Surface treatment of thin-film materials to allow dialogue between endothelial and smooth muscle cells and the effective inhibition of platelet activation
Roman Major*a,
Franz Bruckertb,
Juergen M. Lacknerc,
Jan Marczakd and
Boguslaw Majora
aInstitute of Metallurgy and Materials Science, Polish Academy of Sciences, Reymonta St. 25, 30-059 Cracow, Poland. E-mail: r.major@imim.pl; Fax: +48-12-2952804; Tel: +48-12-2952800 ext. 2841
bLaboratoire des Matériaux et du Génie Physique, Grenoble Institute of Technology – Minatec, 3, Parvis Louis Néel, BP 257, 38016 Grenoble Cedex 1, France
cJoanneum Research Forschungsges mbH, Institute of Surface Technologies and Photonics, Functional Surfaces, Leobner Strasse 94, A-8712 Niklasdorf, Austria
dInstitute of Optoelectronics, Military University of Technology, 2 Gen. S. Kaliskiego Street, 00-908, Warsaw, Poland
First published on 30th October 2013
Abstract
This work aims to reproduce the structure of a blood vessel. The surface modifications that were carried out concerned the creation of an appropriate environment for communication between tissues. Cell–material interactions were studied in this work. Samples for examination were prepared on polished silicon substrates and the sample surface was covered with different biocompatible coatings such as: diamond like carbon (a-C:H), titanium (Ti), titanium nitride (Ti:N), titanium carbonitride (a-C:H:Ti:N), titanium oxide (Ti:O), silicon doped diamond like carbon (a-C:H:Si) and titanium doped diamond like carbon (a-C:H:Ti). Physical vapour deposition (hybrid pulsed laser deposition) was chosen for coating fabrication and the coating thickness ranged from 200 nm to 1 μm. Based on a dynamic analysis using blood, which considered aggregate formation, platelet consumption and blood platelet activation, the most haemocompatible coating was chosen. The study also considered channels for the migration of cells. Using the laser ablation method, migration channels were fabricated. The width of each individual channel depended on the cell size, which was close to 10–30 μm. Cells were deposited directly on the migration channels. An influence of the heat affected zone on cell proliferation was observed. Porous coatings were deposited as the next step after channel formation. The coatings were stabilized by a cross linking chemical reaction using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and N-hydroxysuccinimide. Smooth muscle cells were deposited on the surface of samples without the porous coating, directly onto the migration channels. Human umbilical endothelial cells were deposited on the top of the synthetic porous coating. A de novo surface design for cardiovascular purposes with the function of providing oriented cell growth as well as allowing a dynamic dialogue between tissues was proposed.
1. Introduction
Solutions found in nature seem to be the best inspiration for novel material designs. This study describes an attempt to create an appropriate environment on the surface of solid materials for dynamic dialogue between tissues. Materials can be surface modified using biological, mechanical, or physicochemical methods.1,2 When biomaterial implants come into contact with host tissues, the initial response of cells mostly depends on the surface properties of the implanted materials;3 the proper physical and chemical properties promote cell adhesion and cell growth. A number of different surface modification techniques have been considered to improve the biological properties of materials.4–7 Among these techniques, laser technologies offer a high degree of process controllability and flexibility. The overall objective of the present study was to optimize the surface of biomaterials dedicated to cardiovascular system regeneration using laser techniques to propose a novel surface design based on the architecture of natural blood vessels, which can effectively inhibit the activation of the blood clotting cascade. The surface state of a material is critical for its biocompatibility. Migration channels for controlled cellular proliferation were prepared. The surface modification of any material is based on a controlled, selective and targeted semi-melting or ablation process.8 Surface treatments using laser techniques are widely described. C. De Marco et al. have studied the modifications of surfaces for use in microfluidics,9 preparing femtosecond laser irradiated structures for engineered microfluidic devices. Laser radiation could also be applied for polymer based materials or to produce porosity for biological purposes.10,11The high spatial and temporal coherence of laser light allows for the extreme orientation of the laser beam and its appropriate focusing. Monochrome laser light, together with wavelength tuning, allows for the highly selective excitation of numerous materials. Topographical changes and the generation of micro/nano and 1–3D structures, as well as hierarchical structures, strongly affect the properties and functionalities of materials.8
Channels and ridges in a surface structure guide the migration and orientation of cells, providing healthy tissue organization and mechanical strength.12 An oriented structure fabricated on a surface influences the directional proliferation of cells. The formation of micro-patterns on the surface of the material can be effectively controlled to a desired size by selecting appropriate parameters for the laser treatment. Using the ablation technique, it is possible to change the topography and wetting characteristics, as well as to promote a good adhesion of the cells to the substrate. As a first step, this work considered the design of migration channels for controlled cellular migration and proliferation. In the second stage, a non-thrombogenic surface for use in vascular grafts was prepared, in order to construct an intact luminal endothelial cell layer. In general in a blood vessel, the smooth muscle cells are separated by an extracellular matrix from the endothelial cells, which are in direct contact with blood. A schematic diagram of a blood vessel is presented in Fig. 1.13
2. Materials and methods
Taking into account the blood vessel structure, the following stages of surface modification were proposed:(i) Numerous biocompatible coatings were prepared and the most promising coatings were further modified.
