An anti-galvanic reduction single-molecule fluorescent probe for detection of Cu(II)†
Shuxin Wang,
Xiangming Meng,
Yang Feng,
Hongting Sheng and
Manzhou Zhu*
Department of Chemistry, Anhui University, Hefei, Anhui 230601, P. R. China. E-mail: zmz@ahu.edu.cn; Fax: +86-551-63861487; Tel: +86-551-63861487
First published on 29th January 2014
Abstract
An anti-galvanic reduction method using [Ag62S13(SBut)32]4+ nanoclusters as the metal ion probe for detecting Cu2+ is reported here. We found [Ag62S13(SBut)32]4+ can reduce more reactive Cu2+ with a quantitative relationship. The single molecule fluorescence imaging shows that Ag62 NCs could be used as a single molecule probe for detecting Cu2+.
Fluorescent nanomaterials have recently attracted increasing attention in optical sensing1 and biolabeling.2 Among these materials, much attention has been paid to noble metal clusters (Ag, Au) consisting of a few to dozens of metal atoms.3 Several ultra-small fluorescent nanoparticles (NPs) of noble metal such as gold and silver have been synthesized by using different ligands like DHLA,4 dendrimers,5 polymers,6 DNA,7 peptides and proteins.8 They are particularly stable and can exhibit fluorescence ranging from the visible to the near infrared region depending on the number of metal atoms.9 Meanwhile, Single-nanoparticle imaging has been widely used to elucidate the optical properties of nanoparticles by correlating the optical responses with their well-defined structures and morphologies.10 However, up to now, the applications of these NPs in analyzing and sensing are mainly based on the fluorescence resonance energy transfer (FRET) mechanism with NPs used as the reporting groups, which means an organic fluorophores would be connected as a receptor group.11 Now, researchers pay more attention to the direct detection of the target ions,12 but a fundamental understanding of its process is still far from complete.
Recently, Wu reported an anti-galvanic reduction (AGR) in which the reducing activity of the ultra-small silver nanoparticles (less than 3 nm) is drastically enhanced and they can even reduce some more reactive metal ions (e.g. reducing Cu2+ to Cu0).13 It is very interesting to answer questions such as: (i) how does the fluorescence change during the AGR process? (ii) Could this changing process meet the requirements of ion recognition, such as a fast, accurate and quantitative determination?
Herein, we synthesized [Ag62S13(SBut)32]4+ NCs referring to the reported method with the definite structure in the proper size,14 and the ligand species of [Ag62S13(SBut)32]4+ also meet the requirements of the AGR process. Meanwhile, we found that the Cu2+ ions can be reduced by [Ag62S13(SBut)32]4+ NCs with a quantitative relationship. Both the optical properties and the single-molecule fluorescence of [Ag62S13(SBut)32]4+ NCs are studied. This discovery will help us design nanocluster sensors for detecting metal ions.
Ion selectivity study was performed in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
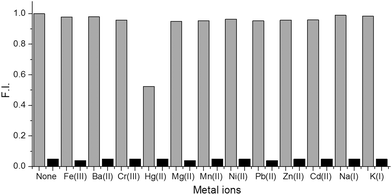
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution (as shown in Fig. 1). The data also illustrate that Co2+, Cd2+, Mn2+, Hg2+, Fe2+, Ni2+, and Cu2+ cause negligible interference to the fluorescence of [Ag62S13(SBut)32]4+ while Hg2+ had a slight effect. The aqueous solutions of [Ag62S13(SBut)32]4+ with the addition of different ions clearly demonstrated that Cu2+ changed the color of the solution dramatically, while other ions caused no perceivable changes. This result suggests that AGR process is too limited to reduce metal ions which have much higher reducing activity (e.g. Pb2+ and Fe3+).
1 solution (as shown in Fig. 1). The data also illustrate that Co2+, Cd2+, Mn2+, Hg2+, Fe2+, Ni2+, and Cu2+ cause negligible interference to the fluorescence of [Ag62S13(SBut)32]4+ while Hg2+ had a slight effect. The aqueous solutions of [Ag62S13(SBut)32]4+ with the addition of different ions clearly demonstrated that Cu2+ changed the color of the solution dramatically, while other ions caused no perceivable changes. This result suggests that AGR process is too limited to reduce metal ions which have much higher reducing activity (e.g. Pb2+ and Fe3+).
It is imperative to confirm whether the reaction of [Ag62S13(SBut)32]4+ and Cu2+ ions is quantitative, which is a prerequisite for the quantitative measurement. To illustrate this, absorption (Fig. 2a) and fluorescence (Fig. 2b) spectra were collected in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution. As shown in Fig. 2a, the absorption spectrum of [Ag62S13(SBut)32]4+ exhibits two shoulders in the UV region and a distinct absorption peak in the visible region (543 nm), which is consistent with the reported results.14 Upon addition of Cu2+, the absorption peak at 543 nm shifted to 534 nm gradually, with a color change of the solution from red to yellow. After addition of Cu2+, the emission at 598 nm was quenched (Fig. 2b). The change of fluorescence is not the same as Au NCs. Li reported that the fluorescence of Au16@BSA had a blue shift and was enhanced after the addition of Ag+.12d The mechanism of the fluorescence quenching by Cu2+ ions still needs to be studied in future work. It should be noted that the minimal fluorescence intensity emerges when the [Ag62S13(SBut)32]4+/Cu2+ molar ratio reaches 1 with a detection limit of 0.5 nM at a signal-to-noise ratio (S/N) of 3. But with the increase of the concentration of [Ag62S13(SBut)32]4+ NCs (e.g. 50 μM), more copper ions will be required to render the fluorescence completely quenched. We deduced that one [Ag62S13(SBut)32]4+ molecular could react with more than one copper ions, thereby complete quenching fluorescence of [Ag62S13(SBut)32]4+ needs a large amount of copper ions.
1 solution. As shown in Fig. 2a, the absorption spectrum of [Ag62S13(SBut)32]4+ exhibits two shoulders in the UV region and a distinct absorption peak in the visible region (543 nm), which is consistent with the reported results.14 Upon addition of Cu2+, the absorption peak at 543 nm shifted to 534 nm gradually, with a color change of the solution from red to yellow. After addition of Cu2+, the emission at 598 nm was quenched (Fig. 2b). The change of fluorescence is not the same as Au NCs. Li reported that the fluorescence of Au16@BSA had a blue shift and was enhanced after the addition of Ag+.12d The mechanism of the fluorescence quenching by Cu2+ ions still needs to be studied in future work. It should be noted that the minimal fluorescence intensity emerges when the [Ag62S13(SBut)32]4+/Cu2+ molar ratio reaches 1 with a detection limit of 0.5 nM at a signal-to-noise ratio (S/N) of 3. But with the increase of the concentration of [Ag62S13(SBut)32]4+ NCs (e.g. 50 μM), more copper ions will be required to render the fluorescence completely quenched. We deduced that one [Ag62S13(SBut)32]4+ molecular could react with more than one copper ions, thereby complete quenching fluorescence of [Ag62S13(SBut)32]4+ needs a large amount of copper ions.
XPS is employed to determine whether the Cu2+ is reduced to Cu0 or not. After an equivalent of Cu2+ was added, the [Ag62S13(SBut)32]4+ NCs were washed with deionized water and treated by vacuum drying before XPS measurement. The Ag 3d peak (Fig. 3a) in [Ag62S13(SBut)32]4+–Cu2+ (367.32 eV) was observed on the oxidation side comparing to that of [Ag62S13(SBut)32]4+ NCs (367.45 eV), indicating that the Ag atoms in Ag62–Cu2+ are positively charged. In contrast, the Cu 2p peak (Fig. 3b) in Ag62–Cu2+ is 932.7 eV, which is assigned to Cu 2p3/2, indicating that the incorporated copper is neutral (932.6 eV for pure copper Cu 2p3/2). The observation implies that copper ions can be reduced by more inert Ag NCs.
 | ||
| Fig. 3 (a) Binding energies of Ag 3d in [Ag62S13(SBut)32]4+ NCs and Ag62–Cu2+, respectively. (b) Binding energies of Cu 2p in Ag62–Cu2+. | ||
TEM can effectively determine whether the size of clusters has changed after reaction, in particular whether the incorporation of Cu2+ decomposes the clusters. As shown in Fig. S3a,† the TEM images reveal that the synthesized [Ag62S13(SBut)32]4+ NCs are in high monodispersity. After an equimolar amount of Cu2+ was added (Fig. S3b†), no significant size change was observed, which indicates that the fluorescence quenching is not induced by the decomposition or size growth of the [Ag62S13(SBut)32]4+ NCs.
