The chemistry and biological activities of natural products from Northern African plant families: from Ebenaceae to Solanaceae
Joseph N. Yong†
*a and
Fidele Ntie-Kang†
*ab
aDepartment of Chemistry, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon. E-mail: joseph.yong@ubuea.cm
bDepartment of Chemistry, Chemical and Bioactivity Information Centre, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon. E-mail: ntiekfidele@gmail.com; fidele.ntie-kang@ubuea.cm; Tel: +237 677915473
First published on 3rd March 2015
Abstract
Traditional medicinal practices significantly affect the livelihoods of populations in countries with developing economies. The aim of this survey was to validate the use of traditional medicine within Northern African communities. In this review series, we summarize the ethnobotanical uses of selected plant species from Northern African flora and attempt to correlate the activities of the isolated bioactive principles with known local uses of the plant species in traditional medicine. The literature is covered for the period 1971 to 2014. Part II of this series focuses on plant families with names beginning with letters E to S, with the ethnobotanical uses of 30 plant species from 17 families compared with the bioactivities of 133 compounds identified.
1 Introduction
The region of Northern Africa covers a surface area of about 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 935
935![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 659 km2, comprising the countries Algeria, Egypt, Libya, Morocco, North Sudan, South Sudan, Tunisia and Western Sahara. This region includes a population of about 198
659 km2, comprising the countries Algeria, Egypt, Libya, Morocco, North Sudan, South Sudan, Tunisia and Western Sahara. This region includes a population of about 198![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 996
996![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 people.1 In Northern Africa, Egypt in particular, the use of plant-derived remedies for handing a wide range of ailments locally, is common practice. Herbal shops are very common even in big cities, despite the presence of modern or Western medicine. Thus, the use of African traditional medicine (ATM) in such societies is often without the need of scientific evidence.2 This is because before the advent of Western medicine, people from the societies of Northern Africa have had a long history of the use of plant-derived medicines, which dates back to the prehistoric Egyptian civilization.2 In effect, it is documented that the apothecaries of ancient Egypt had been familiar with a catalogue of plant-derived traditional remedies. A wide range of plant parts, including the roots, rhizomes, flowers, leaves, fruits, seeds, and oils in the form of powders, pills, suppositories, creams, pastes, and ointments, or sometimes combinations of these, were commonly used.3,4 The knowledge of herbal remedies from Ancient Egypt has therefore affected the practice of traditional medicine in the entire Northern Africa and the Middle East.
526 people.1 In Northern Africa, Egypt in particular, the use of plant-derived remedies for handing a wide range of ailments locally, is common practice. Herbal shops are very common even in big cities, despite the presence of modern or Western medicine. Thus, the use of African traditional medicine (ATM) in such societies is often without the need of scientific evidence.2 This is because before the advent of Western medicine, people from the societies of Northern Africa have had a long history of the use of plant-derived medicines, which dates back to the prehistoric Egyptian civilization.2 In effect, it is documented that the apothecaries of ancient Egypt had been familiar with a catalogue of plant-derived traditional remedies. A wide range of plant parts, including the roots, rhizomes, flowers, leaves, fruits, seeds, and oils in the form of powders, pills, suppositories, creams, pastes, and ointments, or sometimes combinations of these, were commonly used.3,4 The knowledge of herbal remedies from Ancient Egypt has therefore affected the practice of traditional medicine in the entire Northern Africa and the Middle East.
Northern Africa is mostly desert (the Sahara), covering a surface area of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619
619![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 km2, which is >50% of the total area occupied by the region. The rest is made of oases and grasslands, with a few species of trees.5 Vilà et al. have reported the existence of a total of 343 vascular plant species from 69 families non-native to this region.5 The aforementioned paper reported the existence of alien species, ranging from 143 in Algeria to 60 in Tunisia, a majority originating from the regions around the Mediterranean Sea and North America.5
260 km2, which is >50% of the total area occupied by the region. The rest is made of oases and grasslands, with a few species of trees.5 Vilà et al. have reported the existence of a total of 343 vascular plant species from 69 families non-native to this region.5 The aforementioned paper reported the existence of alien species, ranging from 143 in Algeria to 60 in Tunisia, a majority originating from the regions around the Mediterranean Sea and North America.5
There has been a recent surge of publications on traditional knowledge-based de-replication of bioactive compounds for use in the Western or modern medicine. In addition to the increasing global interest in natural products (NPs), it is recommended traditional medicinal concepts be dealt with in detail, with the view of laying the ground work for further scientific investigations. However, unlike modern medicine, the knowledge on traditional medicine is often handed down the ancestral tree and not often properly documented in pharmacopoeia. In our previous study, emphasis has been laid on establishing documented evidence for the use of plant-derived remedies in ATM practices in North Africa.6 Earlier studies are attempts to explore the components of African traditional medicines in general, by documenting the current available knowledge on the biological activities of NPs isolated from the African flora (from dispersed data/literature sources). This was with the aim of enhancing drug discovery programs on the continent. A number of NP databases for components of ATM have been developed for use in virtual screening campaigns.7–10 The pharmacokinetic profiling of NP databases from African flora,10–13 the most recent being the AfroCancer database, which includes about 400 chemical components of African flora with promising anticancer-like properties,14 has been established. Several focused reviews on NPs isolated from African medicinal plants, some paying attention to plants from particular countries/regions have been published,15–20 as well as NPs with interesting biological activities against specific diseases/ailments.21–23 In all the aforementioned cases, emphasis has been laid on the NPs whose measured biological activities correlate with the use of the plant in ATM, with the aim of validating the use of these plant species in traditional medicine. This survey is a continuation of the investigation of ethnobotanical uses of the plant species from Northern Africa versus the bioactivities of the derived NPs. As in the previous study,6 this survey is presented by plant family and in alphabetical order. Wherever the bioactivities of the isolated metabolites correlate with the ethnobotanical uses of the plant species, these are highlighted in bold in the Tables. In the current survey, plant families with names beginning with letters E to S are covered.
2 Ebenaceae and Euphorbiaceae
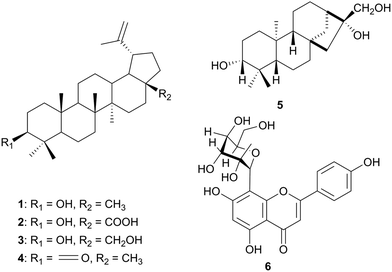
A summary of the medicinal uses and biological activities of the compounds of the Ebenaceae and Euphorbiaceae plant families are shown in Table 1. Diospyros mesipiliformis (Ebenaceae) is a tropical and subtropical tree which has several ethnopharmacological applications in Sudanese folk medicine.24 Different parts of the plants are used to remedy a number of ailments including malaria, pneumonia, syphilis, dermatomycoses, diarrhea, skin infections, toothache and headache. The EtOAc extract of the stem bark of Diospyros mespiliformis afforded lupeol (1), betulinic acid (2), betulin (3) and lupeone (4), Fig. 1.25a These compounds and their semi-synthesized acetate were screened for the inhibition of α-glucosidase enzyme activity as well as against antioxidant activity. Compound 1 and its semi-synthesized acetate, as well as compounds 3 and 4, displayed significant α-glucosidase inhibitory potential. Mohamed et al. also reported a study of Croton zambesicus (Euphorbiaceae). The MeOH extract of the dried fruit of this plant yielded compounds 1, 2 and 3, in addition to the diterpene ent-kaurane-3β,16β,17-triol (5) and vitexin (6). Only compound 6 exhibited strong antioxidant activity when screened along with the other compounds from both species. Vitexin is also widely used in increasing cardiac output and reducing total peripheral vascular resistance and coronary resistance.25b The roots of Croton zambesicus have been implicated in the treatment of malaria and diabetes in Nigeria and in the relief of menstrual pains in Sudan.26–29 The claims of the therapeutic value of C. zambesicus roots are, however, still to be supported by solid scientific evidence.| Plant family | Plant name (Country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Ebenaceae | Diospyros mespiliformis (Sudan) | Fever, whooping cough, pneumonia, syphilis, leprosy, dermatomycoses, diarrhea, headache, toothache | Stem bark | 1–4 | α-glucosidase inhibition, antioxidant | Mohamed et al.25 |