(ii) Migration channels were prepared using the ablation technique to enable controlled cell–material interactions, adhesion and proliferation of smooth muscle cells (SMCs). These cells were deposited directly onto the laser ablated migration channels. Migration channels are fundamental in explaining numerous phenomena involved in cell–material interactions. The controlled SMC growth caused by the changes in the surface roughness and microstructure of the channels was analysed.
(iii) The porous scaffold layer was built from the most appropriate substrate for endothelial cell growth. Human umbilical endothelial cells (HUVECs) were used in this stage of the work. The porous synthetic layer separated the SMCs from the HUVECs. The main aim of the work was to design a novel, functional surface architecture for bulk, blood contacting materials.
Different stages of the work required two types of cells; the first stage, similar to the adventitia side of a blood vessel, required smooth muscle cells. The second stage, the luminal side, required endothelial cells, which are responsible for the effective inhibition of the blood clotting cascade in the natural vessel. The work considered the formation of migration channels for smooth muscle cells and a porous extracellular matrix-like structure under the endothelial cells. Migration channels were prepared at half of the thickness of the haemocompatible ceramic coating deposited by the physical vapour based technique. The work includes computational techniques to properly design the surface structure, which consider and predict thermal and mechanical effects occurring during the surface modification. The finite element method was used to determine the impact of the heat affected zone propagation caused by the laser beam during the migration channel formation.
To allow the application of the materials for the regeneration of the circulatory system, the phase composition of the coating materials was optimized using a dynamic test of haemocompatibility. A laser ablation method was used to create the migration channels for directed cell proliferation. Analysis of the microstructure of the deposited material and the location of the migration channels was done using transmission electron microscopy techniques. Migration channels were used to direct the proliferation of the smooth muscle cells. The monolayer of muscle cells was separated from the endothelial cells in the following stage by means of surface modification using synthetic materials which were similar to the extracellular matrix. The mechanical properties which had a significant impact on the growth of cells were analysed by the finite element method. The results of the numerical simulation were verified in this experiment.
