Highly efficient synthesis of tert-butyl esters using (Boc)2O under solvent/base-free electromagnetic milling conditions: a new reaction model†
Yunxia
Liu
a,
Yan
Zhang
a,
Yingyu
Qu
a,
Qing
Liu
a,
Kai
Cao
*b,
Fachao
Yan
a,
Lizhi
Zhang
a,
Xinjin
Li
 *a,
Zengdian
Zhao
*a and
Hui
Liu
*a,
Zengdian
Zhao
*a and
Hui
Liu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong 255049, China. E-mail: huiliu1030@163.com; zhaozengdian@sdut.edu.cn; lixj@sdut.edu.cn
bPharmacy Department, Zibo Central Hospital, Zibo 255036, China. E-mail: wwwcaokai@126.com
First published on 9th January 2025
Abstract
A novel and efficient method has been developed for the synthesis of tert-butyl esters using (Boc)2O as the tert-butyl source, facilitated by electromagnetic milling. This green and sustainable approach is solvent-free, base-free, and operates without the need for additional heating, making it highly appealing for eco-friendly synthesis. The entirely neutral reaction environment proves particularly advantageous for synthesizing or modifying sensitive drug molecules, especially in late-stage functionalization. In this process, ferromagnetic rods, used as grinding media, become magnetized and charged under a high-speed rotating magnetic field. This magnetization plays a crucial role in bond activation by coordinating with the charged ferromagnetic rods, introducing a novel mechanism for bond activation that could inspire further research in this emerging field.
Green foundation1. This work introduces a novel and efficient method for the synthesis of tert-butyl esters using (Boc)2O as the tert-butyl source, facilitated by electromagnetic milling.2. This solvent-free, base-free, and heating-free approach presents an attractive, green, and sustainable pathway. The neutral reaction environment is especially beneficial for the late-stage modification of sensitive drugs in drug discovery, ensuring both efficiency and compatibility with delicate compounds. 3. In this process, ferromagnetic rods, acting as grinding media, become magnetized and charged under a high-speed rotating magnetic field. The magnetization is key to bond activation, as it interacts with the charged rods, introducing a novel mechanism that could inspire further research in this emerging field. |
Introduction
tert-Butyl is a crucial functional group in organic synthesis, known for its substantial steric hindrance and pronounced spatial effects in reactions. It acts as a large steric group, providing kinetic stability and resisting nucleophilic attacks, which make it an important protective group for carboxylic acids.1 Its utility is especially notable in the synthesis of bioactive compounds like hepatitis C virus NS3 protease inhibitors2 and GABA-T inhibitors.3 Various methods for synthesizing tert-butyl esters have been developed. One of the earliest synthetic methods for preparing tert-butyl esters is the Fischer–Speier esterification, developed in 1895. This method involves the reaction of carboxylic acids with tert-butanol under the catalysis of acids, typically either Lewis acids or Brønsted acids (Scheme 1a).4 It lays the foundation for the development of other tert-butyl esterification methods and remains a classic technique in organic chemistry. Later, Wright and co-workers introduced a method for tert-butyl ester synthesis using sulfuric acid and an excess of tert-butyl alcohol in the presence of anhydrous magnesium sulfate.5 This modification enhanced the reaction efficiency and broadened the scope of applicable substrates, offering an alternative to the Fischer–Speier esterification for preparing tert-butyl esters under different conditions. In 2018, La et al. introduced a BF3·OEt2-promoted tert-butyl esterification process, employing 2-tert-butylpyridine as an innovative tert-butyl surrogate6 (Scheme 1b). This method enables a more controlled esterification process, overcoming the limitation of using strong concentrated acids, which can influence the esterification of substrates bearing acid-sensitive functional groups. Meanwhile, Skrydstrup et al. developed a palladium-catalyzed tert-butyloxycarbonylation reaction of aryl bromides with carbon monoxide, offering a more direct route to tert-butyl esters7 (Scheme 1c). However, despite the efficiency of this process, the use of toxic carbon monoxide posed a significant limitation, highlighting the need for safer alternatives in synthetic methodologies. | ||
| Scheme 1 Research on the synthesis of tert-butyl esters (EA – electron acceptor; ED – electron donor; EMM – electromagnetic milling). | ||
In contrast, di-tert-butyl dicarbonate ((Boc)2O), a safer, more cost-effective, and efficient amino acid protectant, has become widely utilized across several industries, including pharmaceuticals, protein and peptide synthesis, biochemical food production, and cosmetics.8 Due to its broad applicability and ease of use, (Boc)2O has also proven instrumental in the synthesis of tert-butyl esters, offering a safer alternative to methods involving more hazardous reagents, such as carbon monoxide, and reinforcing its importance in modern synthetic chemistry. In 2008, Balsells and colleagues introduced a method for preparing tert-butyl esters at low temperatures by reacting aryl bromides with a magnesium–lithium reagent and (Boc)2O, as shown in Scheme 1d.9 Later, in 2014, Li et al. successfully developed a palladium-catalyzed synthesis process of tert-butyl esters using boric acid or boronic acid esters in combination with (Boc)2O (Scheme 1e).10 These methods offer efficient routes for tert-butyl ester formation under mild conditions. However, the highly moisture and air sensitive metallic reagent and expensive palladium catalyst limited their application.
Therefore, developing a green and efficient method for synthesizing tert-butyl ester compounds under mild reaction conditions presents a significant challenge. In recent years, the field of mechanochemistry has experienced significant advancements, yielding numerous pivotal research findings, such as those by Ito (redox reactions of small organic molecules using ball milling and piezoelectric materials and solid-state cross-coupling of insoluble aryl halides),11 Bolm (the first to perform asymmetric organocatalysis in a ball mill),12 Friščić (constructing porous MOFs using LAG and ILAGs),13 Pilarski (solvent-free catalytic C–H and C–X functionalization without a ball mill),14 Borchardt (photo- or thermo-mechanochemistry),15 Gouverneur (fluorochemicals from fluorspar via a phosphate-enabled mechanochemical process that bypasses HFs),16 Browne (the use of temperature-controlled mechanochemistry to enable mechanochemical nickel-catalyzed Suzuki–Miyaura couplings),17 Wang (covalent and non-covalent functionalization of fullerenes and related materials through mechanochemistry),18 Lian (new reactions with ball milling and piezoelectric materials),19 Wei (mechanochemical synthesis of a-halo alkylboronic esters),20 Zhang and Szostak (the first mechanochemical decarbonylative coupling),21 and Yu and Su (α-C–H functionalization of glycine derivatives under mechanochemically accelerated aging en route to the synthesis of 1,4-dihydropyridines and α-substituted glycine esters).22 Recently, our group has developed a novel multi-energy field coupling apparatus for boronization, Suzuki coupling, and cyanidation in a solvent-free system.23 Electromagnetic milling (EMM) represents an innovative grinding device that utilizes small ferromagnetic particles as the grinding media within a rotating electromagnetic field.24 The EMM consists of an inductor that generates a rotating magnetic field, positioned within a stationary housing that serves as the working chamber. Unlike traditional ball mills, where the housing rotates, the EMM features a stationary housing, while the ferromagnetic rods within the working chamber are set into motion by the vortex electromagnetic field. This motion enhances the mixing of solid reactants and increases the surface area through high-speed collisions of the ferromagnetic rods, leading to improved reaction efficiency compared to conventional ball mills.
Very recently, we discovered that this EMM device can facilitate redox reactions in organic molecules by transferring a single electron between ferromagnetic rods and organic molecules25 (Scheme 1f). On the basis of these results, we designed a method to generate a tert-butyl radical from (Boc)2O via a single electron transfer process under electromagnetic milling conditions, efficiently synthesizing tert-butyl esters with aromatic carboxylic acids (Scheme 2).
 | ||
| Scheme 2 Highly efficient synthesis of tert-butyl esters using (Boc)2O under solvent/base-free electromagnetic milling conditions. | ||
Results and discussion
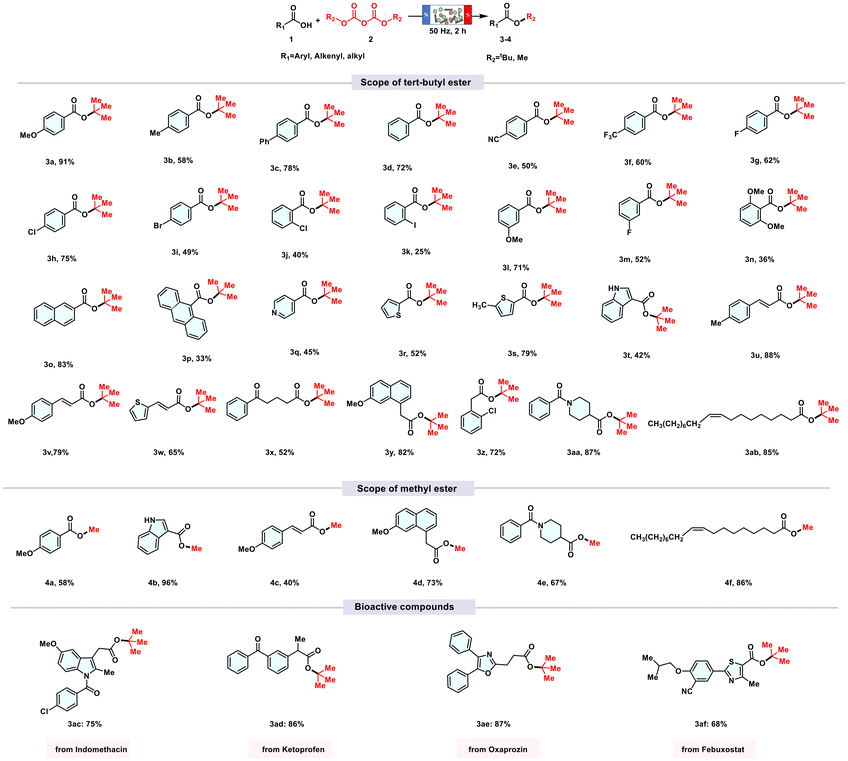
We began the optimization by using p-methoxybenzoic acid as a template substrate for the synthesis of tert-butyl esters. Initially, we explored the effects of ferromagnetic rods on the reaction efficacy. The results revealed that variations in rod specifications had a mild effect on the reaction efficiency (see Fig. 1A). Notably, when using ferromagnetic rods with dimensions of 1.0 × 30.0 mm or 1.0 × 50.0 mm, no target product was obtained, regardless of solvent presence (see Fig. S7†). However, a rod dimension of 0.3 × 5.0 mm resulted in a 76% yield, prompting us to select this specification for further investigation.We speculated that smaller steel needle diameters would result in a greater number of effective collisions. As the reaction progressed, the yield steadily increased, ultimately reaching 91% after 2 hours.
To further explore the scope of the reactions, we investigated various aromatic and alkyl carboxylic acids under the optimized conditions. The results revealed that both electron-rich and electron-deficient carboxylic acids can be efficiently converted into tert-butyl ester products. However, electron-rich carboxylic acids (3a, 3b and 3c) exhibited a higher conversion rate, while benzoic acid (3d) and electron-withdrawing carboxylic acids (3e and 3f) were less efficient. Notably, carboxylic acids containing halogen atoms (3g, 3h and 3i) produced the corresponding products in yields of 62%, 75% and 49%, respectively. It is important to highlight that the 62% yield with p-fluorobenzoic acid (3g) is likely due to the low boiling point of its product, leading to more significant losses during the collection process.
When the functional group on benzoic acid shifted to the ortho-position (3j and 3k), the yield decreased somewhat due to steric hindrance. However, when the functional group was located at the meta-position relative to the carboxyl group, steric hindrance had little effect on the yield, resulting in moderate to excellent yields (3l and 3m). Similarly, compound 3n was obtained with a lower yield (36%) due to significant steric hindrance. Polycyclic compounds such as 2-naphthoic acid and 9-anthracene carboxylic acid were also successfully tert-butyl esterified. The esterification of 2-naphthoic acid was particularly efficient, achieving a yield of 83% (3o). In contrast, 9-anthracene carboxylic acid produced the target product (3p) at a lower yield, again due to steric hindrance. Furthermore, this method has been successfully applied to heterocyclic carboxylic acids, including pyridine (3q), thiophene (3r and 3s), and indole (3t), resulting in excellent tert-butyl ester yields.
Additionally, cinnamic acids were efficiently converted into tert-butyl esters (3u and 3v) under the standard conditions, achieving remarkable yields. Similarly, (E)-3-(thiophen-2-yl)acrylic acid was converted with a 65% yield (3w). Surprisingly, alkyl tert-butyl esters (3x, 3y, 3z and 3aa) can also be synthesized from alkyl carboxylic acids and (Boc)2O under optimal conditions. Notably, long-chain alkyl carboxylic acids, such as oleic acid, underwent tert-butyl esterification with an impressive yield of 85% (3ab). Furthermore, replacing (Boc)2O with dimethyl di-carbonate for the methylation of carboxylic acids, whether aromatic, heteroaromatic, cinnamic, or alkyl, resulted in favorable conversions, yielding moderate to excellent results (4a–4f).
To evaluate the efficacy of this synthetic approach, a variety of complex bioactive compounds were employed in the synthesis of tert-butyl esters. Under the described reaction conditions, the desired tert-butyl esters were successfully obtained. As illustrated in Table 1, the bioactive pharmaceutical agents include nonsteroidal anti-inflammatory drugs such as indomethacin (1ac), ketoprofen (1ad), and oxaprozin (1ae), all known for their antipyretic and analgesic properties, as well as febuxostat (1af), which is recognized for its anti-gout effects.
| a Conditions: 1: 0.5 mmol, 2: 1.0 mmol, high temperature and pressure resistant reaction tube: 10 mL, ferromagnetic rods: SUS304, 5 g, 0.3 × 5.0 mm, reaction time: 2 h, frequency: 50 Hz. |
|---|
|
To investigate the impact of electromagnetic milling (EMM) on the synthesis of tert-butyl esters, we conducted several control experiments under ball milling and solvent-based conditions using traditional magnetic stirring (Table 2). Under EMM conditions, 3a was separated with a yield of 91% after 2 hours (entry 1). However, when the reaction was carried out under ball milling for 2 hours, only trace amounts of the target product 3a were detected regardless of whether agate balls or stainless steel balls were used (entries 2 and 3). Subsequently, we conducted a heating ball milling experiment (entries 4 and 5), which was heated and maintained at 110 °C for 20 minutes. After ball milling for 30 minutes, this process was repeated four times. The results indicated that only trace amounts of 3a were detected regardless of whether agate balls or stainless steel balls were used. Meanwhile, the yield decreased significantly when the reaction was performed at 110 °C without a solvent, and most of the starting materials were recovered (entries 6 and 7) (under EMM conditions, the reaction temperature reached 106 °C; see Fig. 1C). The reaction in toluene proceeded slowly at 110 °C, even after 24 hours, resulting in 14% yield of 3a. These results indicated that the process of EMM is not only based on collision and the heat generated via collision.
| Entry | Solvent | T/°C | t/h | 3a% | 5a% | 6a% | 1a% (recovered) |
|---|---|---|---|---|---|---|---|
Conditions: 1a: 0.5 mmol, 2a: 1.0 mmol, high temperature and pressure resistant reaction tube: 10 mL.a Ferromagnetic rods: SUS304, 5 g, 0.3 × 5.0 mm, reaction time: 2 h, frequency: 50 Hz.b Agate balls (20 g).c Stainless steel balls (20 g).d ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a: 1.0 mmol, 2a: 2.0 mmol agate balls (20 g), 110 °C, 2 h (heated and maintained at 110 °C for 20 minutes, and then ball milling for 30 minutes; this process has to be repeated four times).e 1a: 1.0 mmol, 2a: 2.0 mmol agate balls (20 g), 110 °C, 2 h (heated and maintained at 110 °C for 20 minutes, and then ball milling for 30 minutes; this process has to be repeated four times).e ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a: 1.0 mmol, 2a: 2.0 mmol stainless steel balls (20 g), 110 °C, 2 h (heated and maintained at 110 °C for 20 minutes, and then ball milling for 30 minutes; this process has to be repeated four times).f Oil bath (110 °C).g Oil bath (110 °C) solvent: toluene (2 mL). 1a: 1.0 mmol, 2a: 2.0 mmol stainless steel balls (20 g), 110 °C, 2 h (heated and maintained at 110 °C for 20 minutes, and then ball milling for 30 minutes; this process has to be repeated four times).f Oil bath (110 °C).g Oil bath (110 °C) solvent: toluene (2 mL). |
|||||||
| 1a | — | — (EMM) | 2 | 91 | Trace | Trace | Trace |
| 2b | — | — (ball milling) | 2 | Trace | Trace | Trace | 92 |
| 3c | — | — (ball milling) | 2 | Trace | Trace | Trace | 91 |
| 4d | — | 110 (ball milling) | 2 | Trace | 35 | 28 | 34 |
| 5e | — | 110 (ball milling) | 2 | Trace | 42 | 9 | 45 |
| 6f | — | 110 | 2 | 12 | Trace | 18 | 65 |
| 7f | — | 110 | 24 | 18 | Trace | 27 | 53 |
| 8g | Toluene | 110 | 2 | 5 | 43 | 32 | 19 |
| 9g | Toluene | 110 | 24 | 14 | Trace | 52 | 30 |
To further explore the reaction process, we conducted several control experiments to examine the intermediates and pathways involved. After 30 minutes of the esterification reaction, we identified and successfully isolated intermediates 5a and 6a. It was found that 5a could directly convert into the target product 3a with a 74% yield after 1 hour under electromagnetic milling conditions (Scheme 3). In contrast, under the same conditions, 6a did not produce the target product. However, when 1 mL of tert-butanol was added to the reaction system, 3a was isolated with a 43% yield. Notably, if the reaction was carried out in an oil bath, the addition of tert-butanol did not result in the formation of the target product 3a.
To investigate whether the metals of the ferromagnetic rods contribute to the formation of the target products, we conducted experiments using pure nickel rods and pure iron rods instead of ferromagnetic ones (Scheme 4a). In both cases, 3a was obtained in excellent yields. Additionally, under conventional stirring and heating conditions, less than 20% of the target product was produced, regardless of whether ferromagnetic rods were added (Scheme 4b). This suggests that the presence of ferromagnetic rods is not essential for the synthesis of tert-butyl esters under electromagnetic milling conditions.
Subsequently, a free radical trapping experiment using TEMPO and BHT was conducted; however, 3a was obtained in 89% and 85% yields, respectively. However, no TEMPO-trapped products were detected, indicating that it might be not a radical process; however, it could not be excluded either (Scheme 5-1 and 2). Furthermore, we employed thiobenzoic acid 1ag. After two hours under the standard conditions, the formation of 3ag was detected via GC-MS (see Fig. S8†), indicating that the tert-butyl group but not tert-butyl oxide in the product originates from (Boc)2O (Scheme 5-3). Next, Lewis acid FeCl2 with a stirring bar under EMM conditions was tested, producing the target product 3a in 13% yield. Although the Lewis acid FeCl2 could promote the transformation, the low efficiency indicated that Fe2+ should not be the catalyst which might get generated during the EMM process (Scheme 5-4).
Herein, four reaction mechanism pathways were proposed (Scheme 6). First of all, the carboxylic acid substance A reacts with (Boc)2O in situ to form the tert-butoxycarbonyl intermediate B. When exposed to a high-speed rotating magnetic field, the ferromagnetic rod becomes magnetized and charged, which could form a complex B-Ivia coordination like the working style of a Lewis acid. In path a, the ferromagnetic rod acts as an electron donor, and facilitates the generation of tert-butyl radicals and acyloxy anions via single electron transfer. The ferromagnetic rod subsequently acts as an electron acceptor, receiving electrons from the tert-butyl radicals to form tert-butyl cations. Although no radical adduct was detected, the radical process is still possible.13 Finally, the tert-butyl cation reacts with an acyloxy anion to form the target tert-butyl ester compound H. However, the radical capture experiments did not give the desired results. In path b, intermediate B-I could react with tert-butanol, generated within the system, to yield the target product H. Meanwhile, intermediate D could be formed through the reaction of the starting material A with intermediate B-I (path c). Intermediate D could further deliver the target product H in the presence of tert-butanol. However, this transformation could not be realized in a solvent with heating, indicating the key activation role of the ferromagnetic rod. Furthermore, path d is still an alternative pathway. With the activation of the ferromagnetic rod, complex B-I dissociates into tert-butyl carbocations G and E, accompanied by the release of CO2. Ultimately, the combination of G with E formed the desired product H. Based on the results of Scheme 5-3, path d is the most possible pathway, but we believe paths b and c are also possible. Notably, complex B-I serves as a pivotal intermediate across all these pathways, introducing a novel mechanism for bond activation in synthetic chemistry. Its unique ability to facilitate selective bond activation not only expands the toolkit of synthetic chemists, but also paves the way for more efficient and versatile reaction pathways, offering profound implications for the design of new reaction models.
Conclusions
We have developed a novel and efficient method for synthesizing tert-butyl esters from carboxylic acids and (Boc)2O. This solvent-free, base-free, and heating-free approach offers an appealing green and sustainable pathway. The neutral reaction environment is particularly valuable for late-stage modification of sensitive drugs in drug discovery. The reaction is driven by ferromagnetic rods, which become magnetized and charged under a high-speed rotating magnetic field. Based on the control experiments, four potential reaction pathways have been proposed. The coordination between the charged ferromagnetic rods and the reactants plays a crucial role in bond activation during the transformation. Although several key intermediates have been isolated and further transformed into the target molecules, the exact mechanism of bond activation under electromagnetic milling (EMM) conditions remains unclear. Ongoing research in our lab aims to further elucidate this mechanism, with the hope that it will attract broader attention and establish a new field of research.Author contributions
Yunxia Liu: conceptualization, methodology, data curation, writing – original draft, draft preparation and writing – reviewing and editing. Yan Zhang and Yingyu Qu: data curation; Qing Liu, Fachao Yan and Kai Cao: visualization and investigation; Lizhi Zhang: supervision; and Xinjin Li, Zengdian Zhao and Hui Liu: conceptualization and methodology.Data availability
The data underlying this study are available in the published article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (22078178) and the Youth Innovative Talents Attracting and Cultivating Plan of Colleges and Universities in Shandong Province.References
- (a) A. Kraft, Chem. Commun., 1996, 1, 77–79 RSC; (b) B. Krebs and H. U. Hürter, Acta Crystallogr., Sect. A, 1981, 37, 163 Search PubMed; (c) G. Eulenberger, Z. Naturforsch., B, 1981, 36, 521 CrossRef CAS; (d) G. Solladie, C. Hamdouchi and C. Ziani-Chérif, Tetrahedron:Asymmetry, 1991, 2, 457 CrossRef CAS.
- F. Orvieto, U. Koch, V. G. Matassa and E. Muraglia, Bioorg. Med. Chem. Lett., 2003, 13, 2745–2748 CrossRef CAS PubMed.
- M. Kolb, J. Barth, J. G. Heydt and M. J. Jung, J. Med. Chem., 1987, 30, 267–272 CrossRef CAS PubMed.
- E. Fischer and A. Speier, Untersuchungen aus Verschiedenen Gebieten: Vorträge und Abhandlungen Allgemeinen Inhalts, 1924, pp. 285–291 Search PubMed.
- S. W. Wright, D. L. Hageman, A. S. Wright and L. D. McClure, Tetrahedron Lett., 1997, 38, 7345–7348 CrossRef CAS.
- M. T. La and H. K. Kim, Tetrahedron, 2018, 74, 3748–3754 CrossRef CAS.
- Z. Xin, T. M. Gøgsig, A. T. Lindhardt and T. Skrydstrup, Org. Lett., 2012, 14, 284–287 CrossRef CAS PubMed.
- (a) T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Sytnthesis, John Wiley and Sons, New York, 3rd edn 1999; (b) P. J. Kocienski, Protecting groups, Georg Thieme, New York, 2000; (c) F. Shirini, M. Mamaghani and S. V. Atghia, Catal. Commun., 2011, 12, 1088 CrossRef CAS.
- (a) H. Li and J. Balsells, Tetrahedron Lett., 2008, 49, 2034 CrossRef CAS; (b) J. C. Amedio Jr, G. T. Lee, K. Prasad and O. Repic, Synth. Commun., 1995, 25, 2599 CrossRef; (c) R. C. Larock, Comprehensive Organic Transformations, 1999 Search PubMed; (d) E. Ghera and Y. J. Bendavid, Org. Chem., 1988, 53, 2972 CrossRef CAS; (e) D. Rausch and C. Lambert, Org. Lett., 2006, 8, 5037 CrossRef CAS PubMed; (f) L. D. Bratton, H. Huh and R. A. J. Bartsch, Chem, 2000, 37, 815 CAS.
- X. Li, D. Zou, H. Zhu, Y. Wang, J. Li, Y. Wu and Y. Wu, Org. Lett., 2014, 16, 1836–1839 CrossRef CAS PubMed.
- (a) K. K. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500–1504 CrossRef CAS PubMed; (b) X. Wang, C. Feng, J. Jiang, S. Maeda, K. Kubota and H. Ito, Nat. Commun., 2023, 14, 5561 CrossRef CAS PubMed; (c) K. Kubota, N. Shizukuishi, S. Kubo and H. Ito, Chem. Lett., 2024, 53, upae056 CrossRef; (d) T. Seo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2023, 145, 6823–6837 CrossRef CAS PubMed.
- (a) S. Pan, F. F. Mulks, P. Wu, K. Rissanen and C. Bolm, Angew. Chem., Int. Ed., 2024, 63, e202316702 CrossRef CAS PubMed; (b) S. Biswas and C. Bolm, Org. Lett., 2024, 26, 1511–1516 CrossRef CAS PubMed; (c) C. Sahu, S. Biswas, R. Hommelsheim and C. Bolm, RSC Mechanochem., 2024, 1, 38–42 RSC; (d) V. S. Pfennig, R. C. Villella, J. Nikodemus and C. Bolm, Angew. Chem., Int. Ed., 2022, 61, e202116514 CrossRef CAS PubMed; (e) F. Puccetti, T. Rinesch, S. Suljić, K. Rahimi, A. Herrmann and C. Bolm, Chem, 2023, 9, 1318–1332 CrossRef CAS.
- (a) R. Hernandez, I. Trakakis, J. Cuccia, T. Friščić and P. Forgione, Eur. J. Org. Chem., 2023, e202300374 CrossRef CAS; (b) M. Pérez-Venegas, T. Friščić and K. Auclair, ACS Sustainable Chem. Eng., 2023, 11, 9924–9931 CrossRef.
- (a) M. Hribersek, C. Méndez-Gálvez, M. Huber, P. J. Gates, P. Shakari, A. Samanta and L. T. Pilarski, Green Chem., 2023, 25, 9138–9145 RSC; (b) F. J. Ingner, Z. X. Giustra, S. Novosedlik, A. Orthaber, P. J. Gates, C. Dyrager and L. T. Pilarski, Green Chem., 2020, 22, 5648–5655 RSC.
- (a) S. Hutsch, A. Leonard, S. Graetz, M. V. Höfler, T. Gutmann and L. Borchardt, Angew. Chem., Int. Ed., 2024, e202403649 CAS; (b) M. Wohlgemuth, S. Schmidt, M. Mayer, W. Pickhardt, S. Graetz and L. Borchardt, Angew. Chem., Int. Ed., 2024, e202405342 CAS; (c) H. W. Lee, K. Yoo, L. Borchardt and J. G. Kim, Green Chem., 2024, 26, 2087–2093 RSC.
- C. Patel, E. André-Joyaux, J. A. Leitch, X. Martínez De Irujo-Labalde, F. Ibba, J. Struijs, M. A. Ellwanger, R. Paton, D. L. Browne, G. Pupo, S. Aldridge, M. A. Hayward and V. Gouverneur, Science, 2023, 381, 302–306 CrossRef CAS PubMed.
- (a) L. Pontini, J. A. Leitch and D. L. Browne, Green Chem., 2023, 25, 4319–4325 RSC; (b) R. R. Bolt, S. E. Raby-Buck, K. Ingram, J. A. Leitch and D. L. Browne, Angew. Chem., Int. Ed., 2022, 61, e202210508 CrossRef CAS PubMed.
- (a) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC; (b) H. Xu and G.-W. Wang, J. Org. Chem., 2022, 87, 8480–8491 CrossRef CAS PubMed.
- (a) X. Wang, X. Zhang, X. He, G. Guo, Q. Huang, F. You, Q. Wang, R. Qu, F. Zhou and Z. Lian, Angew. Chem., Int. Ed., 2024, 63, e202410334 CrossRef CAS PubMed; (b) F. You, X. Zhang, X. Wang, G. Guo, Q. Wang, H. Song, R. Qu and Z. Lian, Org. Lett., 2024, 26, 4240–4245 CrossRef CAS PubMed; (c) R. Qu, S. Wan, X. Zhang, X. Wang, L. Xue, Q. Wang and Z. Lian, Angew. Chem., Int. Ed., 2024, 63, e202400645 CrossRef CAS PubMed; (d) X. Wang, X. Zhang, L. Xue, Q. Wang, F. You, L. Dai and Z. Lian, Angew. Chem., Int. Ed., 2023, 62, e202307054 CrossRef CAS PubMed.
- (a) Y. Zhao, Z. Yang, X. Wang, Q. Kang, B. Wang, T. Wu, H. Lei, P. Ma, W. Su, S. Wang, Z. Wu, X. Huang, C. Fan and X. Wei, Adv. Sci., 2024, 11, 2404071 CrossRef CAS PubMed; (b) S. Chen, C. Fan, Z. Xu, M. Pei, J. Wang, J. Zhang, Y. Zhang, J. Li, J. Lu, C. Peng and X. Wei, Nat. Commun., 2024, 15, 769 CrossRef PubMed.
- (a) J. Zhang, P. Zhang, Y. Ma and M. Szostak, Org. Lett., 2022, 24, 2338–2343 CrossRef CAS PubMed; (b) J. Zhang, P. Zhang, L. Shao, R. Wang, Y. Ma and M. Szostak, Angew. Chem., Int. Ed., 2022, 61, e202114146 CrossRef CAS PubMed.
- (a) K. Xiang, P. Ying, T. Ying, W. Su and J. Yu, Green Chem., 2023, 25, 2853–2862 RSC; (b) P. Ying, J. Yu and W. Su, Adv. Synth. Catal., 2021, 363, 1246–1271 CrossRef CAS; (c) P. Ying, T. Ying, H. Chen, K. Xiang, W. Su, H. Xie and J. Yu, Org. Chem. Front., 2024, 11, 127–134 RSC; (d) X. Yang, H. Wang, Y. Zhang, W. Su and J. Yu, Green Chem., 2022, 24, 4557–4565 RSC; (e) X. Yang, C. Wu, W. Su and J. Yu, Eur. J. Org. Chem., 2022, e202101440 CrossRef CAS; (f) C. Wu, T. Ying, X. Yang, W. Su, A. V. Dushkin and J. Yu, Org. Lett., 2021, 23, 6423–6428 CrossRef CAS PubMed.
- (a) X. Li, Y. Liu, L. Zhang, Y. Dong, Q. Liu, D. Zhang and H. Liu, Green Chem., 2022, 24, 6026–6035 RSC; (b) Y. Liu, X. Li, Q. Liu, X. Li and H. Liu, Org. Lett., 2022, 24, 6604–6608 CrossRef CAS PubMed; (c) Y. Hou, H. Wang, J. Xi, R. Jiang, L. Zhang, X. Li and H. Liu, Green Chem., 2023, 25, 2279–2286 RSC; (d) Y. Jia, H. Wang, J. Guo, F. Zhang, L. Zhang, X. Li and H. Liu, J. Org. Chem., 2024, 89, 6704–6713 CrossRef CAS PubMed; (e) H. Wang, X. Chi, X. Zhang, L. Zhang, Q. Liu, Z. Zhao and H. Liu, J. Org. Chem., 2024, 89, 5320–5327 CrossRef CAS PubMed.
- (a) S. Styla, Przegl. Elektrotech., 2016, 92, 103–106 Search PubMed; (b) F. S. Bayones, S. M. Abo-Dahab, A. M. Abd-Alla and A. A. Kilany, Methods Appl. Sci., 2021, 44, 9944–9965 CrossRef; (c) M. Wołosiewicz-Glab, S. Ogonowski and D. Foszcz, IFAC-PapersOnLine, 2016, 49, 67–71 CrossRef; (d) M. Wołosiewicz-Glab, D. Foszcz and S. Ogonowski, IFAC-PapersOnLine (Elsevier B.V.), 2017, 50, 14964–14969 CrossRef; (e) M. Pawelczyk, Z. Ogonowski, S. Ogonowski, D. Foszcz, D. Saramak, T. Gawenda and D. Krawczykowski, PL4130416, 2015.
- H. Liu, X. Han, X. Feng, L. Zhang, F. Sun, F. Jia, D. Zhang, H. Liu and X. Li, J. Am. Chem. Soc., 2024, 146, 18143–18150 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc05617e |
| This journal is © The Royal Society of Chemistry 2025 |






