Miceller media accelerated Baylis–Hillman reaction
Balu
Pawar
a,
Vikas
Padalkar
a,
Kiran
Phatangare
a,
Sudhakar
Nirmalkar
a and
Atul
Chaskar
*b
aDepartment of Chemistry, Postgraduate and Research Centre, C. K. Thakur College, Navi Mumbai, Maharashtra, India
bDepartment of Chemistry, National Taiwan University, Taipei 106, Taiwan. E-mail: achaskar@rediffmail.com; Tel: + 886-0917352249
First published on 16th September 2011
Abstract
Triton X-100 aqueous micelle accelerates the rate of Baylis–Hillman reaction and enhances the product yield. The type and concentration of surfactants play prominent roles in the reaction. An aqueous micelle Triton X-100 is easily recovered and reused for four runs without substantial loss in yield. 1,4-diazabicyclo[2.2.2]octane (DABCO) (20 mol%) is found to be a good basic catalyst in aqueous micelle. Apparently, this protocol has several advantages such as green reaction medium, mild reaction condition, ease of product recovery and moderate-to-good yield of product.
Introduction
Since its invention in 1972,1 the Baylis–Hillman reaction has fascinated chemists owing to its carbon-carbon bond forming ability which offers multifunctional adducts by preserving the atom economy.2 The reaction between aldehyde and α-carbon of an electron deficient olefin in the presence of tertiary amine furnishes a multifunctional product which has extensive uses in a myriad of synthetic contexts.3 However, the main shortcomings of this reaction include slow reaction rate, moderate yield and inertness to enones, α,β-substituted aldehydes and hindered aldehydes. The need of improvement in reaction rate, product yield and environmental benign nature was met by using water as reaction medium.4 Hitherto, the reaction is being practised by various methods involving different strategies viz. ionic liquids,5 PEG-400,6 sulpholane,7 supercritical CO2,8 ultrasound,9 high pressure,10 microwave irradiation,11 aqueous acidic media,12etc. Literature data reveal that generally in a Baylis–Hillman reaction, polar solvents are employed as they increase the equilibrium constant of the zwitterionic intermediate, formed during the course of the reaction.13,14 Indeed, low solubility of reactant and incompatibility of intermediate hamper the reaction rate in aqueous medium. This problem could be encountered with use of miceller media, a microheterogenous system. In recent years aqueous micelles are widely used for chemical reactions.15,16Herein, we present our observations about the potential use of miceller solution, TX 100 surfactant in aqueous medium, for a Baylis–Hillman reaction in presence of 1,4-diazabicyclo[2.2.2]octane (DABCO) at room temperature (Scheme 1). It is intriguing to note that the presence of water insoluble reactants within the hydrophobic core of miceller media boost the rate of reaction.
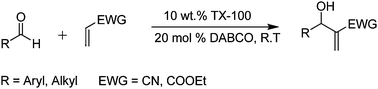 | ||
| Scheme 1 Baylis–Hillman reaction in TX-100 aqueous micelles. | ||
Results and discussion
With regards to optimization of the reaction conditions, the reaction between 2-nitrobenzaldehyde and acrylonitrile was selected as a model reaction. Initially, when this reaction was carried out in the presence of water alone, the reaction was found to be very sluggish, because of poor solubility of aldehydes in water (entry 1, Table 1). Also, in the presence of organic solvent such as THF and dioxan, negligible improvements in the results were observed even though reactions were carried out for a longer period (entries 2, 3, Table 1). Moreover, cationic surfactant cetyl trimethylammonium bromide (CTAB) and anionic surfactant sodium dodecyl sulfate (SDS) offered good conversion and yield at 10 wt% concentration (entries 4, 5, Table 1). Although the ionic surfactants offered good results, the toxicity and cost of these surfactants limited their use. Hence we decided to use non-ionic surfactant TX-100 aqueous micelles. To our delight the best result was obtained with TX-100 aqueous micelles.| Entry | Solvents | Time/h | Conversion (%)b | Yieldc |
|---|---|---|---|---|
| a Reagents and reaction conditions: 2-nitrobenzaldehyde (1 mmol) and acrylonitrile (2 mmol); TX-100 aqueous micelles (10 wt%), DABCO (20 mol%), room temperature. b % conversion was monitored by gas chromatography. c Isolated yield. | ||||
| 1 | Pure water | 12 | 55 | 52 |
| 2 | THF | 48 | 58 | 56 |
| 3 | 1,4-dioxan | 48 | 62 | 60 |
| 4 | 10 wt% CTAB/H2O | 2 | 86 | 78 |
| 5 | 10 wt% SDS/H2O | 2 | 82 | 76 |
| 6 | 5 wt% TX-100/H2O | 2 | 90 | 88 |
| 7 | 10 wt% TX-100/H2O | 1 | 92 | 90 |
| 8 | 20 wt% TX-100/H2O | 1 | 85 | 78 |
| 9 | 50 wt% TX-100/H2O | 1 | 84 | 78 |
Concurrently, we found that the concentrations of TX-100 aqueous micelle influenced the rate of conversion and yield of product. The reaction conversion increased with increase in concentration of TX-100 owing to enlargement of the interfacial area and lower mass transfer resistance15 (entries 6, 7, Table 1). The best conversion of 92% was obtained in 10 wt% TX-100 aqueous micelles. However, further increase in concentration inversely affected the conversion rate (entries 8, 9, Table 1). This was mainly for unused excess TX-100 surfactant, which formed a mask around its own micelle, and hence inhibited the interaction between the substrates.
The catalytic effect of TX-100 micelles in the Baylis–Hillman reaction is illustrated in Fig. 1. In the presence of TX-100 aqueous micelles, the water insoluble substrates migrated into the hydrophobic core of micelle. The enhancements in the reactivity of aldehydes and activated olefins are more in aqueous micellar solution than in pure water and/or different organic solvents. This may be due to the micelles' ability to work as micro- or nanoreactor17 and to stabilize the zwitterionic intermediate, generated from the Michael addition of Lewis base to the activated olefins. The presence of zwitterionic intermediate and aldehyde in the pseudo phase region of micellar solution accelerates the nucleophilic addition, thus offering the Baylis–Hillman adducts in good-to-excellent yield.
 | ||
| Fig. 1 Tentative mechanism of the Baylis–Hillman reaction in micelles. | ||
All the aforementioned results revealed that DABCO in 10 wt% TX-100 aqueous micelles is the best choice for Baylis–Hillman reaction. To explore generality and scope of the protocol, we treated various substituted aldehydes with acrylonitrile/ethyl acrylate (Table 2). The conversion gave the product in good-to-excellent yield under notably practical conditions. The physical and spectral data of all the compounds are in good agreement with the literature.4–12
| Entry | Aldehyde | Activated olefin | Time/h | Yieldb (%) |
|---|---|---|---|---|
| a Reagents and reaction conditions: aldehyde (1 mmol) and acrylonitrile or ethyl acrylate (2 mmol); TX-100 aqueous micelles (10 wt%), DABCO (20 mol%), room temperature. b Isolated yield. | ||||
| 1 |