MALDI-TOF-MS analysis is further carried out to measure the molecular weight changes of the [Ag62S13(SBut)32]4+ NCs. Unlike the most thiolate-protected Au NCs (e.g. Au25, Au38), thiolate-protected [Ag62S13(SBut)32]4+ NCs didn't display a single peak at 9958 Da (Fig. S1†), but a broad peak around 9500 Da, instead. After adding one equivalent of Cu2+, the broad peak slightly shifted to the higher mass, suggesting that Cu is not replacing the Ag atoms in the NCs, but binding to the silver core. Further increasing copper ions until reaching Ag62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1
Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the clusters had decomposed, which also proved from the side, thus a high concentration of [Ag62S13(SBut)32]4+ requires a greater amount of Cu2+ ions to make the fluorescence completely quenched.
4, the clusters had decomposed, which also proved from the side, thus a high concentration of [Ag62S13(SBut)32]4+ requires a greater amount of Cu2+ ions to make the fluorescence completely quenched.
The performance of [Ag62S13(SBut)32]4+ NCs in single-molecule applications was assessed using the previously described immobilization and imaging methods15 (Fig. 4). The single-molecule experiments were carried out with a confocal fluorescence microscope, using a 720 nm laser as the two-photon excitation source. The confocal fluorescence images of [Ag62S13(SBut)32]4+ exhibit many bright spots (Fig. 4a), whereas the [Ag62S13(SBut)32]4+ treated with 1 equiv. of Cu2+ shows no bright spots (Fig. 4b). The extent of surface immobilization for both NCs are indeed similar, thus the difference can only be attributed to the addition of Cu2+ ions which quench the fluorescence of the [Ag62S13(SBut)32]4+ NCs. This result enlightens us that Ag NCs hold potential in single molecule detection.
 | ||
| Fig. 4 Fluorescence images of immobilized [Ag62S13(SBut)32]4+ NCs (a) and after addition of 1 equiv. Cu2+ (b) with pseudo-colors. | ||
In summary, we synthesized [Ag62S13(SBut)32]4+ NCs to reduce more reactive Cu2+ metal ions. The UV-vis and fluorescence spectra suggested that one copper(II) metal ion can quench the fluorescence of one [Ag62S13(SBut)32]4+ nanocluster. The response mechanism was revealed in detail by means of MALDI-TOF mass spectra, XPS and TEM. In addition, single molecule fluorescence imaging illustrated that [Ag62S13(SBut)32]4+ NCs can be used as a single molecule probe for detecting Cu2+. More broadly, this finding gives exciting results for the possible development of a series of new-generation probes for detection of metal ions with the AGR process. A set of single-molecule fluorescence probes with such properties are expected to find widespread applicability as tools in biological studies that utilize fluorescence.
We acknowledges financial support by NSFC (20871112, 21072001, 21372006), Chang Jiang Scholars Program and the Scientific Research Foundation for Returning Overseas Chinese Scholars, State Education Ministry and Ministry of Human Resources and Social Security, Anhui Province International Scientific and Technological Cooperation Project, 211 Project of Anhui University.
Notes and references
- (a) J. J. Shi, Y. F. Zhu, X. R. Zhang, W. R. G. Baeyens and A. M. Garcia-Campana, TrAC, Trends Anal. Chem., 2004, 23, 351 CrossRef CAS; (b) G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433 CrossRef CAS PubMed; (c) G. Q. Wang, Y. Q. Wang, L. X. Chen and J. Choo, Biosens. Bioelectron., 2010, 25, 1859 CrossRef CAS PubMed.
- (a) T. Jamieson, R. Bakhshi, D. Petrova, R. Pocock, M. Imani and A. M. Seifalian, Biomaterials, 2007, 28, 4717 CrossRef CAS PubMed; (b) X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed; (c) M. Mahmoudi, V. Serpooshan and S. Laurent, Nanoscale, 2011, 3, 3007 RSC.
- (a) C. J. Lin, C.-H. Lee, J.-T. Hsieh, H.-H. Wang, J. K. Li, J.-L. Shen, W.-H. Chang, H.-I. Yeh and W. H. Chang, J. Med. Biol. Eng., 2009, 29, 276 Search PubMed; (b) L. Shang, R. M. Dörlich, V. Trouillet, M. Bruns and G. U. Nienhaus, Nano Res., 2012, 5, 531 CrossRef CAS; (c) M. A. H. Muhammed, S. Ramesh, S. S. Sinha, S. K. Pal and T. Pradeep, Nano. Res., 2008, 1, 333 CrossRef CAS; (d) J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS PubMed; (e) X. Yuan, Q. Yao, Y. Yu, Z. Luo, X. Dou and J. Xie, J. Phys. Chem. Lett., 2013, 4, 1811 CrossRef CAS.
- L. Shang, N. Azadfar, F. Stockmar, W. Send, V. Trouillet, M. Bruns, D. Gerthsen and G. U. Nienhaus, Small, 2011, 7, 2614 CrossRef CAS PubMed.
- (a) J. Zheng and R. M. Dickson, J. Am. Chem. Soc., 2002, 124, 13982 CrossRef CAS PubMed; (b) J. Zheng, J. T. Petty and R. M. Dickson, J. Am. Chem. Soc., 2003, 125, 7780 CrossRef CAS PubMed.
- L. Shang and S. J. Dong, Chem. Commun., 2008, 1088 RSC.
- R. Zhou, M. Shi, X. Chen, M. Wang and H. Chen, Chem. – Eur. J., 2009, 15, 4944 CrossRef CAS PubMed.
- (a) W.-Y. Chen, G.-Y. Lan and H.-T. Chang, Anal. Chem., 2011, 83, 9450 CrossRef CAS PubMed; (b) H. Kawasaki, K. Hamaguchi, I. Osaka and R. Arakawa, Adv. Funct. Mater., 2011, 21, 3508 CrossRef CAS; (c) T.-H. Cheng and W.-L. Tseng, Small, 2012, 8, 1912 CrossRef PubMed.
- (a) X. Wen, P. Yu, Y.-R. Toh and J. Tang, J. Phys. Chem. C, 2012, 116, 11830 CrossRef CAS; (b) J. Zheng, P. R. Nicovich and R. M. Dickon, Annu. Rev. Phys. Chem., 2007, 57, 409 CrossRef PubMed; (c) J. Zheng, C. Zhou, M. Yu and J. Liu, Nanoscale, 2012, 4, 4073 RSC.
- (a) T. Funatsu, Y. Harada, M. Tokunaga, K. Saito and T. Yanagida, Nature, 1995, 374, 555 CrossRef CAS PubMed; (b) V. Vukojevic, M. Heidkamp, Y. Ming, B. Johansson, L. Terenius and R. Rigler, Proceedings of the National Academy of Sciences, 2008, 105, 18176 CrossRef CAS PubMed; (c) T. Ha and P. Tinnefeld, Annu. Rev. Phys. Chem., 2012, 63, 595 CrossRef CAS PubMed; (d) S. K. Yang, X. Shi, S. Park, T. Ha and S. C. Zimmerman, Nat. Chem., 2013, 5, 692 CrossRef CAS PubMed.
- (a) P. C. Ray, G. K. Darbha, A. Ray, J. Walker and W. Hardy, Plasmonics, 2007, 2, 173 CrossRef CAS; (b) F. Liu, J. Y. Choi and T. S. Seo, Biosens. Bioelectron., 2010, 25, 2361 CrossRef CAS PubMed; (c) E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759 RSC.
- (a) J. Xie, Y. Zheng and J. Y. Ying, Chem. Commun., 2010, 46, 961 RSC; (b) M. Wang, Z. Wu, J. Yang, G. Wang, H. Wang and W. Cai, Nanoscale, 2012, 4, 4087 RSC; (c) Z. Wu, M. Wang, J. Yang, X. Zheng, W. Cai, G. Meng, H. Qian, H. Wang and R. Jin, Small, 2012, 8, 2028 CrossRef CAS PubMed; (d) H.-W. Li, Y. Yue, T.-Y. Liu, D. Li and Y. Wu, J. Phys. Chem. C, 2013, 117, 16159 CrossRef CAS.
- Z. Wu, Angew. Chem., Int. Ed., 2012, 51, 2934 CrossRef CAS PubMed.
- G. Li, Z. Lei and Q.-M. Wang, J. Am. Chem. Soc., 2012, 132, 17678 CrossRef PubMed.
- W. Luo, K. He, T. Xia and X. Fang, Anal. Bioanal. Chem., 2013, 405, 43 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, TEM, XPS and MALDI-TOF. See DOI: 10.1039/c3ra46877a |
| This journal is © The Royal Society of Chemistry 2014 |