A study of the interaction of cationic dyes with gold nanostructures†
Abstract
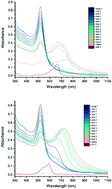
The interaction of methylene blue and crystal violet dyes with a range of gold nanoparticles (AuNPs), gold nanoclusters and gold/silver nanoclusters is reported. It is found that 20 nm citrate-capped AuNPs have strong interactions with these two dyes that result in red-shifted absorption peaks in their electronic absorption spectra. Transmission electron microscopy and dynamic light scattering measurements show that this can be attributed to these AuNPs combining into large agglomerates. Eventually, precipitation is observed. The agglomeration process is triggered when the dye reaches or exceeds a threshold concentration and then does not stop until all the AuNPs have agglomerated into large particles and precipitated. Calculations suggest that the threshold concentration corresponds to having sufficient dye molecules to form a monolayer on the surface of AuNPs. We also observe similar red-shifting in the absorption peaks of the electronic absorption spectra of 11–50 nm citrate-capped AuNPs formed by both single step and seeded growth methods. No such interactions were observed in the UV-vis spectra of the dyes with Tris-capped AuNPs, gold nanoclusters or gold/silver nanoclusters.


 Please wait while we load your content...
Please wait while we load your content...