| Euphorbiaceae | Croton zambesicus (Sudan) | Menstral pains, malaria, diabetes, fever, dysentery, cough | Fruit | 5 and 6 | α-glucosidase inhibition, antioxidant, compound 6 increases cardiac output and reduces total peripheral vascular resistance and coronary resistance | Mohamed et al.25 |
3 Fabaceae (Leguminosae) and Hypericaceae
A summary of the medicinal uses and biological activities of the compounds of the above plant families above are indicated in Table 2. Plants of the Leguminosae or Fabaceae are known to be rich in prenylated flavonoids,30,31 alkaloids32 and triterpenoid saponins.33 The isolated compounds have shown diverse biological activities and the plant materials have been used to treat a number of diseases and ailments.| Plant family | Plant name (country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and reference |
|---|---|---|---|---|---|---|
| Fabaceae | Tephrosia purpurea (Egypt) | Used as a tonic, laxative, anthelminthic for children. Plants from this genus are noted for piscidal, insecticidal and anticancer properties | Aerial parts | 7–11 | Cytotoxicity | Kassem et al.35 |
| Lupinus tassilicus (Algeria) | Antidiabetic | Seeds | 12–13 | Exhibits hyperglycaemic properties | Ainouche et al.38 | |
| Astragalus caprinus (Tunisia) | 16–28 | Not tested | Semmar et al.39 | |||
| Hedysarum species and Sulla (Tunisia, Algeria and Morocco) | Protection and enrichment of soil, or for pastoral production | Seedlings | Zitouna et al.40 | |||
| Erythrina lysistemon (Egypt) | Female infertility, stomach pain, gonorrhoea | Stem bark | 29–38 | Anticancer | El-Masry et al.30 | |
| Erythrina abyssinica (Sudan) | Microbial infections | Seeds | 39–43 | Anti-HIV, cytotoxicity | Mohammed et al.32 | |
| Tephrosia apollinea (Sudan) | Members of genus possess piscicidal, insecticidal, anticancer properties | Aerial parts | 10 | Cytotoxicity | Hassan et al.31 | |
| Gledistia caspica (Egypt) | Carbuncle, scabies, skin diseases, apoplexy, headache productive cough | Fruit | 46–50 | Cytotoxicity | Melek et al.33 | |
| Hypericaceae | Hypericum triquetrifolium | Burns, gastroenteritis; used as a sedative, anti-inflammatory, antioxidant | Aerial parts | Essential oils | Antibacterial, antifungal, cytotoxicity, antiviral | Rouis et al.51 |
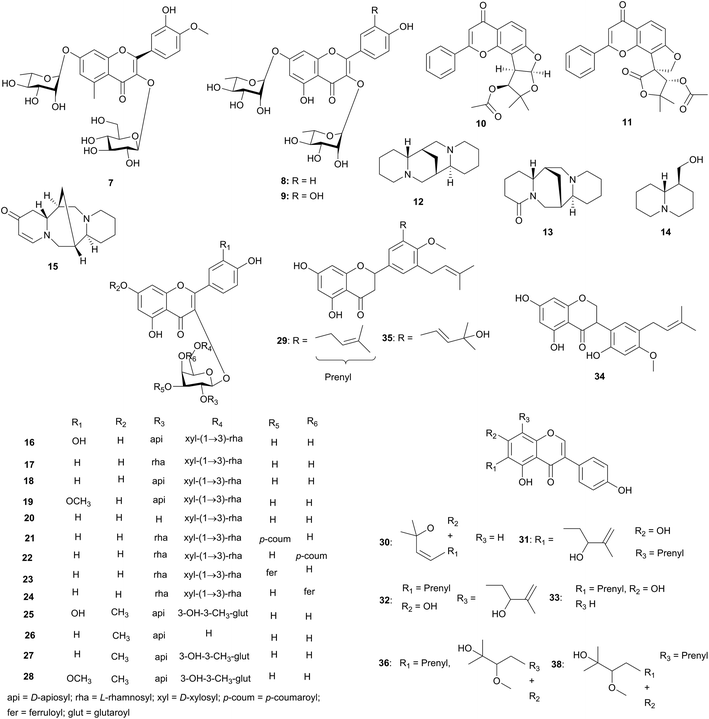
The Tephrosia species (Fabaceae) from Northern Africa are known to possess dynamic pharmacological activities, e.g. piscicidal, insecticidal and anti-cancer properties.34 Kassem et al. isolated and characterized a new naturally occurring flavonol diglycoside, tamarixetin 3-O-β-glucopyranoside-7-O-α-rhamnopyranoside (7), together with kaempferol 3,7-di-O-α-rhamnopyranoside (8) and quercetin 3,7-di-O-α-rhamnopyranoside (9) from the aerial parts of Tephrosia purpurea, Fig. 2.35 Bioassay-guided fractionation of the light petroleum extract, which showed antitumor activity, yielded two bioactive 5-deoxyflavones; pseudosemiglabrin (10) and glabratephrin (11). In vitro screening of pseudosemiglabrin and glabratephrin for cytotoxicity against the human cell lines U251 brain, MCF7 breast and Hepg2 liver carcinoma indicated that both showed cytotoxicity to the Hepg2 hepatic human cell line with IC50 values of 0.87 and 4.03 μg mL−1, respectively and are inactive against U251 (brain tumor) and the MCF7 (breast carcinoma) cell lines.
Lupinus tassilicus (Fabaceae) is considered to be an endangered herbaceous species endemic to the Saharan desert (in the Algerian region of Tassili). Debitted seeds of this plant are believed to possess antidiabetic properties.36 The seeds of this plant contain quinolizidine alkaloids; sparteine (12) and lupanine (13), exhibiting moderate hypoglycemic effects on alloxan-diabetic rats, but not on normal rats.37 Ainouche et al. have also identified epilupinine (14) and multiflorine (15) as the main alkaloids in the species harvested from Algeria.38 The presence of alkaloids like lupinine, anagyrine, sparteine and hydroxylupanine makes this plant potentially toxic if consumed in huge amounts, probably because these compounds induce hypoglycemia.36
In an attempt to establish a chemotaxonomic classification of Astragalus caprinus (Fabaceae) from Tunisia growing under different climatic conditions, Semmar et al. have isolated fourteen flavonol glycosides, although only thirteen of the compounds (16–28) were fully characterized.39 The identified chemical structures are shown in Fig. 3, but a link between the ethnobotanical uses of the plant and the biological activities of the derived compounds is still to be established. Zitouna et al. also carried out a similar study on Hedysarum and Sulla species (Fabaceae) and established a genetic diversity relationship between the two genera, for species growing under different climatic conditions in Tunisia Algeria and Morocco.40 Moreover, nineteen flavonoids and isoflavonoids have been identified or tentatively identified with species from the Adenocarpus genus (Leguminosae), harvested in Mediterranean and tropical African regions.41 These include flavones di-C-glycosides, luteolin derivatives, apigenin derivatives, daidsein derivatives, genistein derivatives, kaempferol derivatives and quercetin derivatives. The biological activities of the isolated compounds is yet to be correlated with the uses of the plant species in traditional medicine.
Erythrina lysistemon (Leguminosae), grown in Egypt as an ornamental plant, has been associated with the treatment of infertility in women, stomach pain and gonorrhoea. Three prenylated flavonoid derivatives, 5,7,4′-trihydroxy-8-(3′′′-methylbut-2′′′-enyl)-6-(2′-hydroxy-3′-methylbut-3′′enyl) isoflavone (isoerysenegalensein E) (31), 5,7,2′-trihydroxy-4′-methoxy-5′-(3′′-methylbut-2′′-enyl) isoflavanone (lysisteisoflavanone) (34), 5,4′-dihydroxy-6-(3′′′-methylbut-2′′′-enyl)-2′′-hydroxyisopropyl dihydrofurano [4′′,5′′:8,7] isoflavone (isosenegalensin) (36), together with the four known flavonoids abyssinone V-4′-methylether (29), alpinumisoflavone (30), wighteone (33) and burttinone (35) were isolated from the stem bark of this plant (Fig. 4).30 Meanwhile, compounds 32 and 38 were isolated from the Cameroonian species.42–44 Compounds 35 and 36 were evaluated for anticancer activity. The IC50 values for compound 35 were less 50 μM against 43 cell lines with maximum cytotoxicity against colon cancer cell line HCC2998 (IC50 = 20 μM), while the IC50 values were higher than 50 μM in all the five tested leukemia lines. Compound 36 was significantly less cytotoxic than compound 35 but the IC50's were a little less 50 μM only against four cell lines, namely, ovarian, non-small cell lung cancer, colon cancer, and renal cancer.30 Both compounds were nonselective in their action.
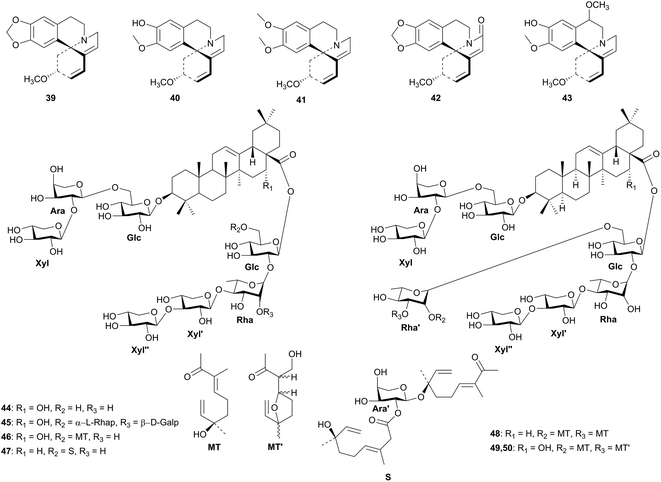
The bark of Erythrina abyssinica (Leguminosae) has been used to cure cough, skin diseases, ulcers, abdominal pain, liver inflammation, colic, trachoma and elephantiasis; the flowers have been used for the treatment of dysentery and as an abortifacient; the leaves are used for the treatment of peptic ulcers, arthralgia and diarrhea; the roots are being used in preparing remedies for epilepsy, malaria and syphilis, while the fruit is used to cure asthma.45 The bioassay-guided fractionation of the alkaloidal fraction of the seeds of E. abyssinica yielded erythraline (39), erysodine (40), erysotrine (41), 8-oxoerythraline (42), and 11-methoxyerysodine (43) on purification, Fig. 3.32 The crude alkaloidal extract was evaluated for in vitro cytotoxicity activity against the cell lines HeLa, Hepg2, HEG-2, HCT116, MCF7 and HFB4. This extract showed potential activity with IC50 values of 13.8, 10.1, 8.16, 13.9, 11.4 and 12.2 μg mL−1, respectively, against the aforementioned cell lines. The pure compounds, 39–43, were screened for in vitro cytotoxicity activity against Hepg2 and this resulted in IC50 values of 17.60, 11.80, 15.80, 3.89 and 11.40 μg mL−1, respectively. These compounds were also evaluated for cytotoxicity activity against HEP-2 cell line and these gave IC50 values of 15.90, 19.90, 21.60, 18.50 and 11.50 mg mL−1, respectively. The anti-HIV-1 activity of the alkaloidal fraction, using the methyl thiazol tetrazolium (MTT) method, was evaluated. This resulted in the reduction in the viability of mock-infected MT-4 cells by CC50 = 53 μM and achieved 50% protection on MT-4 cells from the HIV-1-induced cytopathogeneticy by EC50 ≥ 53 μM, compared with EFV as positive control (which showed CC50 = 45 μM and EC50 = 0.003 μM).32 The cytotoxicity of mock-infected MT cells has been attributed to the presence of isoquinoline-type alkaloids, which inhibit the replication cycle of the HIV-1 virus by adsorption or reverse transcription process,46 thus disrupting the enzyme interaction with template primers.47 This confirms that the cytotoxicity and anti-HIV activity of the crude extract is due to the presence of the pure compounds, although the modes of action of the isolated metabolites is still under investigation.
As previously mentioned, plant species from the genus Tephrosia exhibit a wide range of pharmacological activities, including piscicidal, insecticidal and anti-cancer properties.34 The flavone (−)-pseudosemiglabrin, (10), has also been purified from the aerial parts of Tephrosia apollinea (Leguminosae), harvested in Khartoum, Sudan and tested against nine tumor cell lines.31 The compound indicated selective cytotoxicity against six cancer cell lines, namely, MCF7 (breast cancer, IC50 18.24 μM), PC3 (human prostate cancer, IC50 6.11 μM), HL-60 (human promyelocytic leukemia, IC50 15.7 μM), K562 (human immortalized myelogenous leukemia, IC50 22.5 μM), U937 (human hystiocytic leukemia, IC50 5.76 μM) and HCT116 (human colorectal tumor, IC50 19.6 μM) cell lines. The compound shows, either moderate or poor cytotoxic effects against HT-29 (human leukemia cell line) and Hepg2 (human hepatic carcinoma) with IC50 values 135.12 and >300 μM, respectively. Additionally, this compound was nontoxic to normal fibroblast (CCD-18Co) cells (IC50 = 327.5 μM). It can be concluded that the compound has shown selective anti-proliferative action against leukemia, prostate, breast and colon cancer cell lines and may be a good starting point for synthetic modifications towards anticancer drug development. In addition, the in vitro anticancer properties of compound 10 validate the use of this plant in ATM to treat cancer-related ailment.
Gleditsia species (Leguminosae) have been widely used in traditional medicine, e.g. the thorns of G. sinensis are used in China for the treatment of abscesses, scabies and skin diseases, while the mature pods and anomalous fruits are mainly used for treating cerebral stroke (caused by hemorrhage in the brain), headache, cough and asthma. G. japonica dried fruits have long been known in oriental medicine as a diuretic and expectorant. G. caspica is a tree that is grown in public gardens in Egypt and it is associated with cytotoxic activity.33 Seven bisdesmosidic triterpenoid saponins named caspicaosides E–K (44–50), were isolated from the methanolic fruit extract of G. caspica, harvested from a public garden in Giza, Egypt. The chemical structures were exhaustively elucidated by 1D and 2D NMR spectroscopy as well as high resolution mass spectrometry and acid hydrolysis. The acylated saponins G–K (46–50) were screened for their in vitro cytotoxicity property against the tumor cell lines HCT116, Hepg2 and MCF7, using the acid phosphatase assay. Significant activity was observed against MCF7 cell line (IC50 1.6–2.1 μM), while the other two cell lines were moderately susceptible towards the tested isolates.33
Hypericum triquetrifolium (Hypericaceae), endemic in Eastern Europe and the Mediterranean area, has been used in folk medicine for its sedative, antihelminthic, anti-inflammatory, and antiseptic effects.48 The potential uses of its essential oil and crude extracts as drugs have been reported, mainly in the treatment of burns, gastroenteritis, and as antinociceptive and antioxidant drugs.49,50 Rouis et al. evaluated the essential oils of Hypericum triquetrifolium harvested from different localities in Tunisia for their antimicrobial activities, using micro-broth dilution methods against bacterial and fungal strains.51 In addition, the cytotoxic effects and the antiviral activities of these oils were determined using Vero cell lines and coxsackievirus B3. The results revealed good antibacterial activities against a wide range of bacterial strains, with MIC values varying between 0.39–12.50 μg mL−1 and MBC values between 1.56–25.0 μg mL−1. In addition, the essential oils exhibited potential antifungal activities, with MIC values ranging between 0.39 μg mL−1 and 12.50 μg mL−1; MFC values ranged between 3.12 μg mL−1 and 25.00 μg mL−1; a considerable anticandidal activity was noted (MIC values comprised between 0.39 μg mL−1 and 12.50 μg mL−1). Although their cytotoxic effect was low (CC50 ranged between 0.58 mg mL−1 and 12.00 mg mL−1), the essential oils did not show antiviral activity against coxsakievirus B3. The study confirms the multipurpose medicinal use of this plant in various communities in Tunisia.
4 Labiatae and Lamiaceae
A summary of the medicinal uses and biological activities of the compounds of the plant families above are indicated in Table 3.| Plant family | Plant name (country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and reference |
|---|---|---|---|---|---|---|
| Labiatae | Nepeta cataria (Egypt) | Used as anti-inflammatory, anticancer, antioxidant | Extracts | Antioxidant, antidiabetic | Naguib et al.52 | |
| Lamiaceae | Marrubium vulgare (Morocco) | It is known to have antinociceptive and expectorant properties | Aerial parts of flowering plant | 51 and 52 | Vasorelaxant | El Bardai et al.53 |
| Salvia argentea var. aurasiaca (Algeria) | Bacterial infections, inflammations, tumors, HIV | Aerial parts | 53–62 | Not tested | Lakhal et al.57 | |
| Lavandula stoechas | Epilepsy, headache; has analgesic, antiseptic, antispasmodic and antimicrobial properties | Cavanagh et al.58 | ||||
| Lavanduala multifida | Rheumatism, cold; has hypoglycaemic and anti-inflammatory properties | El-Hilaly et al.59 |
Nepeta cataria (Labiatae) is native to Eurasia but has been naturalized in North America and other parts of the globe. Members of Nepeta genus are noted for different classes of natural products whose biological activities have been evaluated. Extracts of N. cataria in different solvents have been investigated in vitro for antioxidant activity and carbohydrate-hydrolyzing enzymes with diabetes mellitus (α-amylase, β-galactosidase, α-glucosidase). The results were positive for the different successive extracts with 70% ethanol, petroleum ether and chloroform extracts showing the most potent inhibitory activities. However, EtOAc and pure ethanol extracts revealed moderate to low reducing activities.52
The widespread use of Marrubium vulgare (Lamiaceae) in folk medicine, led to the phytochemical investigation of the species and eventually led to the isolation and structural elucidation of furanic diterpenes; marrubiin (51) and marrubenol (52), Fig. 4.53 These compounds were tested, in vitro, for their antihypertensive activity. The results show that marrubenol was more potent than marrubiin (IC50 values were 7.7 ± 1.9 μM and 24 ± 2.3 μM for marrubenol and marrubiin, respectively). Both compounds were much less potent than the standard drug, verapamil: IC50 ratios of 199 and 64 were obtained for marrubiin and marrubenol respectively compared to verapamil. The presence of these compounds however justifies the vasorelaxant activity of the aqueous extract of the aerial parts of M. vulgare.
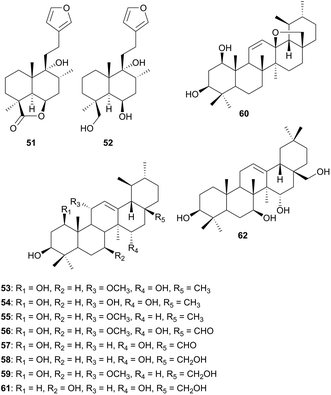
Plants of the Salvia genus (Lamiaceae) are known to have several secondary metabolites which are implicated in the biological activities of the genus. This includes flavonoids,54 diterpenoids,55 tritepenoids and other compounds.56 From the exudates of Salvia argentea var. aurasiaca (Lamiaceae), ten new triterpenoids were isolated and their structures elucidated by extensive spectroscopic methods.57 The compounds include, 11α-methoxyurs-12-ene-1β,3β,15α-triol (53), urs-12-ene-1β,3β,11α,15α-tetraol (54), 11α-methoxyurs-12-ene-1β,3β-diol (55), 1β,3β,15α-trihydroxy-11α-methoxyurs-12-en-28-al (56), 1β,3β,15α-trihydroxyurs-12-en-28-al (57), urs-12-ene-1β,3β,15α,28-tetraol (58), 11α-methoxyurs-12-ene-1β,3β,28-triol (59), 13β,28-epoxyurs-12-ene-1β,3β-diol (60), urs-12-ene-3β,7β,15α,28-tetraol (61) and olean-12-ene-3β,7β,15α,28-tetraol (62). These triterpenoids were not screened for any specific biological activity. From this family, Lavanda stoechas and Lavanda multifida are mostly used in traditional medicine for the treatment of several diseases. L. stoechas is used for the remedy of epilepsy and headaches58 and has analgesic, antiseptic, antispasmodic and antimicrobial properties. L. multifida is used to treat rheumatism colds59 and has anti-inflammatory properties.60 The validation of these ethnobotanical uses is still under investigation.
5 Meliaceae and Moraceae
A summary of the medicinal uses and biological activities of the compounds of the plant families above are indicated in Table 4.| Plant family | Plant name (Country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Meliaceae | Khaya senegalensis (Sudan) | Malaria | Stem | 63–65 | Antiplasmodial, antileishmanial | Khalid et al.,61 |
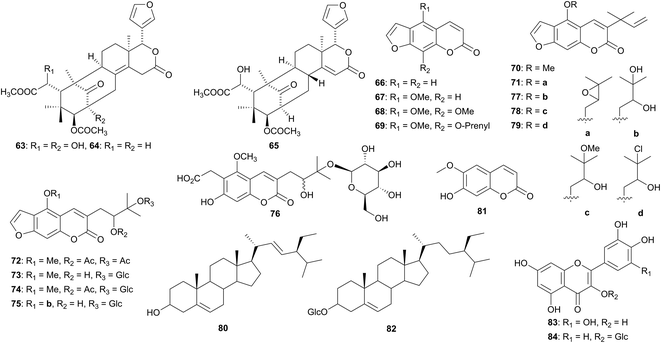
| Moraceae | Dorstenia foetida (Yemen) | Skin diseases, leishmaniasis, leprosy, liver disease, intestinal worms | Leaves | 66–79 | Antifungal, antibacterial, cytotoxicity | Heinke et al.65 |
| Ficus auriculata (Egypt) | Microbial infections, cancer, diabetes, liver diseases, inflammations | Leaves and fruit | 1, 2, 80–84 | Antibacterial, anti-inflammatory, antioxidant | El-Fishawy et al.66 |
Khaya senegalensis (Meliaceae) is very popular African folk medicine and its bark is used to treat malaria. A bioassay-guided fractionation of the chloroform extract of the bark of a plant sample collected in Khartoum, Sudan afforded three tetranortriterpenoids (limonoids) of the mexicanolide group, one of which is new, namely, 2,6-dihdroxyfissinolide (63) and the other two are known compounds: fissinolide (64) and methyl 3β-acetoxy-6-hydroxy-1-oxomeliac-14-enoate (65).61 All three compounds were subjected to antiplasmodial and antileishmanial in vitro tests against the chloroquine-resistant strains of Plasmodium falciparum and the promstigotes of Leishmania major, respectively. Fissinolide (64) was moderately active in vitro against Plasmodium falciparum (IC50 48 ± 3 μM) and promastigotes of Leishmania major (IC50 69 ± 13 μM). Compound 63 exhibited IC50 0.12 ± 0.08 μM against P. falciparum and IC50 > 0.20 mM against L. major. The limonoid 65 exhibited no significant antiprotozoal activity (IC50 > 0.20 mM) against either P. falciparum or L. major (Fig. 5).61
Among the plants surveyed in the Moraceae in North Africa, the aerial parts of Dortsenia foetida is used in Yemen to treat skin diseases.62 The roots are used in Ethiopia to treat leprosy, liver disease and intestinal worms63 and in Oman the roots are used as food.64,65 The extracts of the aerial parts of Ficus auriculata, another Moraceae of North Africa, has shown antimicrobial, antioxidant, anti-inflammatory, antidiabetic and hepaproctective properties.66 The phytochemical investigation of the leaves of Dorstenia foetida yielded four new furanocoumarins and one carboxymethyl coumarin derivative, along with six known previously isolated compounds.65 The known compounds include, psoralen (66), bergapten (67), isopimpellinin (68), phellopterin (69), 5-methoxychalepensin (70) and turbinatocoumarin (73). The new compounds are, 5-(2,3-epoxy-3-methylbutoxy)-chalepensin (71), 5-methoxy-3-(3-methyl-2,3-dihydroxybutyl)-psoralen-diacetate (72), 5-methoxy-3-[3-(β-D-glucopyranosyloxy)-2-acetyloxy-3-methylbutyl]-psoralen (74), 5-(3-methyl-2,3-dihydroxybutyloxy)-3[3-(β-D-glucopyranosyloxy)-2-hydroxy-3-methylbutyl]-psoralen (75) and 7-hydroxy-5-methoxy-6-carboxymethyl-3-[3-(β-D-glucopyranosyloxy)-2-hydroxy-3-methylbutyl]-coumarin (76). Three new derivatives were obtained from the fraction containing compound 71: 5-(2,3-dihydroxy-3-methylbutoxy)-chalepensin (77), 5-(2-hydroxy-3-methoxy-3-methybutoxy)-chalepensin (78) and 5-(3-chloro-2-hydroxy-3-methybutoxy)-chalepensin (79). The n-heptane and EtOAc extracts of D. foetida have antifungal potential. Psoralen derivatives have been known to exhibit antifungal activities due to their genotoxic reaction with DNA under the influence of UV-A light.67,68 The plant extract was tested against the intestinal worms, Caenorhbditis elegans. The n-heptane extract displayed no anthelminthic activity but instead protected the worms compared to the negative control. The n-heptane extract showed significant antibacterial activity against Bacillus subtilis, shown by the growth inhibition of 60.5% at a concentration of 100 μg mL−1. More polar extracts indicated only slightly higher activity. The n-heptane extract also demonstrated cytotoxicity activity against HT29 and PC3 cancer cell lines by a cell growth inhibition of 80% at a concentration of 50 μg mL−1. The antimicrobial and cytotoxicity activities of D. foetida justify its use in folk medicine.
Eight known compounds were isolated from Ficus auriculata collected in Giza, Egypt. The chemical structures elucidated by spectroscopic methods and by comparison with known data.66 The compounds include betulinic acid (2), lupeol (1), stigmasterol (80), bergapten (67), scopoletin (81), β-sitosterol-3-O-β-D-glucopyranoside (82), myricetin (83), and quercetin-3-O-β-D-glucopyranoside (84). The leaves and fruit extracts of the plant were screened against a host of bacteria (agar well diffusion method) and they were found effective against Gram-positive bacteria (Staphylococcus aureus and Bacillus aureus) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa). The alcohol leaf extract at a dose of 500 mg kg−1, showed in vitro anti-inflammatory activity using carrageenin-induced rat hind paw oedema model, as well as antioxidant activity. The fruit extract showed higher antioxidant activity than the leaf. Both extracts (fruit and leaves) exhibited moderate hepatoprotective and antidiabetic properties.66 Thus, the use of this plant in traditional African medicine is supported by the study reported by El-Fishawy and coworkers.
6 Orobanchaceae, Poaceae and Podocarpaceae
A summary of the medicinal uses and biological activities of the compounds of the plant families above are indicated in Table 5.| Plant family | Plant name (Country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Orobanchaceae | Striga hermonthica | Malaria, diabetes, leprosy ulcer, pneumonia, jaundice, microbial infections, trypanosomiasis | Whole plant | Extracts | Antiplasmodial, anitmicrobial, antifertility, antitrypanocidal | Koua69 |
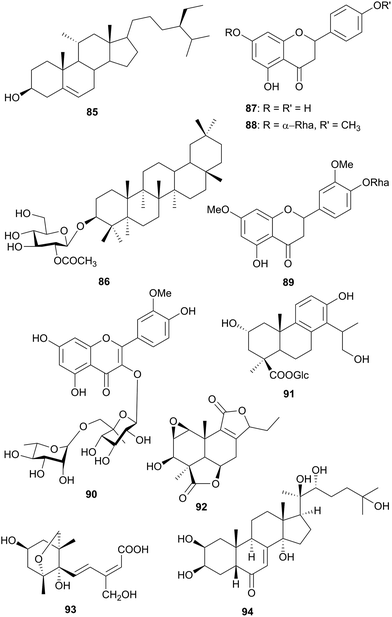
| Poaceae | Eragrostis tef (Egypt and Saudi Arabia) | Diabetes, osteoporosis | Seeds | 85–90 | Not tested | El-Alfy et al.76 |
| Podocarpaceae | Podocarpus gracilior (Egypt) | Fevers, asthma, coughs, cholera, distemper, chest complaints, veneral diseases | 91–93 | Not tested | Faiella et al.78 |
Koua has reviewed the medicinal uses of Striga hermonthica (Orobanchaceae),69 a ubiquitous parasitic weed of the grain family. Although this plant grows widely in rice, millet or maize farms, it has its benefits. The aqueous extract of this plant has exhibited antioxidant activity and a decoction or infusion of the roots is taken orally in East Africa as an abortifacient and in the treatment of pneumonia.70 Fungal infections are treated with a decoction of the plant in Northern Nigeria and the flowers are used to prevent conception.71 The methanol extract of the whole plant exhibited weak activity with IC50 274.8 μg mL−1 against the plasmodium parasite although the same extract exhibited a higher intrinsic activity (in vivo) against chloroquine-sensitive Plasmodium berghi with a dose of 400 mg kg−1 weight of mice.72 Moreover, S. Hermonthica has also shown antitrypanocidal properties against Trypanosoma cruzi and Trypanosoma congolense73 as well as antibacterial properties against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans.74 The aqueous extract has also shown weak antiheminthic activity against Rhavaditid nematode and Caenorhabditis elegans as well as insecticidal properties .75
Eragrostis tef (Poaceae) is one of the ancient grains of the world. It originated in Ethiopia and it is widely used in the Horn of Africa as food. Three types of the grain are known, the white, red and brown. The phytochemistry of the red type has led to the isolation and characterization of seven compounds, namely, β-sitosterol (85), β-amyrin-3-O-(2′-acetyl-glucoside) (86), β-sitosterol-3-O-β-D-glucoside (84), naringenin (87), naringenin-4′-methoxy-7-O-α-L-rhamnoside (88), eriodictyol-3′,7-dimethoxy-4′-O-β-D-glucoside (89) and isorhamnetin-3-O-rhamnoglucoside (90).76 The ethanol extract of the plant indicated anti-hyperlipidaemic and anti-hyperglycaemic properties and the oils from the seeds increased the calcium levels in the blood of rats.76
The conifer, Podocarpus gracilior (Podocarpaceae), grows in East and Central Africa and many species are resistant to insect attack, therefore, they are important trees. In folk medicine various parts of the tree have been used for the treatment of fevers, asthma, coughs, cholera, distemper, chest pain and venereal diseases.77a Three new terpenoids, 2α,16-dihydroxy-4β-carboxy-O-β-D-glucopyranosyl-19-nor-totarol (91), nagilactone K (92), and 15-hydroxy phaseic acid (93), along with nine known compounds, were isolated and characterized from the leaves of Podocarpus gracilior. The following known compounds were identified by comparison with published spectroscopic data: nagilactone B, nagilactone C, nagilactone D, podolactone B, blumenol C glucoside, erythro-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-oxyneolignan, threo-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-oxyneolignan, glochidiobioside, and 20-hydroxyecdysone (94). Some of these compounds have been subjected to a chemical proteomics study as a strategy for the molecular target identification of natural bioactive compounds, setting up a method for the research of their molecular targets. A fishing for partners study reveals Hsp70 and PPARγ as potential proteic target of 15-ketoatractyligenin methyl ester, a semi-synthetic ent-kaurane, while studies based on limited proteolysis-mass spectrometry strategy, free cell assays, and pro-apoptotic activity of hardwiickik acid in human monocytes allowed to validate Hsp27 as target for the clerodane diterpene (Fig. 6).77b
7 Resedaceae, Rhamnaceae and Rutaceae
A summary of the medicinal uses and biological activities of the compounds of the plant families above are indicated in Table 6.| Plant family | Plant name (Country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and reference |
|---|---|---|---|---|---|---|
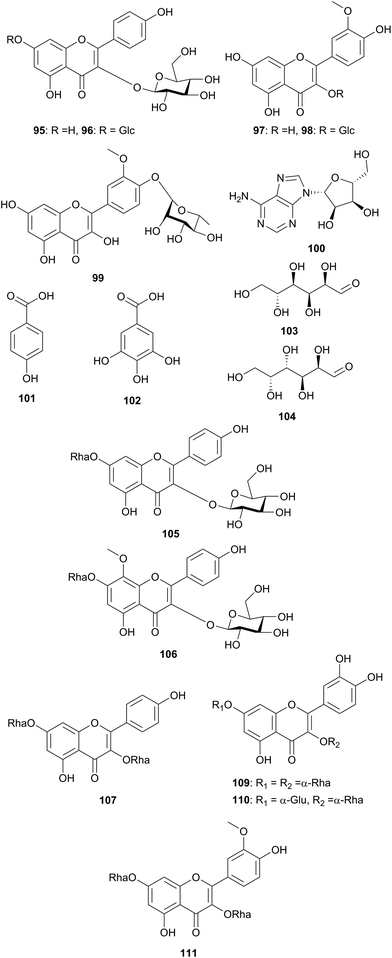
| Resedaceae | Oligomeris linifolis (Egypt) | Aerial parts | 94–104 | Hussein et al.79 | ||
| Randonia africana (Algeria) | Scorpion stings | Aerial parts | 95, 105–111 | Berrehal et al.80 | ||
| Rhamnaceae | Scutia myrtina (Libya) | Stomach problems, salpingitis, malaria, bilharziasis, gonorrhoea, intestinal worms, tumors, inflammations, liver disorders | Aerial parts, roots, leaves | Extracts | Antitumor, antioxidant | Kumar et al.85 |
| Rhamnus alaternus (Tunisia) | Tumors, inflammatioms, asthma, bacterial infections | Leaves | Extracts | Apoptotic | Ammar et al.88 | |
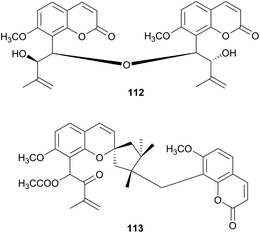
| Rutaceae | Murraya exotica (Egypt) | Skin disease, dysentery, fever, emmenagouge | Vegetative branches | 112–113 | Not tested | Negi et al.90 |
In the survey of the Resedaceae, two species were found: Oligomeris linifolia79 and Randonia africana.80 R. africana has been used to cure scorpions' stings. The phytochemical investigation of O. linifolia yielded one new flavonol monoglycoside: isorhamnetin-4′-O-rhamnopyranoside (99), in addition to 10 compounds; β-sitosterol-3-O-β-glucopyranoside (84), kaempferol-3-O-β-glucopyranoside (95), kampferol-3,7-di-O-β-glucopyranoside (96), isorhamnetin (97), isorhamnetin-3-O-β-glucopyranoside (98), adenosine (100), p-hydroxy benzoic acid (101), gallic acid (102) and two free sugars; glucose (103) and galactose (104). The new glycoside, compound 101, was not tested for any activity. Nine flavonoid glycosides were isolated and characterized from the aerial parts of R. africana, namely kaempferol-3-O-β-glucopyranoside (95), kaempferol-3-O-β-glucopyranoside-7-O-α-rhamnopyranoside (105), 8-hydroxykaempferol 8-methyl ether 3-O-β-glucopyranoside-7-O-α-rhamnopyranoside (sexangularetin) (106), kaempferol-3,7-di-O-α-rhamnopyranoside (107), kaempferol-3-O-β-xylopyranosyl-(1′′′→2′)-O-α-rhamnopyranoside-7-O-α-rhamnopyranoside (sagittatin A) (108), quercetin-3,7-di-O-α-rhamnopyranoside (Fig. 7).
(19), quercetin-3-O-β-glucopyranoside-7-O-α-rhamnopyranoside (110), isorhamnetin-3-O-β-glucopyranoside (98) and isorhamnetin-3,7-di-O-α-rhamnopyranoside (111).
Scutia myrtina (Rhamnaceae), which is widely available in India, East Africa and South Africa, is an important medicinal plant. The aerial parts used for the remedy of stomach problems and salpingitis. The roots and leaves of the plant are traditionally used as an antihelmintic.70 The alcohol extract of the aerial part of the plant possesses antiviral activity.81 The root bark of S. myrtina is used to treat fever and an infusion of the plant is used to treat malaria. In eastern Tanzania, the leaves and root bark of this plant are used for the treatment of bilharziasis, gonorrhea, intestinal worms and fever.82 The tribal people of Kolli Hills in Tamil Nadu (India) used this plant for the treatment of various types of tumours, inflammation and liver disorders. The plant has been reported to show anti-inflammatory and antimicrobial activity.83 An alkaloid nitidine with potent antimalarial activity has been isolated from a Kenyan herbal remedy.84 The ethanol extract of S. myrtina has been investigated for its antitumor and antioxidant activity against Ehrlich ascites carcinoma in Swiss albino rats. The results indicate that the extract has potent antitumor and antioxidant properties and justifies the use of this plant in traditional medicine.85
Another species of the Rhamnoceae with important medicinal uses in the Mediterranean region is Rhamnus alaternus commonly called “oud el khir” in Tunisia. The Rhamnus species have been utilized for the remedy of inflammations, constipation, cancers and asthma.86 The biological screening of pure compounds and extracts of this plant has shown that it has antiproliferative and antibacterial, antimutagenic, antigenotoxic, antioxidant activities.87 Further studies investigate the apoptotic effect of extracts (EtOAc and total oligomer flavonoids) from Rhamnus alaternus in human K562 leukemia cells and on lipid peroxidation.88 The results show that total oligomer flavonoids and ethyl acetate extracts induce apoptotic death in human chronic myelogenous leukemia K562 cell line and effectively protected lipid peroxidation with IC50 values of 196 and 237 μg mL−1, respectively.
Murraya species (Rutaceae) have been used for the remedy of diseases in India, Australia and Africa. A decoction of the leaves has been used to treat bruises, skin diseases, toothache, dysentery and diarrhoea. The leaves have also been used as a tonic, an emmenagogue, a stimulant and an astringent.89 Murraya exotica has been investigated for its potential anticancer, cytotoxic, thrombolytic and antioxidant activities.90 Two new biscoumarins, bismurrangatin (112) and murramarin A (113) have isolated and characterized from the vegetative branches of M. exotica but they have not been tested for biological activity (Fig. 8).
8 Salicaceae, Sapindaceae and Solanaceae
A summary of the medicinal uses and biological activities of the compounds of the plant families above are indicated in Table 7.| Plant family | Plant name (country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and reference |
|---|---|---|---|---|---|---|
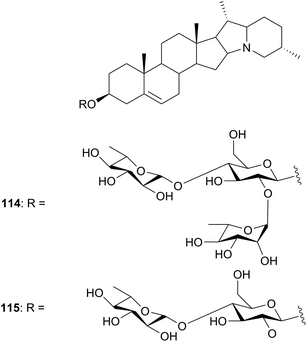
| Solanaceae | Solanum distichum (Egypt) | Fungal infections, herpes | Berries | 114, 115 | Not tested | Abouzid et al.91 |
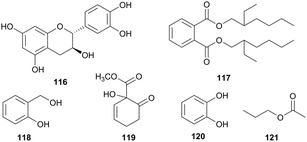
| Saliacaceae | Salix subserrata (Egypt) | Constipation, stomach pains, fever, headache, rabies | Leaf and bark | 84, 85, 116–121 | Antimicrobial | Hussain et al.92 |
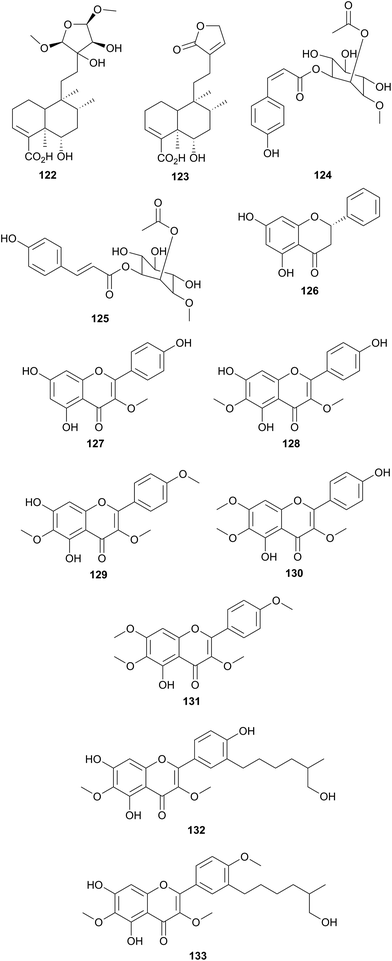
| Sapindaceae | Dodonaea viscose (Egypt) | Cold, fever, rheumatism, indigestion, ulcers, diarrhea, constipation, intestinal worms, dysmenorrhea, irregular menstration | Aerial parts | 126, 131 and 133 | Mostafa et al.93 |
The berries of Solanium distichum (Solanaceae) are used for the treatment of several chronic diseases. In general, Solanum species are known to have steroidal glycoalkaloids which possess many biological properties. They prevent fungal growth94 and inactivate Herpes simplex virus95 and has been shown to have molluscicidal activity.96 The fractionated ethanol extract of the berries of S. distichum yielded two steroidal glycoalkaloids,91 namely; Solanidine 3-O-[α-L-rhamnopyranosyl-(1′′→4)-[α-L-rhamnopyranosyl-(1′→2)]-β-D-glucopyranoside] (114) and Solanidine 3-O-[α-L-rhamnopyranosyl-(1′′→4)-β-D-glucopyranoside] (115) commonly known as α-chanonine and β2-chanonine. Their structures were determined by spectroscopic methods and they were not tested for any biological activities (Fig. 9).
The leaves of Salix subserrata (Synonyms S. safsaf) (Salicaceae), while being used as a laxative in human and veterinary medicine has provided a black dye for local mats in Sudan.97 The roots are used in concoctions that remedy stomach pains, fever and headaches97 and the leaves are used along with milk to treat patients with rabies.98 The EtOAc fraction of the extract of the bark of S. subserrata yielded catechin (116), 1,2-benzenedicarboxylic acid, bis(2-ethylhexyl) ester (117), salgenin (118), methy 1-hydroxy-6-oxocyclohex-2-enecarboxylate (119), and catechol (120). The n-hexane extract afforded propyl acetate (121), β-sitosterol (85) and the chloroform fraction of the bark gave β-sitosterol glucopyranoside (84). The crude extracts and compounds 116 and 85 were tested by an agar diffusion method for antibacterial (Escherichia coli, Bacillus magaterium), antifungal (Microbotyrum violaceum) and antialgal (Chlorella fusca) activities.91 The crude extracts exhibited promising antialgal activity against Chlorella fusca except the EtOAc extract of the leaf but moderate activity against the fungus. All extracts exhibited potential antibacterial activity against Bacillus megaterium but the EtOAc extract of both the leaf and bark of S. subserrata were inactive against fungal Microbotyrum violaceum. Compounds 116 and 85 indicated good activity against Chlorella fusca and antibacterial activity against Gram-positive bacterium, Bacillus megaterium and Gram-negative bacterium Escherichia coli. The tested compounds only showed moderate antifungal activity against Microbotyrum violaceum. A mixture of compounds 118 and 119 also showed good algal and antibacterial properties but moderate antifungal activity against Microbotyrum violaceum.92 These studies underscore the necessity to properly evaluate plants for their biological properties in order to establish a scientific base for their utility in folk medicine. Dodonea viscosa (Sapindaceae) is used as used as a medicinal plant worldwide to cure sore throat, cold, fever, rheumatism, diarrhea, ulcers, indigestion, headache, toothache to expel intestinal worm and to soothe itching.99 The seeds are used in India as fish poison100 and in East Africa, the roots are eaten by women to activate breast milk and treat dysmenorrheal and irregular menstruation (Fig. 10).99
The bioassay-guided fractionation of the aerial parts of D. viscosa (Sapindaceae) from Egypt resulted in the isolation and identification of three new compounds, including two new clerodane diterpenoids 122 and 123 and a new myo-inositol derivative 124, along with nine known compounds 125–133.93 The new compounds were identified as 13,14-dihydroxy-15,16 dimethoxy-(−)-6α-hydroxy-5α, 8α,9α,10α-cleroda-3-en-18-oic acid (122), (−)-6α-hydroxy-5α,8α,9α,10α-cleroda-3,13-dien-16,15-olid-18-oic acid. (123), 1-L-O-methyl-2-acetyl-3-p-coumaryl-myo-inositol (124), and the known compounds as 1-L-1-O-methyl-2-acetyl-3-p-E-coumaryl-myo-inositol (125), pinocembrin (126), isokaemferide (127), 6-methoxy isokaemferide (128), 5,7-dihydroxy-3,6,4′-trimethoxy flavone (129), penduletin (130), 5-hydroxy-3,6,7,4′-tetramethoxy flavone (131), aliarin (132) and 5,7-dihydroxy-3′-(4-hydroxy-3-methylbutyl) 3,6,4′-trimethoxyflavone (133). All isolated compounds were screened for antimicrobial activity, in vitro, against Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591 (MRS), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Mycobacterium intracellulare ATCC 23068, Candida albicans ATCC 90028, Candida glabrata ATCC 90030, Candida krusei ATCC 6258, Cryptococcus neoformans ATCC 90113, and Aspergillus fumigatus ATCC 204305 using ciprofloxacin and amphotericin B as positive controls for bacteria and fungi, respectively. All isolated compounds were equally checked for antileishmanial activity, in vitro, against a culture of Leishmania donovani promastigotes using pentamidine and amphotericin B as a standards. All isolated compounds were investigated for antimalarial activity in vitro against chloroquine sensitive (D6, Sierra Leone) and resistant (W2, Indo China) strains of Plasmodium falciparum by measuring plasmodial LDH activity. Chloroquine was used as positive control. The results concluded that compound 130 displayed good antifungal activity against Cryptococcus neoformans with an IC50 value of 6.11 mg mL−1. Compounds 131 and 132 exhibited reasonable antileishmanial activity against Leishmania donovani with IC50 values of 16.6 and 19.06 mg mL−1, respectively (Fig 11).
9 Conclusions
African medicinal plants are an enormous repository of bioactive NPs with a huge potential for drug discovery programs. As an example, previous studies on Central7,8,15,16 and West Africa,17–20 have revealed a diversity of NPs with a broad range of biological activities. In the Congo Basin, located in Central Africa, several regions in Cameroon serve as typical biodiversity hotspots, with a strong traditional medicinal background and presence. Cameroon's diversity spans hotspots from Mount Cameroon to the Dja reserve, along with several important investigations on traditional plants based on their usage in traditional medicine, some of which have been cited in the aforementioned reviews. Madagascar is another hotspot for NP lead discovery on the continent of Africa and will be discussed in detail in our review series focused on the Southern African region.The results for 30 plant species belonging to 17 families (beginning with letters E to S) from North Africa have been discussed in the current review. This involves 142 compounds with intriguing chemical structures and diverse biological activities. An attempt to correlate the biological activities of the isolated compounds with the uses of the plants in traditional medicine has been carried out. However, such an endeavour could prove to be challenging. One of the reasons may be because the traditional methods (steam baths, boiling, use of total extracts as mixtures, etc.) do not match the typical laboratory extraction methods (isolation in polar or non-polar solvents, separation of fractions, isolation of pure compounds, testing of pure isolated compounds, etc.). A quick look at Tables 1–7 clearly shows many discrepancies (with the exception of a few correlations marked in bold). This contributes to reducing the true impact of the “value” of traditional knowledge derived from ATM in the current scenario in natural products research. The present results however show the usefulness of traditional medicine drug discovery programs. This review paper does not claim to be exhaustive, the reason being that its principal objective is to establish a baseline for further investigations of North African flora. Data on plant sources, geographical collection sites, chemical structures of pure compounds as well as their spectroscopic data, were retrieved from literature sources comprising data collected from major international journals on natural products and some available PhD theses, spanning the period 1971 to 2014. The current survey consisted in collecting data from the literature sources, mainly using author queries in major natural product and medicinal chemistry journals. The collected data includes plant sources, uses of plant material in traditional medicine, plant families, region of collection of plant material, isolated metabolites and type (e.g. flavonoid, terpenoid, etc.), measured biological activities, etc. The data was collected on an Excel sheet and analyzed. We further propose to build a searchable database for compounds isolated from Northern African flora, in order to assist in drug discovery programs on the continent of Africa.
Acknowledgements
Financial support is acknowledged from Lhasa Ltd, Leeds, UK through the Chemical and Bioactivity Information Centre (CBIC), University of Buea, Cameroon. FNK acknowledges a Georg Forster fellowship for postdoctoral researchers from the Alexander von Humboldt Foundation.Notes and references
- United Nations Organization, Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm, assessed on 8th July 2014.
- N. S. Abdel-Azim, K. A. Shams, A. A. A. Shahat, M. M. El Missiry, S. I. Ismail and F. M. Hammouda, Res. J. Med. Plant, 2011, 5, 136 CrossRef.
- A. A. Shahat, L. Pieters, S. Apers, N. M. Nazeif, N. S. Abdel-Azim, D. V. Berghe and A. J. Vlietinck, Phytother. Res., 2001, 15, 593 CrossRef CAS PubMed.
- L. Dagmar, International trade in medicinal and aromatic plants, actors, volumes and commodities, plants, in. Medicinal and aromatic plants, ed. R. J. Bogers, L. E. Craker and D. Lange, Springer, Berlin, Heidelberg, 2006 Search PubMed.
- M. Vilà, Y. Meggaro and E. Weber, Orsis, 1999, 14, 9 Search PubMed.
- F. Ntie-Kang and J. N. Yong, RSC Adv., 2014, 4, 61975 RSC.
- F. Ntie-Kang, J. A. Mbah, L. M. Mbaze, L. L. Lifongo, M. Scharfe, J. Ngo Hanna, F. Cho-Ngwa, P. A. Onguéné, L. C. O. Owono, E. Megnassan, W. Sippl and S. M. N. Efange, BMC Complementary Altern. Med., 2013, 13, 88 CrossRef PubMed.
- F. Ntie-Kang, P. A. Onguéné, M. Scharfe, L. C. O. Owono, E. Megnassan, L. M. Mbaze, W. Sippl and S. M. N. Efange, RSC Adv., 2014, 4, 409 RSC.
- F. Ntie-Kang, P. A. Onguéné, G. W. Fotso, K. Andrae-Marobela, M. Bezabih, J. C. Ndom, B. T. Ngadjui, A. O. Ogundaini, B. M. Abegaz and L. M. Mbaze, PLoS One, 2014, 9(3), e90655 Search PubMed.
- F. Ntie-Kang, D. Zofou, S. B. Babiaka, R. Meudom, M. Scharfe, L. L. Lifongo, J. A. Mbah, L. M. Mbaze, W. Sippl and S. M. N. Efange, PLoS One, 2013, 8(10), e78085 CAS.
- F. Ntie-Kang, L. L. Lifongo, J. A. Mbah, L. C. O. Owono, E. Megnassan, L. M. Mbaze, P. N. Judson, W. Sippl and S. M. N. Efange, In Silico Pharmacol., 2013, 1, 12 CrossRef PubMed.
- F. Ntie-Kang, J. A. Mbah, L. L. Lifongo, L. C. O. Owono, E. Megnassan, L. M. Mbaze, P. N. Judson, W. Sippl and S. M. N. Efange, Org. Med. Chem. Lett., 2013, 3, 10 CrossRef PubMed.
- P. A. Onguéné, F. Ntie-Kang, J. A. Mbah, L. L. Lifongo, J. C. Ndom, W. Sippl and L. M. Mbaze, Org. Med. Chem. Lett., 2014, 4, 6 CrossRef PubMed.
- F. Ntie-Kang, J. N. Nwodo, A. Ibezim, C. V. Simoben, B. Karaman, V. F. Ngwa, W. Sippl, M. U. Adikwu and L. M. Mbaze, J. Chem. Inf. Model., 2014, 54(9), 2433 CrossRef CAS PubMed.
- F. Ntie-Kang, L. L. Lifongo, L. M. Mbaze, N. Ekwelle, L. C. O. Owono, E. Megnassan, P. N. Judson, W. Sippl and S. M. N. Efange, BMC Complementary Altern. Med., 2013, 13, 147 CrossRef PubMed.
- D. Zofou, F. Ntie-Kang, W. Sippl and S. M. N. Efange, Nat. Prod. Rep., 2013, 30, 1098 RSC.
- L. L. Lifongo, C. V. Simoben, F. Ntie-Kang, S. B. Babiaka and P. N. Judson, Nat. Prod. Bioprospect., 2014, 4, 1 CrossRef PubMed.
- F. Ntie-Kang, L. L. Lifongo, C. V. Simoben, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 28728 RSC.
- F. Ntie-Kang, L. L. Lifongo, C. V. Simoben, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 35348 RSC.
- C. V. Simoben, F. Ntie-Kang, L. L. Lifongo, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 40095 RSC.
- P. A. Onguéné, F. Ntie-Kang, L. L. Lifongo, J. C. Ndom, W. Sippl and L. M. Mbaze, Malar. J., 2013, 13, 449 CrossRef PubMed.
- F. Ntie-Kang, P. A. Onguéné, L. L. Lifongo, J. C. Ndom, W. Sippl and L. M. Mbaze, Malar. J., 2014, 13, 81 CrossRef PubMed.
- J. N. Yong and F. Ntie-Kang, Anti-Infect. Agents, 2014, 12, 178 CrossRef CAS.
- F. R. Irvine, Woody plants of Ghana, Oxford University Press, London, 1961 Search PubMed.
- (a) I. E. Mohamed, E. El Bushra, El Nur, M. I. Choudhary and S. N. Khan, Rec. Nat. Prod., 2009, 3(4), 198 CAS; (b) O. K. Vasant, B. G. Vijay, S. R. Virbhadrappa, N. T. Dilip, M. V. Ramahari and B. S. Laxamanrao, Iran. J. Pharm. Res., 2012, 11, 621 Search PubMed.
- A. El-Hamidi, Planta Med., 1970, 18, 278 CrossRef PubMed.
- J. E. Okokon and P. A. Nwafor, J. Ethnopharmacol., 2009, 121, 74 CrossRef PubMed.
- J. E. Okokon, K. C. Ofodum, K. K. Ajibesin and B. K. S. Danladi, Indian J. Pharmacol., 2005, 37, 243 CrossRef PubMed.
- J. E. Okokon, A. L. Bassey and J. Obot, Afr. J. Tradit., Complementary Altern. Med., 2006, 31, 21 Search PubMed.
- S. El-Masry, M. E. Amer, M. S. Abdel-Kader and H. H. Zaatout, Phytochemistry, 2002, 60, 783 CrossRef CAS.
- L. E. A. Hassan, K. K. A. Mohamed, K. Ahamed, A. S. A. Majid, M. A. Iqbal, F. S. R. Al Suede, R. A. Haque, Z. Ismail, O. C. Ein and A. M. S. A. Majid, PLoS One, 2014, 9(3), e90806 Search PubMed.
- M. M. D. Mohammed, N. A. Ibrahim, N. E. Awad, A. A. Matloub, A. G. Mohamed-Ali, E. E. Barakat, A. E. Mohamed and P. L. Colla, Nat. Prod. Res., 2012, 26(17), 1565 CrossRef CAS PubMed.
- F. R. Melek, I. A. A. Kassem, T. Miyase and W. Fayad, Phytochemistry, 2014, 100, 110 CrossRef CAS PubMed.
- V. Gulecha and T. Sivakuma, Asian Pac. J. Trop. Med., 2011, 4, 526 CrossRef.
- M. E. S. Kassem, M. Sharaf, M. H. Shabana and N. A. M. Saleh, Nat. Prod. Commun., 2006, 1(11), 953 CAS.
- Traditional herbal medicines for modern times, Traditional medicines For Modern Times: Antidiabetic Plants, ed. A. Soumyanath, Taylor and Francis, CRC Press, Boca Raton, FL, 2006 Search PubMed.
- B. E. P. Oliver-Bever, Medicinal plants in Tropical West Africa, Cambridge University Press, Cambridge, 1986 Search PubMed.
- A. Ainouche, R. Grienwald, L. Witte and A. Huon, Biochem. Syst. Ecol., 1996, 24(5), 405 CrossRef CAS.
- N. Semmar, M. Jay, M. Farman and R. Chemli, Biochem. Syst. Ecol., 2005, 33, 187 CrossRef CAS PubMed.
- N. Zitouna, S. Marghali, M. Gharbi, H. Chennaoui-Kourda, A. Haddioui and N. Trifi-Farah, Biochem. Syst. Ecol., 2013, 50, 129 CrossRef CAS PubMed.
- R. S. Essokne, R. J. Grayer, E. Porter, G. C. Kite, M. S. J. Simmonds and S. L. Jury, Biochem. Syst. Ecol., 2002, 42, 49 CrossRef PubMed.
- J. Wandji, Z. T. Fomum, F. Tillequin, E. Seguin and M. Koch, Phytochemistry, 1994, 35, 245 CrossRef CAS.
- J. Wandji, Z. T. Fomum, F. Tillequin, A. L. Skaltsounis and M. Koch, Planta Med., 1994, 60, 178 CrossRef CAS PubMed.
- A. E. Nkengfack, M. Meyer, M. S. Tempesta and Z. T. Fomum, Planta Med., 1991, 57, 488 CrossRef CAS PubMed.
- H. D. Neuwinger, African Traditional Medicine; A Dictionary of Plant Use and Applications, Medpharm Scientific, Stuttgart, Germany, 2000, pp. 207–208 Search PubMed.
- A. J. Vlietinck, T. De Bruyne, S. Apers and L. A. Pieters, Planta Med., 1998, 64, 97 CrossRef CAS PubMed.
- T. G. Tan, A. D. Kinghorn, S. H. Hughes and J. M. Pezutto, J. Biol. Chem., 1991, 266, 23529 Search PubMed.
- S. Inouye, M. Takahashi and S. Abe, Int. J. Essent. Oil Ther., 2008, 2, 139 CAS.
- S. Apaydin, U. Zeybek, I. Ince, G. Elgin, C. Karamenderes, B. Ozturk and I. Tuglular, J. Ethnopharmacol., 1999, 67, 307 CrossRef CAS.
- F. Conforti, G. A. Statti, R. Tundis, F. Menichini and P. Houghton, Fitoterapia, 2002, 73, 479 CrossRef CAS.
- Z. Rouis, N. Abid, S. Koudja, T. Yangui, A. Elaissi, P. L. Cioni, G. Flamini and M. Aouni, BMC Complementary Altern. Med., 2013, 13, 24 CrossRef PubMed.
- A. M. M. Naguib, M. E. Ebrahim, H. F. Aly, H. M. Metawaa, A. H. Mahmoud, E. A. Mahmoud and F. M. Ebrahim, Nat. Prod. Res., 2012, 26(23), 2196 CrossRef CAS PubMed.
- S. El Bardai, N. Morel, M. Wibo, N. Fabre, G. Llabres, B. Lyoussi and J. Quetin-Leclerq, Planta Med., 2003, 69, 75 CrossRef CAS PubMed.
- Y. Lu and L. Y. Foo, Phytochemistry, 2002, 59, 117 CrossRef CAS.
- A. Kabouche, Z. Kabouche, R. Touzani and C. Bruneau, Chem. Nat. Compd., 2008, 44, 824 CrossRef CAS PubMed.
- Y. B. Wu, Z. Y. Ni, Q. W. Shi, M. Dong, H. Kiyota, Y. C. Gu and B. Cong, Chem. Rev., 2012, 112, 5967 CrossRef CAS PubMed.
- H. Lakhal, A. Kabouche, A. A. Magid, L. Voutquenne-Nazabadioko, D. Harakat and Z. Kabouche, Phytochemistry, 2014, 102, 145 CrossRef CAS PubMed.
- H. M. A. Cavanagh and J. M. Wilkinson, Phytother. Res., 2002, 16, 301 CrossRef CAS PubMed.
- J. El-Hilaly, M. Hmammouchi and B. Lyoussi, J. Ethnopharmacol., 2003, 86, 149 CrossRef.
- S. Sosa, G. Altinier, M. Politi, A. Braca, I. Morelli and R. della Loggia, Phytomedicine, 2005, 12(4), 271 CrossRef CAS PubMed.
- S. A. Khalid, G. M. Friedrichsen, A. Kharazmi, T. G. Theander, C. E. Olsen and S. B. Christensen, Phytochemistry, 1998, 49(6), 1769 CrossRef CAS.
- A. N. A. Ali, M. Alhaj and H. Bakthir, Int. J. Nat. Appl. Sci., 2005, 2, 74 Search PubMed.
- M. Giday, T. Teklehaymanot, A. Animut and Y. Mekonnen, J. Ethnopharmacol., 2007, 110, 516 CrossRef PubMed.
- R. G. Marwah, M. O. Fatope, R. Al Mahrooqi, G. B. Varma, H. Al Abadi and S. K. S. Al-Burtamani, Food Chem., 2007, 101, 465 CrossRef CAS PubMed.
- R. Heinke, K. Franke, A. Porcel, L. A. Wessjohann, N. A. A. Ali and J. Schmidt, Phytochemistry, 2011, 72, 929 CrossRef CAS PubMed.
- A. El-Fishawy, R. Zayed and S. Afifi, J. Nat. Prod., 2011, 4, 184 CAS.
- F. Dall'Acqua, M. Terbojevich, S. Marciani, D. Vedaldi and M. Recher, Chem.-Biol. Interact., 1978, 21, 103 CrossRef.
- I. Quinto, D. Averbeck, E. Moustacchi, Z. Hrisoho and J. Moron, Mutat. Res., 1984, 136, 49 CAS.
- F. H. M. Koua, J. Appl. Pharm. Sci., 2011, 01(07), 01 Search PubMed.
- J. O. Kokwaro, in Medicinal Plants in East Africa, ed. L. Van Puyvelde and D. Geysen, East African Literature Bureau, Nairobi, Kenya, 1976, pp. 92–93, 203 Search PubMed.
- M. K. Choudhury, A. L. Phillips and A. Mustapha, Phytother. Res., 1998, 12, 141 CrossRef.
- L. C. Okpako and E. O. Ajaiyeoba, Afr. J. Med. Sci., 2004, 33, 73 CAS.
- S. E. Atawodi, T. Bulus, S. Ibrahim, D. A. Ameh, A. J. Nok, M. Mamman and M. Galadima, Afr. J. Biotechnol., 2003, 2, 317 CrossRef.
- F. H. M. Koua, H. A. A. Babiker, A. Halfawi, R. O. Ibrahim, F. M. Abbas, E. I. Elgaali and M. M. Khalafallah, Res. Pharm. Biotechnol., 2011, 3, 85 Search PubMed.
- A. M. Ibrahim, Phytother. Res., 1992, 6, 155 CrossRef.
- T. S. El-Alfy, S. M. Ezzat and A. A. Sleem, Nat. Prod. Res., 2012, 26(7), 619 CrossRef CAS PubMed.
- (a) H. S. Abdillahi, J. F. Finnie and J. van Staden, J. Ethnopharmacol., 2011, 136, 496 CrossRef CAS PubMed; (b) L. Faiella, Isolation and structural characterization of diterpenoidic compounds and identification of their molecular targets, PhD Thesis, Università di Pisa, 2013.
- L. Faiella, A. Temraz, T. Siciliano, N. de Tommasi and A. Braca, Phytochem. Lett., 2012, 5, 297 CrossRef CAS PubMed.
- S. R. Hussein, A. Elkhateeb, M. M. Marzouk, L. F. Ibrahim and S. A. Kawashty, Biochem. Syst. Ecol., 2013, 49, 73 CrossRef CAS PubMed.
- D. Berrehal, A. Khalfallah, A. Kabouche, Z. Kabouche, A. Karioti and A. R. Bilia, Biochem. Syst. Ecol., 2010, 38, 1007 CrossRef CAS PubMed.
- M. L. Dhar, M. M. Dhar, B. N. Dhawan, B. N. Mehrotra and C. Ray, Indian J. Exp. Biol., 1968, 6, 232 CAS.
- I. Hedberg, O. Hedberg, K. E. Mashigeni, E. N. Mshiu and G. Samuelsson, J. Ethnopharmacol., 1983, 9, 237 CrossRef CAS.
- R. S. Kumar, N. Kritheka, V. Senthil, N. V. Murthy, R. S. Sundram and P. Perumal, Orient. Pharm. Exp. Med., 2008, 8, 400 CrossRef.
- D. M. N. Gakunju, E. K. Mberu, S. F. Dossaji, A. I. Gray, R. D. Waigh, P. G. Waterman and W. M. Watkins, Antimicrob. Agents Chemother., 1995, 39, 2606 CrossRef CAS.
- R. S. Kumar, K. A. Kumar and N. V. Murthy, Nat. Prod. Res., 2012, 26(16), 1504 CrossRef CAS PubMed.
- J. S. Jiang, C. H. M. Shih, S. H. Wang, T. T. Chen, C. H. N. Lin and W. C. H. Ko, J. Ethnopharmacol., 2006, 103, 281 CrossRef CAS PubMed.
- R. Ben Ammar, W. Bhouri, M. Ben Sghaier, J. Boubaker, I. Skandrani and A. Neffati, Food Chem., 2009, 116, 258 CrossRef CAS PubMed.
- R. B. Ammar, A. Neffati, I. Skandrani, M. B. Sghaier, W. Bhouri, K. Ghedira and L. Chekir-Ghedira, Nat. Prod. Res., 2011, 25(11), 1047 CrossRef PubMed.
- H. L. Walter and P. F. Memory, “Medicinal Plants Affecting Man's Health”, John Wiley, New York, 1977, p. 2505 Search PubMed.
- (a) N. Negi, A. Ochi, M. Kurosawa, K. Ushijima, Y. Kitaguchi, E. Kusakabe, F. Okasho, T. Kimachi, N. Teshima, M. Ju-ichi, A. M. Abou-douh, C. Ito and H. Furukawa, Chem. Pharm. Bull., 2005, 53(9), 1180 CrossRef CAS; (b) A. Khatun, M. Rahman and S. Jahan, Orient. Pharm. Exp. Med., 2014, 14, 223 CrossRef.
- S. Abouzid, N. Fawzy, N. D. Arweesh and Y. Orihara, Nat. Prod. Res., 2008, 22(2), 147 CrossRef CAS PubMed.
- H. Hussain, A. Badawy, A. Elshazly, A. Elsayed, K. Krohn, M. Riaz and B. Schulz, Rec. Nat. Prod., 2011, 5(2), 133 CAS.
- A. E. Mostafa, A. A. El-Hela, A. A. E. I. Mohammad, M. Jacob, S. J. Cutler and S. A. Ross, Phytochem. Lett., 2014, 8, 10 CrossRef CAS PubMed.
- A. M. Fewell, J. G. Roddick and M. Weissenberg, Phytochemistry, 1994, 37, 1007 CrossRef CAS.
- H. V. Thorne, G. F. Clarke and R. Skuce, Antiviral Res., 1985, 5, 335 CrossRef CAS.
- A. W. Wanyonyi, S. C. Chhabra, G. Mkoji, U. Eilert and W. M. Njue, Phytochemistry, 2002, 59, 79 CrossRef CAS.
- H. M. Burkill, The useful plants of west tropical Afric, Families S–Z, Addenda, Royal Botanic Gardens, Kew, Richmond, United Kingdoma, vol. 5, 1985 Search PubMed.
- H. Yineger, D. Yewhalaw and D. Teketay, J. Ethnobiol. Ethnomed., 2008, 4, 11 CrossRef PubMed.
- S. M. Rani, R. S. Pippalla and K. Mohan, Dodonaea viscosa Linn.-an overview, Asian J. Pharm. Res. Health Care, 2009, 1, 97 Search PubMed.
- H. Wagner, C. Ludwig, L. Grotjahn and M. S. Y. Khan, Phytochemistry, 1987, 26, 697 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |