-SMP, which will affect the shape recovery capacity in the recovery process. The stress in MC-SMP can distribute more homogenously than that in CC-SMP owing to the sliding of the cross-linking points, which contributes to complete freezing and activation of the long-range motion of polymer chain segments below and above the Tg, respectively. Thus MC-SMP shows excellent shape memory capacity. When the deformation further increasing, the disentanglement of cross-links and the slippage of molecular chains will occur, which lead poor shape recovery capacity of PC-SMP. Besides, the chemical cross-linking cannot avoid the localization of the stress due to the heterogeneous polymer chains length between fixed cross-links under external force. As a result, the short polymer chains in CC-SMP may be broken, which will cause CC-SMP damaging under large deformation. Whereas the cross-links in MC-SMP can rotate and slide along the polymer chains, the tension of the polymer chains can be equalized, which may diminish the localization of the stress. As a result, the MC-SMP still exhibit excellent shape memory performance at high strain.
Conclusions
In conclusion, we have designed and fabricated a novel type of the SMPs with topologically interlocked slide-ring structure that exhibits an extraordinary combination of outstanding shape memory capability even at high-strain with excellent mechanical properties. The key structural feature of the network is the movable netpoints that enable the whole network more homogeneous and chain–chain interactions tunable, achieving complete freezing and activation of the long-range motion of polymer chain segments below and above Ttrans, respectively. It is expected that this type of SMP will not only meet more requirements of the increasingly complex application, but also stimulate the development of high-performing shape memory polymers.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (Grant no. 51173185 and 51303179) for the financial support of this research.Notes and references
- J. H. Li, J. A. Viveros, M. H. Wrue and M. Anthamatten, Adv. Mater., 2007, 19, 2851–2855 CrossRef CAS PubMed.
- T. Liu, J. Li, Y. Pan, Z. H. Zheng, X. B. Ding and Y. X. Peng, Soft Matter, 2011, 7, 1641–1643 RSC.
- R. Xiao, J. W. Choi, N. Lakhera, C. M. Yakacki, C. P. Frick and T. D. Nguyen, J. Mech. Phys. Solids, 2013, 61, 1612–1635 CrossRef PubMed.
- A. Lendlein, H. Jiang, O. Junger and R. Langer, Nature, 2005, 434, 879–882 CrossRef CAS PubMed.
- H. Koerner, G. Price, N. A. Pearce, M. Alexander and R. A. Vaia, Nat. Mater., 2004, 3, 115–120 CrossRef CAS PubMed.
- X. F. Luo and P. T. Mather, Soft Matter, 2010, 6, 2146–2149 RSC.
- H. B. Lu, Y. J. Liu and J. S. Leng, Macromol. Mater. Eng., 2012, 297, 1138–1147 CrossRef CAS PubMed.
- R. Mohr, K. Kratz, T. Weigel, M. Lucka-Gabor, M. Moneke and A. Lendlein, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 3540–3545 CrossRef CAS PubMed.
- M. Y. Razzaq, M. Behl and A. Lendlein, Adv. Funct. Mater., 2012, 22, 184–191 CrossRef CAS PubMed.
- J. R. Kumpfer and S. J. Rowan, J. Am. Chem. Soc., 2011, 133, 12866–12874 CrossRef CAS PubMed.
- Z. Q. Dong, Y. Cao, Q. J. Yuan, Y. F. Wang, J. H. Li, B. J. Li and S. Zhang, Macromol. Rapid Commun., 2013, 34, 867–872 CrossRef CAS PubMed.
- R. Xiao and T. D. Nguyen, Soft Matter, 2013, 39, 9455–9464 RSC.
- M. C. Serrano and G. A. Ameer, Macromol. Biosci., 2012, 12, 1156–1171 CrossRef CAS PubMed.
- R. Vaia and J. Baur, Science, 2008, 319, 420–421 CrossRef CAS PubMed.
- H. J. Yoo, Y. C. Jung, N. G. Sahoo and J. W. Cho, J. Macromol. Sci. Phys., 2006, 45, 441–451 CrossRef CAS.
- J. L. Hu, Y. Zhu, H. H. Huang and J. Lu, Prog. Polym. Sci., 2012, 37, 1720–1723 CrossRef CAS PubMed.
- J. L. Hu and S. J. Chen, J. Mater. Chem., 2010, 20, 3346–3355 RSC.
- J. W. Xu and J. Song, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7652–7657 CrossRef CAS PubMed.
- Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485–587 CrossRef CAS.
- A. B. Imran, T. Seki, T. Kataoka, M. Kidowaki, K. Ito and Y. Takeoka, Chem. Commun., 2008, 41, 5227–5229 RSC.
- J. Araki, T. Kataoka and K. Ito, Soft Matter, 2008, 4, 245–249 RSC.
- A. B. Imran, T. Seki, K. Ito and Y. Takeoka, Macromolecules, 2010, 43, 1975–1980 CrossRef.
- R. C. Ball, M. Doi, S. F. Edwards and M. Warner, Polymer, 1981, 22, 1010–1018 CrossRef CAS.
- Y. P. Liu, K. Gall, M. L. Dunn, A. R. Greenberg and J. Diani, Int. J. Plast., 2006, 22, 279–313 CrossRef CAS PubMed.
- G. Q. Liu, X. B. Ding, Y. P. Cao, Z. H. Zheng and Y. X. Peng, Macromolecules, 2004, 37, 2228–2232 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Fig. S1–S8 and Table S1–S3. See DOI: 10.1039/c4ra00165f |
| This journal is © The Royal Society of Chemistry 2014 |