|
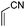
|
2 | 92 |
| 2 |

|
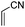
|
1.5 | 94 |
| 3 |

|

|
1.5 | 95 |
| 4 |

|

|
1.5 | 90 |
| 5 |

|

|
2.5 | 85 |
| 6 |

|
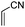
|
5.0 | 72 |
| 7 |

|

|
2 | 90 |
| 8 |

|

|
1.5 | 87 |
| 9 |
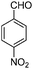
|

|
1.5 | 92 |
| 10 |
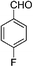
|
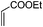
|
2.0 | 87 |
| 11 |

|

|
2.5 | 82 |
| 12 |

|
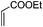
|
3.0 | 88 |
| 13 |
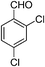
|

|
4.0 | 75 |
| 14 |
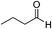
|
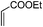
|
4.5 | 80 |
| 15 |

|
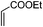
|
4.5 | 64 |
The investigation of recyclability of reaction media revealed that the reaction media could be recycled and reused for four consecutive cycles offering the same conversions and negligible loss in yield (Table 3). Upon completion of the reaction the product and unutilized starting materials were extracted using diethyl ether and thus separated micellar media were reused for the next cycle.
| Runs | Conversion (%)b | Yieldc |
|---|---|---|
| a Reagents and reaction conditions: 2-Nitrobenzaldehyde (1 mmol) and acrylonitrile (2 mmol); TX-100 aqueous micelles (10 wt%), DABCO (20 mol%), room temperature. b % conversion was monitored by gas chromatography. c Isolated yield. | ||
| 1 | 92 | 90 |
| 2 | 92 | 88 |
| 3 | 91 | 87 |
| 4 | 92 | 83 |
Conclusion
In summary, micellar media accelerated inventive synthetic protocol has been successfully established for the Baylis–Hillman reaction. The reaction is catalyzed by 10 mol% DABCO in micellar media. The process has several advantages over the many documented procedures from economical and environmental points of view, such as operational simplicity, short reaction time, excellence in yield and recyclability of the reaction media. We believe that this will provide a better and more practical alternative to the existing methodologies for large scale production.Experimental
All commercial reagents and solvents were procured from s. d. fine chemicals (India) and were used without further purification. However, 1,4-diazabicyclo[2.2.2]octane (DABCO) was purchased from Ms. Spectrochem Ltd., Mumbai. The reaction was monitored by TLC using 0.25 mm E-Merck silica gel 60 F254 precoated plates, which were visualized with UV light. Analysis was performed on gas chromatograph (chemito 8610) with flame ionization detector. A 4 m long i.d. S.S. column packed with OV-17 on chromosorb WHP was employed for the analysis. Nitrogen was used as carrier gas at a flow rate of 30 mL m−1. Infrared spectra were recorded on a Perkin-Elmer spectrum 100 FT-IR spectrometer. 1H NMR spectra were recorded on Bruker Avance 400 MHz spectrometer in CDCl3, using TMS as an internal standard.General procedure for Baylis–Hillman in TX-100 aqueous micelles
Into a stirred solution of aldehyde (1 mmol) and acrylonitrile/ethyl acrylate (2 mmol) in 10 wt% TX-100 aqueous micelles (5 mL) was added DABCO (20 mol%) and the vigorous stirring was continued at room temperature. The progress of the reaction was monitored by TLC. After completion of reaction, as indicated by TLC and GC, the product was extracted with diethyl ether (3 × 10 mL). The collective organic phase was dried and concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography.Representative spectral data
FT IR (cm−1): 3434, 2861, 2228, 1608, 1524, 1347, 1185, 859, 789, 731.
Mass (m/z): theoretical 204, observed 204, (M − 1) 203.
Acknowledgements
The authors are greatly thankful to RSIC, I.I.T Mumbai for providing the 1H NMR and mass spectroscopy facilities, and Dr S.T. Gadade for his benevolent support.References
- A. B. Baylis and M. E. D. Hillman, German patent, 2155113, 1972 Search PubMed; A. B. Baylis and M. E. D. Hillman, Chem. Abstr., 1972, 77, 34174q Search PubMed.
- B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS.
- (a) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS; (b) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS; (c) D. Basavaiah, P. D. Rao and R. S. Hyma, Tetrahedron, 1996, 52, 8001 CrossRef CAS; (d) P. Langer, Angew. Chem., Int. Ed., 2000, 39, 3049 CrossRef CAS.
- (a) H. S. Byun, K. C. Reddy and R. Bittman, Tetrahedron Lett., 1994, 35, 1371 CrossRef CAS; (b) F. Rezgui and M. M. E. Gaied, Tetrahedron Lett., 1998, 39, 5965 CrossRef CAS; (c) J. Auge, N. Lubin and A. Lubineau, Tetrahedron Lett., 1994, 35, 7947 CrossRef CAS; (d) D. Basavaiah, M. Krishnamacharyulu and A. J. Rao, Synth. Commun., 2000, 30, 2061 CrossRef CAS; (e) C. Z. Yu, B. Liu and L. Q. Hu, J. Org. Chem., 2001, 66, 5413 CrossRef CAS.
- J. N. Rosa, C. A. M. Afonso and A. Santos, Tetrahedron, 2001, 57, 4189 CrossRef CAS.
- S. Chandrasekhar, C. Narsihmulu, B. Saritha and S. S. Sultana, Tetrahedron Lett., 2004, 45, 5865 CrossRef CAS.
- P. R. Krishna, A. Manjuvani, V. Kannan and G. V. M. Sharma, Tetrahedron Lett., 2004, 45, 1183 CrossRef CAS.
- P. M. Rose, A. A. Clifford and C. M. Rayner, Chem. Commun., 2002, 968 RSC.
- F. Coelho, W. P. Almeida, D. Veronese, C. R. Mateus, E. C. S. Lopes, R. C. Rossi, G. P. C. Silveira and C. H. Pavam, Tetrahedron, 2002, 58, 7437 CrossRef CAS.
- Y. Hayashi, K. Okado, I. Ashimine and M. Shoji, Tetrahedron Lett., 2002, 43, 8683 CrossRef CAS.
- M. K. Kunda, S. B. Mukherjee, N. Balu, R. Padmakumar and S. V. Bhat, Synlett, 1994, 444 CrossRef.
- P. Caumul and H. C. Hailes, Tetrahedron Lett., 2005, 46, 8125 CrossRef CAS.
- (a) J. Cai, Z. Zhou, G. Zhao and C. Tang, Org. Lett., 2002, 4, 4723 CrossRef CAS; (b) R. O. M. A. de Souza, V. L. P. Pereira, P. M. Esteves and M. L. A. A. Vaconcellos, Tetrahedron Lett., 2008, 49, 5902 CrossRef CAS.
- E. Ciganek, The Morita Baylis Hillman reaction, in Organic reactions, ed. L. A. Paquette, John Wiley & Sons, New York, 1997, vol. 51, pp. 201–247 Search PubMed.
- T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., Int. Ed., 2005, 44, 7174 CrossRef CAS.
- (a) K. Manabe, S. Limura, X. M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2002, 124, 11971 CrossRef CAS; (b) G. Lu and C. Cai, Colloids Surf., A, 2010, 355, 193 CrossRef CAS; (c) A. Kumar, M. Gupta and M. Kumar, Tetrahedron Lett., 2010, 51, 1582 CrossRef CAS; (d) A. A. Jafari, F. Moradgholi and F. Tamaddon, Eur. J. Org. Chem., 2009, 1249 CrossRef CAS; (e) A. Chaskar, V. Padalkar, K. Phatangare, B. Langi and C. Shah, Synth. Commun., 2010, 40, 2336 CrossRef CAS; (f) H. Firouzabadi and N. Iranpoor, Adv. Synth. Catal., 2009, 351, 755 CrossRef CAS; (g) B. H. Lipshutz and R. Alexander, Org. Lett., 2008, 10, 5329 CrossRef CAS.
- J. Z. Jiang and C. Cai, J. Colloid Interface Sci., 2006, 299, 938 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
