Mechanism and kinetic properties for the complete series reactions of chloro(thio)phenols with O(3P) under high temperature conditions†
Abstract
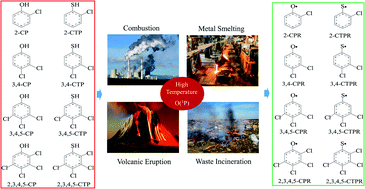
Polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/Fs) and polychlorinated dibenzothiophenes/thianthrenes (PCDT/TAs) are two groups of dioxin-like compounds with oxygen and sulfur substitution, respectively. Chlorophenols (CPs) and chlorothiophenols (CTPs) are direct precursors in PCDD/F and PCDT/TA formation. The formation of chlorophenoxy radicals (CPRs) and chlorothiophenoxy radicals (CTPRs) from chlorophenols (CPs) and chlorothiophenols (CTPs) with O(3P) is an important initial step for the formation of PCDD/Fs and PCDT/TAs, respectively. In this paper, the formation of CPRs/CTPRs from the complete series reactions of 19 CP/CTP congeners with O(3P) was studied using the density functional theory (DFT) method. The rate constants of each reaction were calculated using canonical variational transition state (CVT) theory along with a small-curvature tunneling (SCT) contribution over a wide temperature range of 600–1200 K. The effect of the chlorine substitution pattern on the structural parameters, thermochemical properties and rate constants in both CPs and CTPs was discussed. This study shows that the reactions between CPs and O(3P) can be affected by the chlorine substitution at the para-position, and the reactions between CTPs and O(3P) are mostly influenced by both ortho-substitutions. The thiophenoxyl-hydrogen abstraction from CTPs by O(3P) is more likely to occur than the phenoxyl-hydrogen abstraction from CPs by O(3P). Comparison of the reactivity of CP/CTPs with O(3P) with our previous work on CP/CTPs with H and OH shows that the order for phenoxyl-hydrogen abstraction potential is CP + OH > CP + O(3P) > CP + H, and the order for thiophenoxyl-hydrogen abstraction potential is CTP + O(3P) > CTP + H > CTP + OH.


 Please wait while we load your content...
Please wait while we load your content...