2.1 Numerical methods in surface design
The finite element method is gaining popularity among numerical techniques in engineering.14,15 The finite elements program has a rapidly developing number of analytical functions, offering a user-friendly interface and CAD software TRANSOR.16,17 For the analysis, the Adina program was used. This system enabled us to perform a simulation of structures.18 Two-dimensional calculations were chosen as a much more efficient alternative to three-dimensional ones because of the lower mesh complexity. The simulated layer consisted of a a-C:H:Ti:N layer, modified with laser ablation to form migration channels. The mesh near the indenter and the interface layer had to be very fine to be able to describe the deformation and the stress distribution with sufficient accuracy. Numerical methods were used to create a simplified model of the porous coatings. Porosity was simulated by using voids in the coating structure.2.2 Surface preparation
Based on preliminary studies,8 the following materials (in the form of thin coatings) were chosen for pre-selection with respect to their biocompatibility: titanium nitride (Ti:N), metallic titanium (Ti), diamond like carbon (a-C:H), titanium carbonitride (a-C:H:Ti:N), titanium oxide (Ti:O), silicon doped diamond like carbon (a-C:H:Si), and titanium doped diamond like carbon (a-C:H:Ti).Variable energetic conditions were attained by direct current (DC) and pulsed DC magnetron sputtering with increasing plasma energy and pulsed laser deposition (PLD). Titanium oxide and titanium carbonitride films were deposited by hybrid PLD (Nd:YAG laser, 1064 nm wavelength) from a pure Ti source (>99%) assisted with unbalanced DC magnetron sputtering in argon–nitride and argon–oxide atmospheres. a-C:H and a-C:H:Si layers were obtained by pulsed DC magnetron sputtering in inert argon conditions, while Si-doping was carried out using a silicon target in a C2H2 atmosphere. Film growth was achieved at a range between room temperature and 50 °C. A detailed description of the method is given elsewhere.8
2.3 Microstructure
Analysis of microstructure is a crucial element in biomaterial design. A cross section analysis of the microstructure was carried out for the as-deposited coatings as well as for the processed surfaces, in areas where migration channels were made. Thin foils for TEM observation were prepared directly from the place of interest using the focused ion beam technique (equipped with an in situ micromanipulator). A Quanta 200 3D DualBeam was used for thin film preparation. Microstructure characterization was performed using transmission electron microscopy (TEM) on thin film cross sections. A Tecnai G2 F20 (200 kV) equipped with a field emission gun (FEG) was used for this analysis. A detailed description of TEM microstructure analysis is presented elsewhere.192.4 Surface modification
In the stage dedicated to migration channel preparation, the reflectance R of the beam varies from about 0.091, 0.099 to 0.114, for wavelengths of 1064 nm, 532 nm and 355 nm, respectively, with a perpendicular laser beam incidence. The parameters were indicated from the estimation of the reflection coefficients of carbon based layers.Taking into account the value of the extinction coefficient of the layer to the wavelength, the depth of penetration (epidermal thickness) was 3389 nm, 847 nm and 94 nm, respectively, for the wavelengths above. The penetration depth was adjusted for a 355 nm wavelength of radiation. This condition allowed the evaporation of material from a depth of half of the thickness of the coating.
The next stage in the functional surface fabrication was the deposition of the porous layer. Schematic diagrams of the architecture of the functional layers are shown. Fig. 16a presents the porous layer deposited on the line-like channels, Fig. 16b presents the design of the porous layer deposited on the well-like channels. A natural-like structure of the surface was prepared in two stages. The first one was a porous coating using 12 bi-layers of polyelectrolyte (PLL/HA), stabilized with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysulfosuccinimide (NHS).20 The EDC–sulfo-NHS mixture (EDC at 400 mM and sulfo-NHS at 100 mM were mixed v/v) was freshly prepared in a 0.15 M NaCl solution at pH 5.5. The coupling chemistry is based on the reaction of activated carboxylic sites with primary amine groups. The film coated substrate was put in contact with the EDC–sulfo-NHS solution for 12 hours. The final coating on the top was PLL.
Porous coatings were a key part of the surface optimisation and they were deposited with the “Layer by Layer” method using oppositely charged polyelectrolytes.21 A new material should be biocompatible and biologically stable. In all of the surface modification steps, much attention was focused on ensuring that all elements were biocompatible.22
The last phase of surface functionalisation was the adsorption of the corresponding protein for endothelial cells. The characteristic protein of the extracellular matrix, fibronectin, was adsorbed to the surface. The fibronectin was prepared with a final concentration of 50 μg mL−1 and the final step was the endothelial layer deposition. The following stages of confluent monolayer formation are presented in Fig. 18a to c.
2.5 Bioengineering
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL. The cells were stored in liquid nitrogen until use.
000 cells per mL. The cells were stored in liquid nitrogen until use.100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–125
000–125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were plated in a 25 cm2 flask. From each vial, it was possible to prepare 4 or 5 flasks. Cells were re-suspended in an endothelial cell culture basal medium mixed with cell growth and survival supplements (bullet kit growth mixture purchased from Lonza, including cell growth promoting serum, vitamins, and antibiotics). Before adding the cells, the medium was warmed at 37 °C in a water bath. Cells were taken from the liquid nitrogen container and placed into a 37 °C water bath for 2–3 min. Under a laminar air flow, a maximum of 1 mL of medium mixed with the bullet kit was added. Everything was then pipetted into a 15 mL Falcon tube and diluted to 4 or 5 mL, to receive 100
000 cells were plated in a 25 cm2 flask. From each vial, it was possible to prepare 4 or 5 flasks. Cells were re-suspended in an endothelial cell culture basal medium mixed with cell growth and survival supplements (bullet kit growth mixture purchased from Lonza, including cell growth promoting serum, vitamins, and antibiotics). Before adding the cells, the medium was warmed at 37 °C in a water bath. Cells were taken from the liquid nitrogen container and placed into a 37 °C water bath for 2–3 min. Under a laminar air flow, a maximum of 1 mL of medium mixed with the bullet kit was added. Everything was then pipetted into a 15 mL Falcon tube and diluted to 4 or 5 mL, to receive 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 or 125
000 or 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per flask, respectively. 1 mL of re-suspended cells was taken from the Falcon tube and introduced into a 25 cm2 cultivation flask. Then each flask was filled up to 6 mL of medium with supplements.
000 cells per flask, respectively. 1 mL of re-suspended cells was taken from the Falcon tube and introduced into a 25 cm2 cultivation flask. Then each flask was filled up to 6 mL of medium with supplements.
SMCs were deposited directly on the surfaces of the migration channels. The cells were incubated at 37 °C, with a 5% CO2/95% air flow and 100% humidity. After three days the cells were fixed in 4% paraformaldehyde. Then the cells were permeabilised in the detergent Triton X-100 (0.2%) for 4 min. The actin cytoskeleton was marked with AlexaFluor Phalloidin and the nucleus with DAPI. Fluorescent dyes were excited with appropriate laser wavelengths, 488 nm for actin cytoskeleton visualization and 405 nm to visualize the nucleus of the cell.
3. Results
3.1 Finite element modeling – material design
This section considers the work of determining the theoretical architecture of the coating. The idea was to prepare an architecture of the coating that was similar to the structure of a blood vessel. Depending on the thickness of the deposited layer it was possible to classify the models of the film growth operating at the early and late stages of deposition.8 A numerical analysis was performed to estimate the temperature distribution for the coating directly deposited on the substrate, which was responsible for creating the appropriate surface charge. A homogeneous temperature distribution would not significantly affect the stress concentration, hence, there would be a greater risk of cracking. A uniform temperature distribution was possible for the layers deposited with the 2D model of thin film nucleation (Fig. 2). No evidence of temperature concentration was visible. | ||
| Fig. 2 Simulation of the temperature distribution for the layers deposited with the 2D model of thin film nucleation from the gas phase. | ||
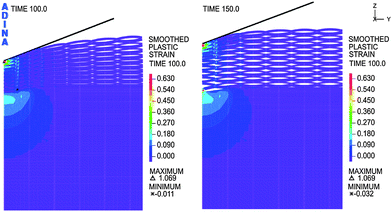
A complex analysis was performed, considering the local temperature distribution during the partial evaporation of the material to create migration channels. (Fig. 3). The local temperature distribution influenced the heat affected zone formation. The partial surface evaporation, re-sputtering effect and heat affected zone were taken under consideration in the numerical analysis as the key points affecting cell–material interactions.
 | ||
| Fig. 3 Simulation of the temperature distribution for the partial evaporation of the material to create migration channels. | ||
The numerical model of the final porous functional coating and estimations of the mechanical properties of the coating are presented in Fig. 4 and 5, respectively.
3.2 The chemical composition of the thin coatings and their selection on the basis of protein and blood–material interactions
The chemical composition of the thin coatings was analysed using X-ray photoelectron spectroscopy (XPS) analysis 20 nm below the surface after sputtering in an argon atmosphere. The results are presented in Table 1.| Phase | Composition |
|---|---|
| Ti | ∼97 at% Ti, the rest is O, N, Ar from deposition atmosphere |
| Ti:N | ∼52 at% Ti, 46% N, the rest is O and Ar |
| a-C:H | Pure carbon (>95 at% without H analysis), about 20 at% H (from ERDA analysis) |
| a-C:H:Si | ∼92 at% C, 3% Si, the rest is O and Ar |
| a-C:H:Ti | ∼87 at% C, 10.5% Ti, the rest is O and Ar |
| a-C:H:N:Ti | ∼83 at% C, 4 at% N, 10 at% Ti, the rest is O and Ar |
The chemical composition influences the properties of the surface, particularly the surface energy, which makes an important contribution in cell–material interactions. The analysis of the surface energy of the tested materials is shown in Fig. 6.
One of the most essential responses after the introduction of the material into the environment of tissue is protein adsorption.25 The materials described here are designed in terms of blood-contact applications. About 80% of blood plasma protein is albumin. The results of a protein–material interaction experiment are shown in Fig. 7.
The following pre-selection step for the thin coatings was performed based on the dynamic tests with human blood. It considered blood cells as a “reference” of the applied material. On the basis of this research, thin coatings were selected for further analysis.26
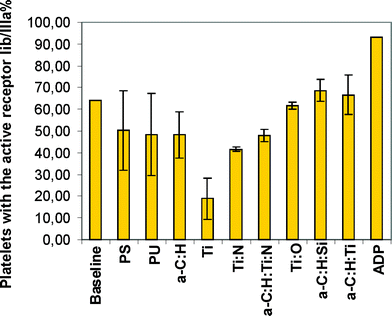
Along with the investigated samples, the same amount of blood was activated with adenosine diphosphate (ADP) as a positive control. The blood–material interactions were examined. Blood is a complex tissue, being comprised of plasma and cells. The blood plasma is an isotonic electrolyte solution corresponding to 0.9% sodium chloride and has relatively high protein content (40–60 g L−1). Albumin comprises more than half of the total plasma protein, but many low-level blood constituents are critical for the function of the whole organism, e.g., clotting factors, anti-proteases, transporter proteins, and immunoglobulins. The Impact-R test was chosen for the experiment and material selection, which is a simplified method to assess haemocompatibility under high shear-stress conditions. This study was designed to evaluate the performance of an alternative in vitro dynamic test for haemocompatibility. The material selection was performed based on several aspects of the blood response, such as: change in the number of platelets (Fig. 8), the percentage of platelet aggregates (Fig. 9), the percentage of platelets with activated IIb/IIIa receptors (Fig. 10), and the percentage of platelets expressing P-selectin (Fig. 11) after exposure to shear force. Based on the dynamic analysis with blood, the silicon doped diamond like carbon was selected for further examination.
Using the scheme shown in Fig. 12, the following values of the refractive coefficient ν and extinction coefficient κ for the carbon based coatings were taken (Table 2):
 | ||
| Fig. 12 Schemes of the values of the refractive coefficient ν and extinction coefficient κ as a function of wavelength for the carbon based coatings. | ||
| Refractive coefficient values | Extinction coefficient values |
|---|---|
| ν for 1064 nm wavelength = 1.86 | κ for 1064 nm wavelength = 0.025 |
| ν for 523 wavelength = 1.92 | κ for 532 nm wavelength = 0.05 |
| ν for 355 wavelength = 1.96 | κ for 355 nm wavelength = 0.3 |
Two different geometries for the migration channels for controlled SMC growth and proliferation were considered. Migration channels were prepared in the shape of lines and wells.
The microstructure of the unmodified part (Fig. 13a, Stage II) was amorphous, which was confirmed by the electron diffraction (selected area electron diffraction pattern – SAED). The cross section of the modified part is shown in Fig. 13a, Stage III.
The TEM analysis shows micro-scale inhomogeneity in the migration channel microstructure (Fig. 13a, Stage III). The material from the migration channel was partially removed and the substrate was not fully exposed. The crystallites of the modified surface had a larger size than those from the as-deposited area, which was proved by the SAED analysis. Phase analysis was performed using high resolution transmission electron microscopy (HRTEM) (Fig. 13b). The rings of the diffraction pattern for the modified surface were less blurred than the rings of the diffraction pattern for the as-deposited material. The protective gas caused unbalanced re-crystallization, causing bubbles to form under the surface.
