Transition metal coordination complexes of chrysazin†
Abstract
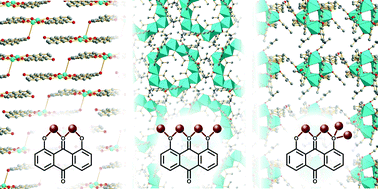
Eleven novel coordination compounds, composed of chrysazin (1,8-dihydroxyanthraquinone) and different first-row transition metals (Fe, Co, Ni, Cu), were synthesised and the structures determined by single-crystal X-ray diffraction. The synthetic trends were investigated using high-throughput synthesis under systematic variation of concentration and reagent stoichiometry: for complexes containing Co, Ni or Cu crystallisation was improved by low ligand : metal ratios, while the effect of concentration depended on the metal used. The compounds crystallise as discrete clusters, apart from two, which contain long Cu–O bonds which may allow the two compounds to be considered one-dimensional coordination polymers. One of these compounds shows a distance between aryl rings of less than 3.26 Å, which is shorter than that in graphite, suggesting applications as an organic–inorganic semiconductor. The compound was found to be insulating by single-crystal and powder AC-impedance measurements, and this result is discussed with reference to the electronic structure calculated using density-functional theory.
- This article is part of the themed collection: In celebration of Tony Cheetham’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...