Temperature-dependent decaying mechanism of BOPET corona films
Liping Ding and
Yongping Bai*
School of Chemical Engineering and Technology, Harbin Institute of Technology, PO Box 401#, No. 92, West Da-Zhi Street, Harbin 150001, People's Republic of China
First published on 5th December 2013
Abstract
BOPET corona films undergo a decrease in surface energy with time, a so called decaying effect, which has become a serious and widely known problem which prohibits their downstream industrial applications and needs to be carefully understood. Herein, the decaying behaviors and dynamics of decaying BOPET corona films in hot air were studied carefully by measuring water contact angle and surface tension and variable angle XPS. It was found that the molecular mechanism for the decaying effect involves migration of polar groups from the surface to the interior, and the decaying process is highly dependent on temperature. In addition, it was also found that such a temperature-dependent decay process is related to the glass transition of BOPET, and the glass transition temperature (Tg) can be predicted through analysis of the dynamics.
Introduction
Biaxially-oriented polyethelene terephthalate (BOPET) film is widely used in many fields because of its excellent and comprehensive properties. The surface energy of BOPET film is low due to the rigid molecular chain of PET and lower content of polar groups existing on the surface. This lower surface energy greatly limits the applications of BOPET film in industries such as printing, coating, gold stamping and so on. Therefore, additional treatment is needed to raise the surface energy.1–15 Different methods exist to modify the surface properties of the polymer films such as chemical treatment, graft polymerization, corona discharge treatment and so on. Recently, plasma treatment and corona treatment have been widely used in industry to raise the surface energy of BOPET film16–24 because they are environmentally friendly and mild treatments. Moreover, these modifications will not destroy the bulk properties of BOPET films. Nevertheless, these modifications are not permanent since the surface energy will decline after a period of time, known as the “decaying effect”. When BOPET corona film is left in humid, hot air for some time, its hydrophilicity will decline significantly and even return to the level of untreated BOPET film. The “decaying effect” of BOPET corona film greatly affects its downstream applications. It is therefore necessary to investigate the decay mechanism in order to prohibit or delay the decaying process.Some work has been done to investigate the “decaying effect” of BOPET film. Pandiyaraj and coworkers25 studied the ageing process of plasma treated films and showed that the decay is due to migration of polar groups into the bulk polymer. Morra and coworkers26 pointed out that the decay could be attributed to surface contamination, orientation of polar groups, blooming of additives and absorption of ubiquitous contaminations. Geyter and coworkers27 found that after storage in air, the wettability of the PET films decreased since the induced polar groups reoriented from the surface into the bulk of the material. Yang28 and Donnio29 pointed out that the ageing effect of the modified PET surface could be summarized as the reorientation of polar groups at the surface layer, diffusion of non-polar groups from the sub-surface to the surface, and reactions of free radicals at the surface. Other work13,30 only presented the phenomenon of decay.
Although there are some correlating reports about the decaying effect of BOPET film, the temperature-dependent dynamics of the decaying process has not been studied carefully. In this study, the decaying effect of BOPET corona film was investigated systematically by analyzing the evolution of the water contact angle and of surface tension on the surface of the BOPET film with temperature and time. In addition, the variation in surface chemical composition with time at different sample depths was also studied using XPS. A mechanism for the decay of BOPET corona film was put forward based on these results. Most interestingly, it was found that the glass transition temperature (Tg) of BOPET can be predicated by analyzing the decay dynamics, based on the relaxation time and temperature, which may provide a novel method to predicate the Tg of polymers.
Experimental
Preparation of the corona films
The 12 μm thick BOPET corona films were provided by Fuwei Films Co. Ltd (Shandong, China). These were commercial films and were obtained from the Brückner biaxially-oriented stretching production line (Germany). The glass transition temperature (Tg) of these BOPET films was 77 °C (Netzsch DSC 204 thermal analyser).Decay of the BOPET corona film in air
Some pieces of BOPET corona film were left in hot air at temperatures of 50 °C, 70 °C and 90 °C for different time periods. These films were then added to a block of ice to freeze their surface configurations after removing them from the hot air.Secondary decay of the BOPET corona film
Some pieces of BOPET corona film which have achieved decaying balance in water were taken out and dried in a block of ice. Then the films would be further treated in hot air of 100 °C, 120 °C, 140 °C, 160 °C and 180 °C.Water contact angle measurements
Water contact angle measurements were performed with a goniometer (JY-125) by the sessile drop method. Distilled water was used as the working liquid and the volume of the water drops was maintained at 0.5 μl. Each contact angle value was taken as an average value of five different points on the same sample surface. The standard deviation of the average contact angle was smaller than 0.5°.Surface energy measurements
Surface energy was measured with reference to ASTMD 2578-99a. The specific method used is as follows: mixtures with a variety of surface energy values were prepared and then a clean cotton ball was dipped into each mixture. A liquid film square of 6 cm−2 was used to coat the surface of a BOPET corona film. If the liquid film didn't break after 2 s it indicated that the surface energy of the BOPET film was higher than the liquid mixture used. In this case a solution with a higher surface energy was used to try again. If the liquid film was broken, indicating that the surface energy of the BOPET film was lower than the used liquid mixture, a solution with a lower surface energy was used to try again. When the critical condition was reached, the former mixture could spread while the latter mixture couldn't spread. The surface energy of the former mixture was then studied.X-ray photoelectron spectroscopy
Surface chemical characterization of the BOPET film and the BOPET corona film was carried out by means of X-ray photoelectron spectroscopy (XPS) which was performed on a PERKIN-ELMER, PHI 5300 XPS spectrometer using a monochromatic MgKα (1253.6 eV) photon source at an anode voltage of 10 kV, an anode current of 20 mA and a pressure in the analytical chamber of 10−7 Pa. Data collection, processing and handling were all carried out using the APOLLO SERIES3500 workshop, of the decaying samples of the BOPET corona film left in hot air at 70 °C for different amounts of time. Analysis of the surface elements was performed at take-off angles of 20° and 70°.Results and discussion
Decaying effect of the BOPET corona film in hot air
Fig. 1 shows the evolution of water contact angle and the variation of surface energy of the BOPET corona film as a function of storage time. As shown in Fig. 1, when the storage time is increased, the hydrophilicity declines, the water contact angle increases and the surface energy decreases gradually. After 9 days, the water contact angle and surface energy reach a plateau, and their values are 56° and 44 mN m−1 respectively. This may be attributed to a rearrangement of some of the polar groups from the surface to the bulk. Therefore, the BOPET corona film undergoes a decaying effect at room temperature. After corona discharge treatment, the hydrophilicity of the BOPET film is improved temporarily. As the storage time in air increases, the surface properties of the BOPET film decay gradually. | ||
| Fig. 1 Decaying regulation of surface energy (a) and water contact angle (b) of BOPET corona film in 25 °C air. | ||
Fig. 2 shows the evolution of water contact angle as a function of treatment at different temperatures. As seen from Fig. 2, the surface hydrophilicity of the BOPET corona film declines more quickly with increasing treatment temperature. With increasing hot air treatment temperature, the water contact angle decay accelerates and in a short period of time the decaying effect plateaus. The higher the air temperature, the lower the water contact angle value when it plateaus. When decaying in hot air at temperatures of 50 °C, 70 °C and 90 °C, the water contact angle plateaus at 65°, 62° and 60° respectively. Furthermore, there is a tendency that water contact angle increases to a maximum and then decreases until a plateau is reached which might be attributed to rearrangement of some of the polar groups from the bulk to the surface at higher temperature.
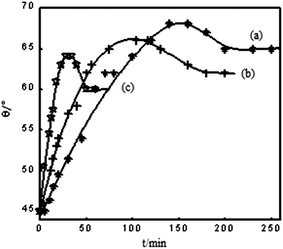 | ||
| Fig. 2 Decaying regulation of water contact angle of BOPET corona film with different treatment temperatures in hot air: (a) 50 °C, (b) 70 °C, and (c) 90 °C. | ||
As can be seen from the experiment, the surface hydrophilicity of BOPET corona film is a dynamic equilibrium process and the essence of decay is correlated to the polar groups on the surface of the BOPET corona films. As the thermal decomposition temperature of PET is much higher than 90 °C, it cannot decompose in such a short period of time below 90 °C in the air, so the polar groups cannot be lost in the air spontaneously. Therefore, the decaying effect can only be attributed to the movement of polar groups from the surface to the bulk, and the migration process is obviously correlated to the thermal movement of macromolecules on the membrane surface. From the view of molecular thermal movement, the temperature of the experiment is lower than Tg, the macromolecular movement is frozen and the movement of polar groups is achieved only by secondary relaxation of macromolecular segments, accompanying the movement of lateral groups, chain links and oxygen-containing groups. Secondary relaxation is a process of dynamic equilibrium which relates to temperature.
When the annealing temperature is higher, the activities of molecular units are enhanced and the relaxation time (τ) is reduced. The movement of polar groups to the bulk and the decrease of the water contact angle are accelerated. But at the same time, the migration of polar groups to the surface is accelerated. Thus, at a higher annealing temperature, an equilibrium between the migration of polar groups from the surface to the interior and the reverse process is reached much faster, and more polar groups are kept after reaching an equilibrium. As a result, the BOPET corona film has a lower contact angle at a higher annealing temperature (Fig. 2). In order to validate this, we performed another control experiment. Some pieces of BOPET corona film which reached a steady level of decay in hot air at 70 °C (contact angle 62°) and 90 °C (contact angle 60°) were placed in hot air at 160 °C for 30 min. We found the contact angle reached 58.5° for the two types of membranes and the surface hydrophilicity increased further. This means that polar groups of the molecule chains in the interior of the film migrate to the surface at high annealing temperatures.
Microscopic mechanism of BOPET corona film decay
PET is a crystalline polymer. After biaxially-oriented stretching, the surface molecules of the BOPET film have a special characteristic structure. The array of molecule chains are loose and are more active than the bulk. The surface is vulnerable to the surrounding environment and changes in order to reach the surface free energy minimum, which is the structural basis for molecular movement. After corona treatment, polar groups were incorporated onto the surface of the BOPET film. It is predicted that these polar groups migrate with the movement of macromolecules.We used variable angle XPS to determine the changes in surface chemical composition with sample depth for the BOPET corona membrane treated in hot air at 70 °C. The depth d can be obtained by changing the take-off angle of the measurements according to the formula:
d = 3λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
After corona treatment, both the oxygen and nitrogen content increased due to the formation of polar groups. However, the content of carbon did not change significantly during this process. So, herein, we used the relative content of oxygen atoms compared to carbon atoms, O1s/C1s, to characterize the changes in polar groups on the film surface (Table 1).
| Take-off angle | Untreated film, % | Corona film, % | Hot air (30 min), % | Hot air (2 h), % |
|---|---|---|---|---|
| 20° | 38.0 | 46.6 | 43.5 | 42.0 |
| 70° | 38.0 | 46.2 | 45.7 | 45.2 |
Three conclusions can be generated from Table 1. Firstly, the relative oxygen content increased from 38% to 46% after corona treatments, indicating the formation of many polar groups on the surface of the membrane after this treatment. Secondly, the relative oxygen content on the membrane surface decreased when it was treated in hot air at 70 °C, for example, it decreased from 46% to 42.0% after being treated for 2 h at a take-off angle of 20°. Thirdly, the relative oxygen content at the outmost surface (take-off angle of 20°) decreased much faster than that at the inner surface (take-off angle of 70°). These results indicate that the polar groups on the BOPET corona film migrate from the outmost surface to the interior.
The variation in O atom content is caused by molecular thermal movement which brings about rotation of oxygen-containing polar groups. This migration is controlled by heat and the thermal movement is a secondary relaxation process. There are possibly several movement units, containing a variety of polar groups, which cause the migration. There are two possible movement mechanisms. One option is that the polar groups on the film surface migrate into the interior of the film, the content of polar groups on the surface decreases and the hydrophilicity declines. The other is that some movement units (containing polar and non-polar groups) of the molecule chain in the interior of the film migrate to the surface. When treated in hot air, the activity of the surface molecules is higher, the first movement takes place and the O atom content at the surface is reduced, resulting in a decline in hydrophilicity and an increase in water contact angle. As the treatment time increases, which is equivalent to raising the ambient temperature according to the time-temperature equivalence principle, the activity of the movement units and the capacity for migration from the surface to the interior of the film are enhanced. At the same time, movement units which are difficult to move are unfrozen and may migrate to the interior of the film or the surface, making some groups return to the surface. As the time spent in hot air increases, migration to the interior of the film becomes the dominant movement and ultimately reaches a maximum value. At this time, the polar group content on the surface of the film is smallest and the water contact angle is largest. Then, migration from the interior of the film to the surface is enhanced. The polar group content on the film’s surface increases slightly, making the water contact angle diminish. Finally, migration to the interior of the film and surface of the film are equal and the water contact angle remains constant. Therefore, when decaying in hot air, as the treatment time increases, the water contact angle first increases to a maximum and then decreases and maintains a constant value.
Decaying dynamics of BOPET corona film in hot air
From the viewpoint of molecular thermal movement, BOPET corona film decay in hot air can seem to be a process of relaxation. Therefore, this decay process could be described by the mechanism of relaxation dynamics. The relaxation is a dynamic equilibrium process. The basic element is relaxation time (τ), and the relaxation could be described by the following equation:
 | (1) |
The water contact angle θ on the surface of BOPET film is the subject under investigation. θ0 is the starting water contact angle, θ∞ is the water contact angle when the rate of decay is constant, θ∞ − θ0 is the variable range of the water contact angle, θt − θ0 is the variable value at t and 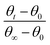 is the percentage of decay at t in the decaying process.
is the percentage of decay at t in the decaying process.
Therefore, the conservation rate of hydrophilicity could be characterized by  .
.
Theoretically, the relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔXt and t is linear and the slope of the straight line is the relaxation time (τ). When decaying in hot air below 90 °C, the macromolecule undergoes secondary relaxation which also follows the above dynamic equation.
ΔXt and t is linear and the slope of the straight line is the relaxation time (τ). When decaying in hot air below 90 °C, the macromolecule undergoes secondary relaxation which also follows the above dynamic equation.
Fig. 3 shows the dynamic features of the BOPET corona film decay in hot air. As can be seen, part of the dynamic curve is linear. The relaxation time can be derived from the linear part, the results for which are shown in Table 2. As seen from Table 2, in lower temperature hot air, the start of the BOPET corona film decay is successful and the regression coefficient is close to 1. In the latter part of the decay, the movement units in the interior of the film migrate to the surface, resulting in the migration of polar groups from the interior to the surface. The mechanism of molecular movement becomes complex, deviating from the relaxation process. Temperature has a great impact on the decaying dynamics, the higher the temperature, the shorter the relaxation time, which satisfies the regularity of polymeric thermal movement, indicating that the surface molecules follow a secondary relaxation process, at least at the beginning of the decaying process.
 | ||
| Fig. 3 The dynamic features induced by changes in contact angle of the BOPET corona film decaying at different treatment temperatures in hot air; (a) 50 °C, (b) 70 °C, and (c) 90 °C. | ||
| Temperature | Regression coefficient | τ, min |
|---|---|---|
| 50 °C | 0.9973 | 80.1238 |
| 70 °C | 0.9986 | 32.1834 |
| 90 °C | 0.9975 | 15.0564 |
Secondary relaxation has a special regularity; the relaxation time and temperature meet the Eyring equation:
 | (2) |
A graph is made by plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ and 1/T, and the slope is the activation energy of the secondary relaxation. The results are shown in Fig. 4. As can be seen, when BOPET corona film decays at lower temperatures, the dynamic data has a good fit with the Eyring equation. Almost all the experimental points fall along the straight line predicted by the Eyring equation, and the linear regression coefficient is 0.9998. This experiment further proves that when BOPET corona film decays at lower temperatures (below 90 °C), molecular movement on the surface is a secondary relaxation process and the activation energy is 40.77 kJ mol−1.
τ and 1/T, and the slope is the activation energy of the secondary relaxation. The results are shown in Fig. 4. As can be seen, when BOPET corona film decays at lower temperatures, the dynamic data has a good fit with the Eyring equation. Almost all the experimental points fall along the straight line predicted by the Eyring equation, and the linear regression coefficient is 0.9998. This experiment further proves that when BOPET corona film decays at lower temperatures (below 90 °C), molecular movement on the surface is a secondary relaxation process and the activation energy is 40.77 kJ mol−1.
 | ||
| Fig. 4 The relationship between relaxation time and temperature detected by the Eyring equation when decaying in hot air. | ||
Secondary decay dynamics of BOPET corona film in hot air after soaking in water
Fig. 5 shows the evolution of the water contact angle of BOPET corona film after secondary decay in hot air at different temperatures. As seen from Fig. 5, (1) BOPET corona film which has reached its decaying limit in water could further decay in hot air (T > Tg); this could be called secondary decay. The water contact angle on the surface increases as the time treated in hot air increases and ultimately reaches a constant value. (2) There is no restoring equilibrium which happens in hot air (T ≤ 90 °C), and the decay directly reaches a constant level, and the water angle is larger than that at lower temperatures, which is close to the level of the untreated BOPET film (WCA = 80°). (3) The higher the temperature, the faster the decay and the quicker the decay plateaus. (4) The water contact angle when the decay plateaus varies with temperature. | ||
| Fig. 5 The changing trend of contact angles of BOPET corona film decaying twice at different temperatures in hot air: (a) 100 °C, (b) 120 °C, (c) 140 °C, (d) 160 °C, and (e) 180 °C. | ||
The migration speed of polar groups from the surface to the interior of the film is faster than migration from the interior to the surface. As the time increases, the number of polar groups which migrate to the interior becomes equal to the number migrating to the surface, resulting in a dynamic balance. Therefore, there is only a decaying balance, not a restoring balance. The higher the temperature, the fewer polar groups there are on the surface and the water contact angle is relatively larger.
The essence of BOPET corona film secondary decay in hot air after soaking in water is the thermal movement of chain segments on the film surface. This is a relaxation process and the dynamic results are shown in Fig. 6 and Table 3. As seen from Fig. 6, the first part of the process is linear but the latter part deviates from the straight line. This phenomenon may be caused by the migration of polar groups from the inner layer to the outer or the irregular distribution of crystalline and amorphous areas. As seen from Table 3, the linear regression coefficients are all close to 1, so the relaxation dynamics describe the decay regularity well. The higher the temperature, the shorter the relaxation time. Compared to Table 2, the relaxation time for secondary decay (T > Tg), the relaxation has declined significantly.
| Temperature | Regression coefficient | τ, min |
|---|---|---|
| 100 °C | 0.9925 | 6.3309 |
| 120 °C | 0.9904 | 5.6904 |
| 140 °C | 0.9981 | 3.5188 |
| 160 °C | 0.9989 | 1.2386 |
| 180 °C | 0.9977 | 0.1980 |
Fig. 7 is a graph of relaxation time and temperature. The graph is similar to the characteristic graph of the glass transition temperature. As the temperature increases, the relaxation time is reduced. There is a significant inflection point when the temperature is between 50 °C and 100 °C. Based on the trends of the graph, two tangents are drawn, and the abscissa of the point of intersection is 81 °C, which is consistent with the Tg of PET. The relaxation time is closely linked to, and is a measurement of, the molecular thermal movement. Therefore, when the polymer takes place the glass transition, the relaxation will have a mutation in theory. The experiment confirms this hypothesis. Therefore, the Tg of PET could be determined by the variable regularity of the relaxation time of the film surface molecular movement and the temperature, which can be applied to other polymers. It is a novel method to predict the Tg of polymers. Because this method is directly correlated to the molecular thermal movement, it could predict the absolute value of the Tg. Up to now, there are no reports of this method. It is known that the Tg of polymer films decreases as the film thickness decreases below 100 nm.31 We also hope the method shown in Fig. 7 could be used to predict the Tg of thinner films in order to disclose the Tg depression.
 | ||
| Fig. 7 The relationship between relaxation time of molecular movement on a BOPET film surface and temperature when decaying in hot air. | ||
The relaxation time and the corresponding temperature of the secondary decay were substituted into the Eyring equation, and the graph of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ and 1/T is shown in Fig. 8. If the relaxation process satisfies the Eyring equation, the graph of ln
τ and 1/T is shown in Fig. 8. If the relaxation process satisfies the Eyring equation, the graph of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ–1/T is a straight line. But the graph deviates from the straight line severely. Therefore, the mechanism of molecular thermal movement can't be secondary relaxation, but can be due to thermal movement of the chain segments.
τ–1/T is a straight line. But the graph deviates from the straight line severely. Therefore, the mechanism of molecular thermal movement can't be secondary relaxation, but can be due to thermal movement of the chain segments.
 | ||
| Fig. 8 The relationship between relaxation time of BOPET film and temperature, calculated using the Eyring equation, when decaying twice in hot air. | ||
When the polymers occur the thermal movement of chain segments, the relaxation time and temperature satisfy the Williams–Landel–Ferry (WLF) equation:
 | (3) |
Equation (3) can be converted into the following equation:
 | (4) |
The derived τ and T are substituted into equation (4). Choosing 100 °C as Ts, the results are shown in Fig. 9. As seen from Fig. 9, when BOPET corona film decays in hot air of T > Tg, the dynamics data derived from the principle of relaxation dynamics satisfy the WLF equation well. The experimental dots almost fall on the straight line predicted by the WLF equation, and the linear regression coefficient is 0.9929. The experiment further proves that the mechanism of surface molecular movement is a relaxation of chain segments when decaying in hot air of T > Tg.
 | ||
| Fig. 9 The relationship between relaxation time of BOPET film and temperature, detected by the WLF equation, when decaying twice in hot air. | ||
Conclusion
BOPET corona film experiences a serious decaying effect in air, and the decaying effect is a dynamic equilibrium process. Temperature and time are the important factors which determine the decay. When the temperature is higher or the time of decay is longer, the decay is greater. Therefore, there is a useful lifetime of BOPET corona film. After a period of time, the hydrophilicity of the film surface declines significantly and it must be retreated. Heat could accelerate the decaying process, so it should be stored in a non-humid environment.The essence of the decaying effect is the molecular movement on the film surface which results in the transfer of polar groups to the interior of the BOPET corona film. When BOPET corona film decays in air, the decay is caused by molecular thermal movement. At lower temperatures, the molecular movement mechanism is secondary relaxation, whilst at higher temperatures (>Tg), the molecular movement mechanism is the cooperative movement of molecular chain segments. This is a relaxation process.
Relaxation dynamic equations can be used to describe the early stages of the decaying effect in air. The Eyring equation and WLF equation can successfully describe the early decaying process in hot air at low and high temperatures. Meanwhile, the relationship between relaxation time and temperature of surface molecular thermal movement can be predicted accurately. As the temperature is raised, the relaxation time of molecular movement on the surface of BOPET corona film is reduced. The movable velocity increases and the activation energy is reduced, resulting in a decrease of surface hydrophilicity.
The variable relationship between relaxation time and temperature can be used to predict the Tg of PET correctly. This method directly reflects the molecular thermal movement on the surface of BOPET corona film and factually reflects the glass transition process. It has the potential to predict the precise value of the Tg of other polymers.
References
- S. M. Pelagade, N. L. Singh, A. Qureshi, R. S. Rane, S. Mukherjee, U. P. Deshpande, V. Ganesan and T. Shripathi, Nucl. Instrum. Methods Phys. Res., Sect. B, 2012, 289, 34–38 CrossRef CAS PubMed.
- S. Guruvenket, G. M. Rao, M. Komath and A. M. Raichur, Appl. Surf. Sci., 2004, 236, 278–284 CrossRef CAS PubMed.
- D. Hegemann, H. Brunner and C. Oehr, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 208, 281–286 CrossRef CAS.
- F. S. Denes and S. Manolache, Prog. Polym. Sci., 2004, 29, 815–885 CrossRef CAS PubMed.
- L.-A. O'Hare, S. Leadley and B. Parbhoo, Surf. Interface Anal., 2002, 33, 335–342 CrossRef CAS.
- M. J. Shenton and G. C. Stevens, J. Phys. D: Appl. Phys., 2001, 34, 2761 CrossRef CAS.
- C. Huang, Y.-C. Chang and S.-Y. Wu, Thin Solid Films, 2010, 518, 3575–3580 CrossRef CAS PubMed.
- L. P. Santos, J. S. Bernardes and F. Galembeck, Langmuir, 2013, 29, 892–901 CrossRef CAS PubMed.
- N. Kalapat and T. Amornsakchai, Surf. Coat. Technol., 2012, 207, 594–601 CrossRef CAS PubMed.
- G. M. Bayley and P. E. Mallon, Polym. Eng. Sci., 2008, 48, 1923–1930 CAS.
- C. A. Cáceres, N. Mazzola, M. França and S. V. Canevarolo, Polym. Test., 2012, 31, 505–511 CrossRef PubMed.
- M. Ragoubi, B. George, S. Molina, D. Bienaimé, A. Merlin, J. M. Hiver and A. Dahoun, Composites, Part A, 2012, 43, 675–685 CrossRef CAS PubMed.
- I. Vlaeva, T. Yovcheva, A. Viraneva, S. Kitova, G. Exner, A. Guzhova and M. Galikhanov, J. Phys.: Conf. Ser. 398, 2012, 012054, DOI:10.1088/1742-6596/398/1/012054 .
- J. Abenojar, R. Torregrosa-Coque, M. A. Martínez and J. M. Martín-Martínez, Surf. Coat. Technol., 2009, 203, 2173–2180 CrossRef CAS PubMed.
- I. Woodward, W. C. E. Schofield, V. Roucoules and J. P. S. Badyal, Langmuir, 2003, 19, 3432–3438 CrossRef CAS.
- J. Lai, B. Sunderland, J. Xue, S. Yan, W. Zhao, M. Folkard, B. D. Michael and Y. Wang, Appl. Surf. Sci., 2006, 252, 3375–3379 CrossRef CAS PubMed.
- Y. Zhao, S. Tang, S.-W. Myung, N. Lu and H.-S. Choi, Polym. Test., 2006, 25, 327–332 CrossRef CAS PubMed.
- Z.-M. Liu, Z.-K. Xu, J.-Q. Wang, Q. Yang, J. Wu and P. Seta, Eur. Polym. J., 2003, 39, 2291–2299 CrossRef CAS.
- A. Yu, M. Grushin, N. Dyatko, I. Kochetov, A. Napartovich, N. Trushkin, D. Tran Minh and S. Descours, J. Phys. D: Appl. Phys., 2008, 41, 235203 CrossRef.
- O. Motret, F. Coursimault, R. Viladrosa and J. M. Pouvesle, J. Phys. D: Appl. Phys., 2003, 36, 2060 CrossRef CAS.
- N. Inagaki, S. Tasaka, K. Narushima and H. Kobayashi, J. Appl. Polym. Sci., 2002, 85, 2845–2852 CrossRef CAS.
- R. Dorai and M. J. Kushner, J. Phys. D: Appl. Phys., 2003, 36, 666 CrossRef CAS.
- D. P. Dowling, J. Tynan, P. Ward, A. M. Hynes, J. Cullen and G. Byrne, Int. J. Adhes. Adhes., 2012, 35, 1–8 CrossRef CAS PubMed.
- M. H. Han, J. P. Jegal, K. W. Park, J. H. Choi, H. K. Baik, J. H. Noh, K. M. Song and Y. S. Lim, Surf. Coat. Technol., 2007, 201, 4948–4952 CrossRef CAS PubMed.
- K. N. Pandiyaraj, V. Selvarajan, R. R. Deshmukh and M. Bousmina, Surf. Coat. Technol., 2008, 202, 4218–4226 CrossRef CAS PubMed.
- M. Morra, E. Occhiello and F. Garbassi, J. Colloid Interface Sci., 1989, 132, 504–508 CrossRef CAS.
- N. De Geyter, R. Morent and C. Leys, Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 3086–3090 CrossRef CAS PubMed.
- S. Yang and M. C. Gupta, Surf. Coat. Technol., 2004, 187, 172–176 CrossRef CAS PubMed.
- A. Carradò, O. Sokolova, B. Donnio and H. Palkowski, J. Appl. Polym. Sci., 2011, 120, 3709–3715 CrossRef.
- N. Knorr, S. Rosselli and G. Nelles, J. Appl. Phys., 2010, 107, 054106–054114 CrossRef PubMed.
- J. A. Forrest and K. Dalnoki-Veress, Adv. Colloid Interface Sci., 2001, 94, 167–195 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |