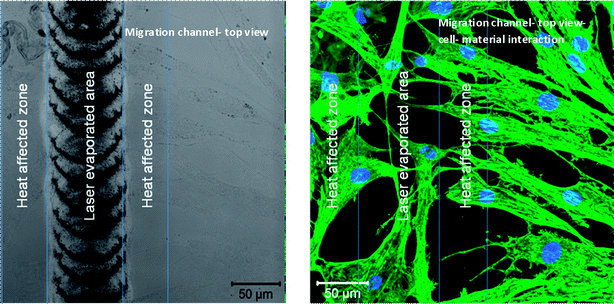
In the spot-like channels, the heat affected zone played an important role in the cell response on the material. This zone was developed around the wells and an overlap was observed between neighboring zones. This area was an obstacle to cellular growth and proliferation (Fig. 15).
Illustrative diagrams of the surface modifications are shown in Fig. 16a and b. Fig. 16a presents the schematic of the porous layer placed on a line-shaped migration channel, while Fig. 16b presents the schematic of the porous layer placed on a well-shaped migration channel.
The porous functional layers were deposited by the “Layer by Layer” technique. The surface, plain view analysis revealed that no change in the geometry of the substrate surface, shaped in the form of channels, was observed (Fig. 17).
Mimicking the internal structure of a blood vessel, the endothelial cells were deposited on the surface in order to inhibit the blood clotting cascade. A few days of observation showed the formation of an appropriate cell monolayer. The following steps of confluent monolayer formation are presented in Fig. 18a to c. Fig. 18a presents 1 day of cell culture, Fig. 18b presents 2 days of cell culture and Fig. 18c presents 3 days of cell culture. The observation after the three days of culture revealed the appropriate confluent monolayer formation. The surface was properly covered with the endothelial layer. Then, the effectiveness of the endothelial layer in the inhibition of the coagulation process was tested.
 | ||
| Fig. 18 (a) Confluent growth after one day of cell culture. (b) Confluent growth after two days of cell culture. (c) Confluent growth after three days of cell culture. | ||
The research was designed to evaluate the performance of an alternative in vitro dynamic test for haemocompatibility. Instead of a linear flow chamber, the test was based on a cone and plate analyzer, an instrument validated for clinical use in the evaluation of thrombotic diseases and the effectiveness of anti-platelet drugs.
The material selection was performed on the basis of several components of the blood response. Results of the blood–material interaction experiments are illustrated in Fig. 19.
4. Discussion
The bio-mimetic approach was used to reproduce the properties of blood vessels on artificial surfaces. In the natural vessel, the dynamic dialogue between the tissues is able to control the life and death of cells. The properly formed monolayer of the endothelium is able to effectively inhibit the activation of the blood-clotting cascade, which was confirmed by the analysis performed in this work. Cell co-culture is an important aspect in the design of new materials. This issue is being studied by many researchers. The solution seems to be the creation of a suitable environment for active communication during tissue creation. Nackman et al. pointed out that the endothelial cells have been noted to affect smooth muscle cell activities related to intimal hyperplasia: proliferation, migration, and extracellular matrix production.27 The authors proposed a fully porous material, where the cells were cultured on both sides. Quite similar observations were found in the publication of J. Whitelock et al., where the authors found that the native endothelial extracellular matrix, which was shown to contain the same proteoglycans (PGs), demonstrated a pronounced stimulatory effect on the proliferation of human smooth muscle cells.28 Their data do not support the hypothesis that human endothelial cells, in vivo, control the activation or proliferation of smooth muscle cells directly by the secretion of a non-proliferative molecule. Instead, they support the hypothesis that the endothelial cells form a barrier from activating factors in the plasma.Taking into account the observations found in the literature, we tried to reproduce the cell co-culture concept, but on solid materials. The environment of the cell co-culture was reproduced on the surface of a solid. Most publications describe an oriented dynamic cellular culture.27–31 In our study, the oriented cell growth of smooth muscle cells was controlled through the migration channels, which were developed and successfully applied as a valuable method to observe and investigate the chemotaxis effect of the substrate on the cell. It should be noted that the migration channels were carried in a coating which was selected in dynamic tests of blood. The determination of the effect of the surface topography and microstructure on cell behavior is essential in understanding cell–substrate interactions. In our work, the pulsed laser ablation technique was used as the main method of surface modification in the first stage of the surface preparation. It cannot be stated that this is the best technique for modifying the surface of bulk biomaterials. Y. Feng et al. investigated photochemical immobilization and photograft polymerization.32 They observed that both techniques are efficient methods for the surface modification of biomaterials. From their observations, a biomimetic surface can be easily achieved via photo-modification by coupling bioactive molecules or peptide sequences. This method, however, does not allow the creation of appropriate conditions for the modification considered in our work. We proposed to construct an extracellular matrix-like (ECM-like) structure in the final step of the surface preparation. The ECM-like structure was prepared using synthetic materials which are deposited using an electrostatic based interaction. The method requires an appropriate surface charge of the substrate on which the porous structure is to be prepared. The smooth muscle cells growing in the migration channels and the endothelial cells were effectively separated by the ECM-like structure. The migration channel topography was not generated through the porous structure. The channels were not visible on the final top view observation of the surface. The surface of the porous coating was covered with endothelial cells in order to slow down the activation of the coagulation cascade. The effectiveness of endothelial cell monolayer formation was examined using a dynamic test with whole blood. As presented in Fig. 19, it can be concluded that in spite of the complicated construction of the coating, reduced activation of the coagulation system was observed.
5. Conclusions
On the basis of the results the following conclusions were formed:- Low platelet degradation was observed on contact with the silicon modified diamond-like carbon coating and titanium carbonitride.
- Materials were pre-selected at the initial stage of modification of the surface for migration channel formation.
- Material surface modification was dependent on the light absorption coefficient; during the fabrication of the migration channel a heat affected zone appeared as a side effect.
- A possible effect of re-sputtering deposition occurred, long time remodelling caused crystallization.
- The influence of the crystallite size and the surface topography changes on cellular behavior were observed, indicating the ability for controlled cellular growth depending on the microstructure.
- The surface modification in the form of migration channels was not generated through the porous, extracellular matrix-like structure; it was observed due to the probable self remodelling function of the synthetic materials.
Acknowledgements
This research was financially supported by project 2011/03/D/ST8/04103 “Self-assembling, biomimetic porous scaffolds in terms of inhibiting the activation of the coagulation system” of the Polish National Center of Science and the Polish–Austrian exchange project 2012–2014 no. 023/2012/2013/2014 8548/R 12/R 14 “The development of biomimetic thin films for cardiac support devices; new strategies based on vacuum based deposited self assembling biomaterials”.References
- D. Buddy Ratner, S. Allan, F. Hoffman, J. Schoen and J. E. Lemon, Biomaterials Science An Introduction to Materials in Medicine, Elsevier Inc, San Diego, California, 2nd edn, 2004 Search PubMed.
- B. M. Leung and M. V. Sefton, Ann. Biomed. Eng., 2007, 35, 2039–2049 CrossRef PubMed.
- X. U. Li, H. U. Kun, J. I. Yanpeng, C. Fuzhai and A. Hongbin, Front. Mater. Sci. China, 2007, 1(4), 388–394 CrossRef PubMed.
- D. G. Castner and B. D. Ratner, Surf. Sci., 2002, 500(1–3), 28–60 CrossRef CAS.
- A. Bandyopadhyay, V. K. Balla, M. Roy and S. Bose, J. Med., 2011, 63, 94–99 CAS.
- F. Platon, P. Fournier and S. Rouxel, Wear, 2001, 250, 227–236 CrossRef.
- C. Liu, Q. Bi and A. Matthews, Surf. Coat. Technol., 2003, 163–164, 597–604 CrossRef CAS.
- J. M. Lackner, Industrially-scaled hybrid Pulsed Laser Deposition at Room Temperature, Orecop sc, Krakow, 2005 Search PubMed.
- C. De Marco, S. M. Eaton, R. Suriano, S. Turri, M. Levi, R. Ramponi, G. Cerullo and R. Osellame, ACS Appl. Mater. Interfaces, 2010, 2(8), 2377–2384 CAS.
- X. Ma, H. Huo, M. Wei, L. Wang, M. Shen, C. Barry and J. Mead, Appl. Phys. Lett., 2011, 98, 171101 CrossRef PubMed.
- F. Baset, Femtosecond laser induced porosity in poly-methyl methacrylate, Appl. Surf. Sci., 2013, 282, 729–734 CrossRef CAS PubMed.
- R. Major, K. Maksymow, J. Marczak, J. M. Lackner, M. Kot and B. Major, Bull. Pol. Acad. Sci.: Tech. Sci., 2012, 60, 337–342 Search PubMed.
- J. A. Davidson, C. M. Asgian, A. K. Mishra and P. Kovacs, in Bioceramics 5, ed. T. Yamamuro, T. Kokubo and T. Nakamura, Kobunshi Kankokai, Kyoto, Japan, 1992, pp. 389–401 Search PubMed.
- R. Major, J. M. Lackner, K. Gorka, P. Wilczek and B. Major, RSC Adv., 2013, 3, 11283–11291 RSC.
- B. Yin, W. Wang and Y. Jin, Comput. Struct., 1997, 64(5–6), 931–938 CrossRef.
- R. Major and P. Lacki, Arch. Metall. Mater., 2005, 50, 379–385 CAS.
- K. J. Bathe, J. Walczak and H. Zhang, Proceedings of the 9th ADINA Conference, 1993, pp. 511–521.
- http://www.adina.com/.
- L. Major, W. Tirry and G. Van Tendeloo, Surf. Coat. Technol., 2008, 202, 6075–6080 CrossRef CAS PubMed.
- L. Richert, F. Boulmedais, P. Lavalle, J. Mutterer, E. Ferreux, G. Decher, P. Schaaf, J.-C. Voegel and C. Picart, Biomacromolecules, 2004, 5(2), 284–294 CrossRef CAS PubMed.
- T. Crouzier, K. Ren, C. Nicolas, C. Roy and C. Picart, Small, 2009, 5(5), 598–608 CrossRef CAS PubMed.
- D. J. Mooney, D. F. Baldwin, N. P. Suht, J. P. Vacantis and R. Larger, Biomaterials, 1996, 17, 1417–1422 CrossRef CAS.
- R. Major, J. Mater. Sci.: Mater. Med., 2013, 24, 725–733 CrossRef CAS PubMed.
- M. Sanak, B. Jakiela and W. Wegrzyn, Bull. Pol. Acad. Sci.: Tech. Sci., 2010, 58(2), 317–322 CAS.
- D. Varont, R. Dardiki, B. Shenkmanl, S. Kotev-Emeth, N. Farzarne, I. Tamarinl and N. Savion, Thromb. Res., 1997, 85(4), 293–294 Search PubMed.
- J. M. Lackner, W. Waldhauser, R. Major, B. Major and F. Bruckert, Hemocompatible, pulsed laser deposited coatings on polymers, Biomed. Tech., 2010, 55, 57–64 CrossRef CAS PubMed.
- G. B. Nackman, M. F. Fillinger, R. Shafritz, T. Wei and A. M. Graham, Surgery, 1998, 124(2), 353–361 CrossRef CAS.
- J. Whitelock, S. Mitchell and P. A. Underwood, Cell Biol. Int., 1997, 21, 181–189 CrossRef CAS.
- F. Kinard, T. Sergent-Engelen, A. Trouet, C. Remacle and Y. J. Schneider, In Vitro Cell. Dev. Biol.: Anim., 1997, 33(2), 92–103 CrossRef CAS.
- V. A. Conejo, R. De Haro, J. Sosa-Melgarejo and J. D. Méndez, Biomed. Pharmacother., 2007, 61(2–3), 173–179 CrossRef CAS PubMed.
- M. F. Fillinger, L. N. Sampson, J. L. Cronenwett, R. J. Powell and R. J. Wagner, J. Surg. Res., 1997, 67(2), 169–178 CrossRef CAS PubMed.
- Y. Feng, H. Zhao, L. Zhang and J. Guo, Front. Chem. Eng. China, 2010, 4(3), 372–381 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |