Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle†
Maria
Lapteva
,
Michael
Möller
,
Robert
Gurny
and
Yogeshvar N.
Kalia
*
School of Pharmaceutical Sciences, University of Geneva & University of Lausanne, 30 Quai Ernest Ansermet, 1211 Geneva, Switzerland. E-mail: Yogi.Kalia@unige.ch; Fax: +41 22 379 3360; Tel: +41 22 379 3355
First published on 19th October 2015
Abstract
Acne vulgaris is a highly prevalent dermatological disease of the pilosebaceous unit (PSU). An inability to target drug delivery to the PSU results in poor treatment efficacy and the incidence of local side-effects. Cutaneous application of nanoparticulate systems is reported to induce preferential accumulation in appendageal structures. The aim of this work was to prepare stable polymeric micelles containing retinoic acid (RA) using a biodegradable and biocompatible diblock methoxy-poly(ethylene glycol)-poly(hexylsubstituted lactic acid) copolymer (MPEG-dihexPLA) and to evaluate their ability to deliver RA to skin. An innovative punch biopsy sample preparation method was developed to selectively quantify follicular delivery; the amounts of RA present were compared to those in bulk skin, (i.e. without PSU), which served as the control. RA was successfully incorporated into micelle nanocarriers and protected from photoisomerization by inclusion of Quinoline Yellow. Incorporation into the spherical, homogeneous and nanometer-scale micelles (dn < 20 nm) increased the aqueous solubility of RA by >400-fold. Drug delivery experiments in vitro showed that micelles were able to deliver RA to porcine and human skins more efficiently than Retin-A® Micro (0.04%), a marketed gel containing RA loaded microspheres, (7.1 ± 1.1% vs. 0.4 ± 0.1% and 7.5 ± 0.8% vs. 0.8 ± 0.1% of the applied dose, respectively). In contrast to a non-colloidal RA solution, Effederm® (0.05%), both the RA loaded MPEG-dihexPLA polymeric micelles (0.005%) and Retin-A® Micro (0.04%) displayed selectivity for delivery to the PSU with 2-fold higher delivery to PSU containing samples than to control samples. Moreover, the micelle formulation outperformed Retin-A® Micro in terms of delivery efficiency to PSU presenting human skin (10.4 ± 3.2% vs. 0.6 ± 0.2%, respectively). The results indicate that the polymeric micelle formulation enabled an increased and targeted delivery of RA to the PSU, potentially translating to a safer and more efficient clinical management of acne.
Introduction
Acne vulgaris is a local skin disease that affects over 80% of adolescents and can continue to have an impact during adulthood. Although a superficial skin condition, acne can strongly affect patient physical appearance and self-esteem, resulting in psychological complications.1 It occurs in the pilosebaceous unit (PSU) and has a complex pathogenesis usually triggered by hormonal changes during puberty. The PSU is a skin appendage comprising the hair shaft and sebaceous gland inside a follicular duct covered by a stratified squamous epithelium. In healthy tissue, this epithelium desquamates and the shed cells are eliminated via the follicular duct together with the sebum. In the case of acne affected skin, the follicular duct infundibulum is occluded by desquamated cells due to increased turnover; the sebum is over-secreted and cannot be evacuated. Bacterial growth is thus promoted inside the PSU and can lead to inflammation and/or rupture of the entire unit. Skin lesions can range from microcomedones (i.e. no inflammation) to inflamed pustules and cysts.1–3Among the different therapeutic agents available to treat acne, retinol (Vitamin A) derivatives play an important role in both systemic and topical therapy. Topically applied retinoids have been shown to act on retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in keratinocytes and sebocytes modulating their proliferation and differentiation.1,4 Retinoids have also been shown to possess anti-inflammatory activity. However, this therapeutic activity is often accompanied by side effects such as skin dryness, peeling or irritation.5 It has recently been suggested that colloidal micro- and nanoparticulate drug delivery systems possess a unique ability to be deposited in the follicular duct; in particular, when accompanied by massage of the formulation on the skin surface which facilitates dispersal and entry into the appendage.5–13 Follicular targeting is of major interest in the treatment of acne since it could increase the efficacy of anti-acne agents and reduce the incidence and severity of local side effects.
All-trans retinoic acid (tretinoin; TRN) is one of the most widely used retinoids for the topical treatment of acne. It is also one of the most widely studied and its formulation in liposomes,14–16 solid lipid nanoparticles17 and microspheres5,18 has been investigated and improvements in skin bioavailability have been observed. Furthermore, a microsphere formulation (Retin-A Micro®), which also showed a favorable irritation profile, has received regulatory approval.5 Given its high lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 6.2) and almost negligible aqueous solubility, TRN is difficult to formulate: excipients able to solubilize TRN can also perturb the stratum corneum lipids and enhance transdermal permeation thereby increasing the risk of systemic side effects. Therefore, the challenge for the formulator is to improve cutaneous delivery of TRN, ideally targeting the PSU, while minimizing transdermal permeation.
P = 6.2) and almost negligible aqueous solubility, TRN is difficult to formulate: excipients able to solubilize TRN can also perturb the stratum corneum lipids and enhance transdermal permeation thereby increasing the risk of systemic side effects. Therefore, the challenge for the formulator is to improve cutaneous delivery of TRN, ideally targeting the PSU, while minimizing transdermal permeation.
Polymeric micelles composed of a novel biodegradable and biocompatible diblock methoxy-poly(ethylene glycol)-poly(hexylsubstituted lactic acid) copolymer (MPEG-dihexPLA) have shown promise as nanocarriers for poorly water soluble, lipophilic drugs. Copolymer degradation resulted in the formation of non-toxic 2-hydroxyoctanoic acid and lactic acid.19 Its biocompatibility was demonstrated in vitro in different cell lines and in vivo using the chick embryo chorioallantoic membrane (CAM) model for both the copolymer (below the CMC) and polymeric micelles up to copolymer concentrations of 20 mg ml−1.20 Moreover, no hemolysis was observed.20
Recent investigations have also shown the potential of these micelles as topical delivery systems for targeted dermatological therapy. Micelles applied to skin were able to deliver “difficult-to-formulate” drugs such as econazole nitrate,21 tacrolimus,22 and ciclosporin A23 to the upper layers of the skin, avoiding undesirable transdermal permeation. The preferential deposition of micelles into the follicular duct, without accompanying skin massage, was observed by confocal laser scanning microscopy.22 This raised the possibility of using micelles for the selective delivery of therapeutic agents to the PSU.
The first aim of this study was to formulate TRN loaded MPEG-dihexPLA polymeric micelles. It is well known that TRN has poor photostability and is readily converted into nine identified cis–trans stereoisomers upon exposure to light.24 Therefore, it is believed that a significant proportion of TRN applied to the skin surface is degraded within one or two hours by sunlight.25 Of the different photoisomers, only isotretinoin (13-cis retinoic acid) and alitretinoin (9-cis retinoic acid) are active and might have a pharmacological effect; indeed, isotretinoin has been shown to possess non-negligible skin deposition.26 Thus, TRN containing micelles underwent a photostability study and the formulation was optimized to improve drug stability. However, given that the main degradation products in both micelle and commercial formulations were isotretinoin and alitretinoin, it was decided to quantify the total amount of the different retinoic acid (RA) isomers in the in vitro skin delivery experiments that made up the second part of the study. It is important to note that in the present work, TRN refers to isomerically pure all-trans retinoic acid (tretinoin) and RA is a collective term used to describe all isomers of retinoic acid. The cutaneous delivery efficiency of RA from the optimal TRN micelle formulation was compared with that from two marketed formulations (Retin-A Micro® (0.04%) and Effederm® (0.05%)) and the ability of the micelle formulation to target the PSU selectively was demonstrated using a newly developed biopsy method.
Experimental
Materials
All-trans retinoic acid (Ph. Eur.; ≥98% purity) and bovine serum albumin (BSA) were purchased from Axon-Lab (Baden-Dättwil, Switzerland). The amphiphilic MPEG-dihexPLA copolymer (methoxy-poly(ethylene glycol) di-(hexyl- substituted polylactide)) was synthesized in-house as described previously.22,23 The MPEG-dihexPLA copolymer had a Mn of 6080 g mol−1 and a polydispersity index (P.I.) of 1.15 according to GPC measurements. The internal standard acitretin (ACT), 9-cis retinoic acid, 13-cis retinoic acid, tartrazine, Quinoline Yellow, Acid Yellow 17, sodium and potassium chloride, sodium and potassium phosphate, trifluoroacetic acid, phosphoric acid and triethylamine were purchased from Sigma Aldrich (Buchs, Switzerland). Acetone and acetonitrile (HPLC grade) were supplied by Biosolve chemicals (Valkenswaar, Netherlands). Ultra-pure water (Millipore Milli-Q® Gard 1 Purification Pack resistivity >18 MΩ cm, Zug, Switzerland) was used. All other chemicals were at least of analytical grade.Analytical methods
| Time (min) | Flow rate (ml min−1) | % A | % B |
|---|---|---|---|
| 0 | 1 | 90 | 10 |
| 2 | 1 | 90 | 10 |
| 2.5 | 0.4 | 80 | 20 |
| 30 | 0.4 | 40 | 60 |
| 35 | 1 | 90 | 10 |
| 45 | 1 | 90 | 10 |
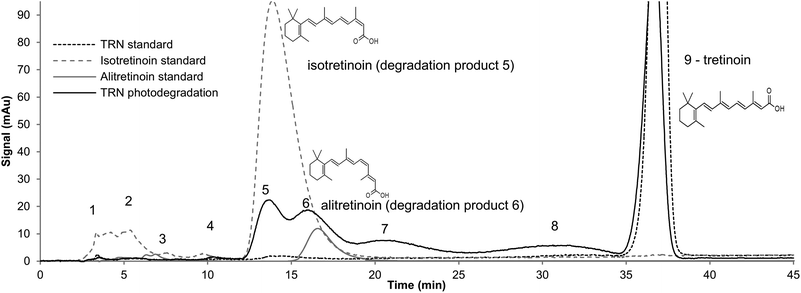
The gradient method was able to separate the 9 stereoisomers of retinoic acid. Fig. 1 shows the chromatograms obtained for (i) TRN standard (100 μg ml−1), (ii) isotretinoin standard (100 μg ml−1), (iii) alitretinoin standard (100 μg ml−1) and (iv) a TRN sample (100 μg ml−1) exposed to light for 1 h. The respective retention times of peaks 1 to 9 were 3.8, 5.1, 7.1, 9.3, 14.5, 16.8, 20.3, 31.1 and 36.6 min, respectively. Based on the signals of the injected standards, isomers 5, 6 and 9 corresponded to isotretinoin, alitretinoin and TRN, respectively. Isomers 1 to 4 were already present in the isotretinoin standard, and were considered as secondary isomers that originated from the isomerization of isotretinoin. Isomers 5, 6, 7 and 8 appeared to be the main products of TRN isomerization. It has been reported that apart from isotretinoin and alitretinoin, the main isomerization products of TRN are: 11-cis retinoic acid; 11,13-cis retinoic acid and 9,13-cis retinoic acid.24 Therefore peaks 7 and 8 could correspond to any of these entities. Tretinoin, isotretinoin and alitretinoin are of major pharmacological interest since they are active at retinoic acid receptors. The remaining isomers will simply be referred to by their respective numbers in the following text.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v). The flow rate and injection volume were 1 ml min−1 and 25 μl, respectively. RA was detected at a wavelength of 348 nm. A peak for RA was obtained at 2.25 min and the run time was 5.0 min. The HPLC-UV method was validated (ESI†).
5 v/v). The flow rate and injection volume were 1 ml min−1 and 25 μl, respectively. RA was detected at a wavelength of 348 nm. A peak for RA was obtained at 2.25 min and the run time was 5.0 min. The HPLC-UV method was validated (ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v). The flow rate was set at 0.6 ml min−1 and the injection volume was 5 μl. As ion suppression was observed in the samples – presumably due to the presence of endogenous compounds released from the skin – acitretrin (ACT) was used as an internal standard. Each injected sample contained a known amount of the internal standard at a concentration of 50 ng ml−1. Mass spectrometric detection was performed with electrospray ionization in negative ion mode using multiple reaction monitoring (MRM). The detection settings for RA and ACT are presented in Table 2.
5 v/v). The flow rate was set at 0.6 ml min−1 and the injection volume was 5 μl. As ion suppression was observed in the samples – presumably due to the presence of endogenous compounds released from the skin – acitretrin (ACT) was used as an internal standard. Each injected sample contained a known amount of the internal standard at a concentration of 50 ng ml−1. Mass spectrometric detection was performed with electrospray ionization in negative ion mode using multiple reaction monitoring (MRM). The detection settings for RA and ACT are presented in Table 2.
| Retinoic acid | Acitretin | |
|---|---|---|
| Nature of parent ion | [M − H]− | [M − H]− |
| Parent ion (m/z) | 299.2 | 325.2 |
| Daughter ion (m/z) | 255.1 | 266.1 |
| Collision energy (V) | 16 | 14 |
| Cone voltage (V) | 30 | 30 |
| Capillary voltage (kV) | 3.00 | 3.00 |
| Capillary temperature (°C) | 500 | 500 |
| Desolvation gas flow (L h−1) | 1000 | 1000 |
| Cone gas flow (L h−1) | 50 | 50 |
| Collision gas flow (L) | 0.15 | 0.15 |
| LM resolution 1 | 2.78 | 2.78 |
| HM resolution 1 | 15 | 15 |
| Ion energy 1(V) | 0.7 | 0.7 |
| LM resolution 2 | 2.74 | 2.74 |
| HM resolution 2 | 14.88 | 14.88 |
| Ion energy 2 (V) | 0.6 | 0.6 |
The different RA isomers were not separated and all were eluted as a single peak at 1.58 min, together with the internal standard ACT. The UHPLC-MS/MS method was also validated (ESI†).
Preparation of the micelle formulation
Micelles with different TRN loading (20, 25 and 30 mg of TRN per g of copolymer) were prepared using the solvent evaporation method (Formulations, A, B and C, respectively).21,27 Briefly, a known quantity of TRN and copolymer was dissolved in 2 ml of acetone. This was then added dropwise under sonication (Branson digital Sonifier® S-450D, Carouge, Switzerland) to 4 ml of ultra-pure water. Acetone was then slowly removed by rotary evaporation (Büchi RE 121 Rotavapor, Flawil, Switzerland). The final copolymer concentration was set to 5 mg ml−1. After equilibration overnight, the micelle solution was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min (Eppendorf Centrifuge 5804, Hamburg, Germany) to remove excess drug and the supernatant was carefully collected. The samples were protected from light throughout the preparation process to prevent photoisomerization.
000 rpm for 15 min (Eppendorf Centrifuge 5804, Hamburg, Germany) to remove excess drug and the supernatant was carefully collected. The samples were protected from light throughout the preparation process to prevent photoisomerization.
Characterization of micelle formulations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 1
20, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 1
50 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilutions in acetonitrile were made for each formulation. The RA content, RA loading and incorporation efficiency were calculated as in previous studies.22,23
100 dilutions in acetonitrile were made for each formulation. The RA content, RA loading and incorporation efficiency were calculated as in previous studies.22,23
Evaluation of the physical stability of the micelle formulations
Micelles of different target drug loading (20, 25 and 30 mgRA gcopolymer−1) were formulated and stored at 4 °C for 6 months. A visual observation of the micelle solutions was performed and the incorporated RA was quantified by HPLC-UV at a series of time-points (Day 1, 5, 7 followed by 1, 2, 3, 4, 5, and 6 months).Evaluation of the photostability of TRN in the micelle formulations
The micelle characterization steps described above did not distinguish between RA isomers. However, it is known that all-trans retinoic acid, tretinoin (TRN), is prone to photodegradation. Thus, when it is applied topically to the skin, TRN undergoes photoisomerization under ambient light conditions after a few hours.25 Therefore, the physically optimal micelle formulation (Formulation A, target 20 mgRA gcopolymer−1) was selected to undergo a photostability study.Micelles and a control formulation (Effederm® solution 0.05%; Sinclair Pharma, France) were placed at a distance of 30 cm in front of a light source (compact fluorescent lamp, BLC-GDU10-7W; neutral white, 4000 K; OSRAM SA, Winterthur, Switzerland). As a control experiment, the two formulations were also kept in the dark. Formulations were sampled at 0, 1, 4, 8 and 12 h. All RA isomers were separated and quantified using the gradient HPLC-UV method.
Several photoprotective agents were tested in order to improve the photostability of the micelle formulation. It has been reported that radiation at 420 nm is the most harmful for TRN leading to its photoisomerization.28 Yellow dyes that absorb visible light in this region of the spectrum have been shown to decrease TRN photodegradation.28 Therefore, Acid Yellow 17, tartrazine and Quinoline Yellow, were selected to be tested as photostabilizers in the TRN micelle formulation based on their maximal absorption wavelength, water solubility and skin tolerability. The photostabilizer concentration in the formulation was 0.005% as this concentration proved to be minimally skin staining. The photostabilizers were added in appropriate amounts to the micelle formulation after the evaporation step to avoid dye incorporation inside the micelle.
Skin preparation
Porcine ears were purchased from a local abattoir (CARRE; Rolle, Switzerland). After washing under running cold water, full-thickness skin samples were harvested and underlying adipose tissue excised. Hair was removed from the skin surface using clippers. Discs corresponding to the formulation application area were punched out (Berg & Schmid HK 500; Urdorf, Switzerland). Skin samples were frozen at −20 °C and stored for a maximum period of 3 months. Prior to the experiment, skin samples were thawed at room temperature and placed for 15 min in 0.9% saline solution for rehydration.Human skin samples were collected immediately after surgery from the Department of Plastic, Aesthetic and Reconstructive Surgery, Geneva University Hospital (Geneva, Switzerland). The study was approved by the Central Committee for Ethics in Research (CER: 08-150 (NAC08-051); Geneva University Hospital). Skin samples originated from breast reduction in a male subject and therefore contained terminal hairs. Hypodermis and fatty tissue were removed and discs corresponding to the permeation area were punched out (Berg & Schmid HK 500; Urdorf, Switzerland). The skin was stored in a biobank at −20 °C for a maximum period of 3 months.
Evaluation of retinoic acid skin delivery in vitro
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min, the permeation samples were analyzed by UHPLC-MS/MS. The diffusion cells were dismantled and each sample was carefully washed in order to ensure removal of the residual formulation from the skin surface. Skin samples were then cut into small pieces and RA deposited in the skin was extracted by soaking the pieces in 4 ml of acetonitrile for 4 h with continuous stirring at room temperature. The extraction procedure was validated (ESI†). The extraction samples were centrifuged at 10
000 rpm for 15 min, the permeation samples were analyzed by UHPLC-MS/MS. The diffusion cells were dismantled and each sample was carefully washed in order to ensure removal of the residual formulation from the skin surface. Skin samples were then cut into small pieces and RA deposited in the skin was extracted by soaking the pieces in 4 ml of acetonitrile for 4 h with continuous stirring at room temperature. The extraction procedure was validated (ESI†). The extraction samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and diluted prior to UHPLC-MS/MS analysis.
000 rpm for 15 min and diluted prior to UHPLC-MS/MS analysis.
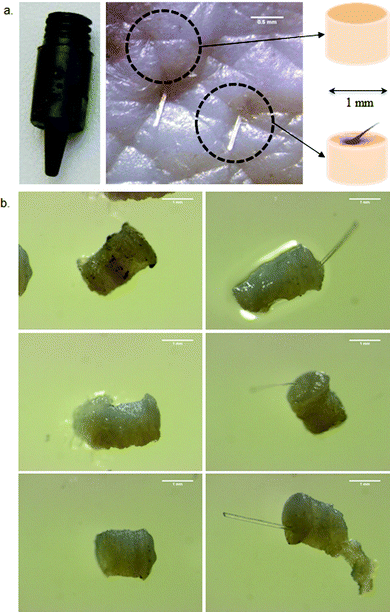
Each harvested sample was inspected visually for the presence of the entire PSU. The hair shaft was also cut to 1 mm above the surface of skin. Each sample was collected in an individual eppendorf tube. RA deposited in each sample was extracted for 2 h with 100 μl of acetonitrile + 0.1% TEA at room temperature and subjected to UHPLC-MS/MS analysis after centrifugation. A similar number of 1 mm skin samples were harvested from bulk skin (PSU-free) to serve as controls (Fig. 2).
Data analysis
Data were expressed as the mean ± SD. Outliers determined using the Dixon test were discarded. Results were evaluated statistically using either Student's t test or analysis of variance (ANOVA) followed by Student Newman Keuls test when necessary as a post-hoc procedure. The level of significance was fixed at α = 0.05.Results
Characterization of micelle formulations
| Formulation | Copolymer content (mg ml−1) | TARGET RA loading (mgRA gcopo−1) | RA loading ± SD (mgRA gcopo−1) | RA content ± SD (mgRA ml−1) | Incorporation efficiency ± SD (%) |
|---|---|---|---|---|---|
| a The optimal formulation was obtained by a dilution of Formulation A and addition of the selected photoprotecting agent. | |||||
| A | 5 | 20 | 18.25 ± 0.15 | 0.091 ± 0.001 | 91.26 ± 0.77 |
| B | 5 | 25 | 22.37 ± 0.21 | 0.112 ± 0.001 | 89.49 ± 0.85 |
| C | 5 | 30 | 26.62 ± 1.35 | 0.133 ± 0.007 | 88.73 ± 0.45 |
| Optimal formulationa | 2.7 | 20 | 18.25 ± 0.15 | 0.050 ± 0.001 | 91.26 ± 0.77 |
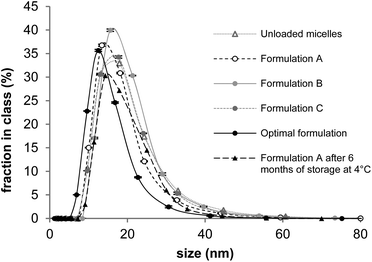
All formulations displayed a unimodal distribution with micelle sizes below 20 nm. Further DLS data can be found in the ESI.†
Physical stability of micelle formulations
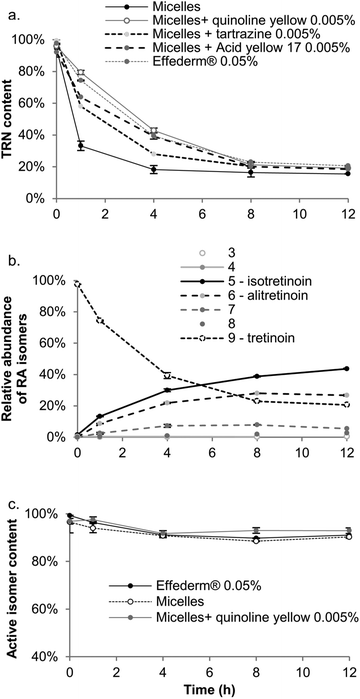
RA content in Formulations A–C was quantified over 6 months at different time points (Fig. 4).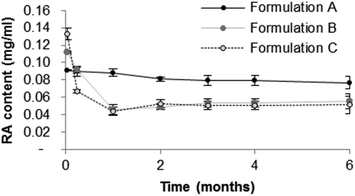 | ||
| Fig. 4 RA content of micelle formulations as a function of time. Data presented as mean ± SD; N = 3. | ||
Formulations B and C were considered to be unstable since RA precipitation was observed 1 month after micelle preparation. Relative to the initial value, RA content fell by 63.6 and 69.2%, respectively. In contrast, Formulation A, proved to be more stable, retaining more than 84.3% of its initial drug content after 6 months of storage. The micelle size in this formulation remained constant over 6 months (dn of 16.6 at day 1 and dn of 17.4 at day 180; Fig. 3). Therefore, Formulation A was selected for further optimization.
Photostabilization of RA in the micelle formulations
The addition of yellow dyes to the micelle formulation led to a significant increase in TRN stability; Quinoline Yellow (0.005%) gave the best results, as TRN was more stable over time in this formulation than in Effederm® solution (0.05%). Indeed, the area under the curve was 394.0%TRN content·h for the micelle formulation stabilized with Quinoline Yellow and 378.8%TRN content·h for Effederm® solution (0.05%). Thus, the micelle formulation stabilized by Quinoline Yellow was selected as the optimal formulation to undergo further experiments.
It is known that isotretinoin and alitretinoin are active isomers of TRN; Fig. 5c shows the proportion of active isomers as a function of light exposure time. It can be observed that after 12 h, the content of active retinoic acid isomers in Effederm® (0.05%) and the micelle formulation was 91.0 ± 0.3% and 90.3 ± 0.6%, respectively. However, the addition of Quinoline Yellow to the latter further increased the active isomer fraction to 93.0%. This was due to a higher isotretinoin content in the stabilized formulation (55.7 ± 1.7%) compared to the commercial formulation (43.6 ± 0.4%) and unprotected micelles (52.1 ± 1.2%). Consequently the Quinoline Yellow stabilized micelle formulation protected TRN against photodegradation and when this did occur, it increased the proportion of active isomers in the formulation.
With this in mind, and given that >90% of the TRN isomers formed after exposure to light were active, the total retinoid content in the skin was quantified following application of the TRN formulations in the subsequent in vitro skin delivery experiments.
Development of a 0.005% micelle formulation
Effederm® 0.05% solution and Retin-A Micro® 0.04% were selected as control formulations in order to have both non-colloidal and colloidal comparators. A simple “head-to-head” comparison between the commercial formulations and the micelle formulation was impossible because of the difference in drug content. Formulation A, which had the optimal physically stable (Table 3), was diluted to 0.005% (i.e. 10 times less drug content than in Effederm®) and stabilized with Quinoline Yellow (0.005%) which was selected as the best photostabilizer. The characterization data for the optimized formulation are presented in Table 3 and Fig. 3. The TEM micrograph of the optimized formulation (Fig. 6) demonstrates that the micelles were spherical in shape with diameters ranging from 10 to 20 nm; these dimensions confirmed the DLS results in Table 3. The pH of the final formulation was 6.3, which was suitable for application to the skin.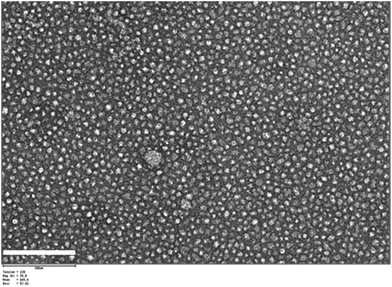 | ||
| Fig. 6 TEM micrograph of the optimal micelle formulation: Formulation A with Quinoline Yellow (0.005%). Scale bar = 200 nm. | ||
Retinoic acid delivery to porcine skin and human skin in vitro
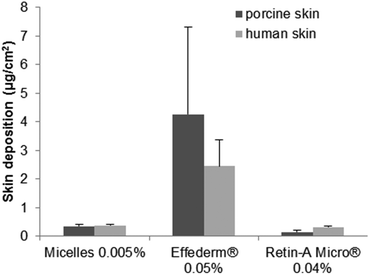
The delivery to porcine skin from Retin-A® Micro (0.04%) (0.14 ± 0.06 μg cm−2) was lower than that yielded by micelles (0.36 ± 0.05 μg cm−2). However, delivery to human skin from both formulations was similar (0.31 ± 0.03 and 0.38 ± 0.04 μg cm−2, respectively). Effederm®, a 0.05% ethanolic solution, outperformed both formulations delivering 4.26 ± 3.04 μg cm−2 of RA to porcine and 2.45 ± 0.94 μg cm−2 to human skins.
However, it is important to note that although the same amounts of each formulation were applied to skin, due to the difference in drug content, the RA doses were different – 5, 40 and 50 μg cm−2 for the micelle formulation, Retin-A® Micro and Effederm®, respectively. For a better comparison of delivery data, results have to be expressed as delivery efficiencies (i.e. the percentage of the applied dose that was delivered to the skin) and this will be described in the Discussion section.
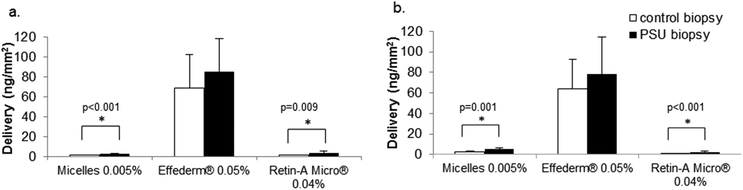 | ||
| Fig. 8 Targeted delivery of retinoids to PSU and control skin samples in (a) porcine and (b) human skin. (N = 15). | ||
In experiments with porcine skin, the optimal micelle formulation yielded 2-fold superior deposition (2.6 ± 0.6 ng mm−2) to the PSU containing biopsy than that to the control (PSU free) skin biopsy (1.3 ± 0.4 ng mm−2), suggesting that micelles could be preferentially retained in the appendage. For human skin, a similar increase (1.9-fold) was observed, 5.2 ± 1.6 ng mm−2 in the PSU containing biopsy and 2.8 ± 1.0 ng mm−2 for the control (PSU free) skin biopsy, confirming that the micelles could target the hair follicle. A similar increase was observed for Retin-A® Micro in both porcine and human skins: 3.9 ± 1.6 ng mm−2 and 2.5 ± 0.8 ng mm−2 were delivered to PSU presenting samples in porcine and human skins, respectively, whereas only 1.4 ± 0.4 ng mm−2 and 1.2 ± 0.3 ng mm−2 was retrieved in the corresponding control samples. In contrast, no significant difference in delivery was observed for Effederm® for either porcine (85.2 ± 33.0 ng mm−2 for PSU-presenting skin and 68.7 ± 33.5 ng mm−2 for the control sample) or human skins (78.1 ± 36.0 ng mm−2 for PSU-presenting skin and 64.1 ± 28.7 ng mm−2 for the control sample). These results clearly demonstrate that the colloidal formulations promote targeted delivery to the PSU whereas a simple solution does not.
Discussion
Micelle formulation development
Drug loadings (mgRA loaded per gcopo) were relatively low in comparison to those obtained with similar formulations studied previously, e.g. 132.25 ± 0.31 mg g−1 in the case of tacrolimus and >300 mg g−1 for ciclosporin A (Table 4).21–23| Fluconazole | Econazole nitrate | CsA | Tacrolimus | Clotrimazole | Retinoic acid | |
|---|---|---|---|---|---|---|
| Mass/mass drug loading (mg g−1) | 268.3 | 293.1 | 333.8 | 132.2 | 58.0 | 18.2 |
| Approximate molar drug loading (molecule of drug per molecule of copolymer) | 4.9 | 3.7 | 1.7 | 1.0 | 0.9 | 0.4 |
| MW (Da) | 306.3 | 444.7 | 1202.6 | 804.1 | 344.8 | 300.4 |
| H donors | 1 | 0 | 5 | 3 | 0 | 1 |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) P P
|
0.4 | 5.2 | 2.7 | 4.7 | 5.9 | 6.2 |
| H acceptors | 8 | 3 | 23 | 13 | 2 | 2 |
| Water solubility (g L−1) | 0.001 | 0.800 | 0.012 | 0.018 | 0.030 | 0.0003 |
| Ionisable site pKa | — | 6.65 | — | — | 5.83 | 4.73 |
A recent study using multiple linear regression suggested that drug loading was influenced by several molecular parameters, in decreasing order: number of H-donors, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, number of H-acceptors and aqueous solubility.21 Indeed, it can be seen that the drugs that were efficiently loaded into micelles (i.e. drug loading >100 mg g−1) possessed a high number of H donors and H acceptors and intermediate log
P, number of H-acceptors and aqueous solubility.21 Indeed, it can be seen that the drugs that were efficiently loaded into micelles (i.e. drug loading >100 mg g−1) possessed a high number of H donors and H acceptors and intermediate log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (0.4–5.2). RA on the other hand, like clotrimazole has few H donors and H acceptors and log
P (0.4–5.2). RA on the other hand, like clotrimazole has few H donors and H acceptors and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 6.2. The presence of an ionizable site could also negatively influence RA incorporation into micelles.
P = 6.2. The presence of an ionizable site could also negatively influence RA incorporation into micelles.
Formulations B and C were able to incorporate enough RA to reach drug contents of 0.112 ± 0.007 mg ml−1 and 0.133 ± 0.007 mg ml−1, respectively. The latter corresponds to a 409-fold increase in RA aqueous solubility (325 ng ml−1; experimental data). However, Formulation A was selected for use in the subsequent studies as it was the most stable formulation; the RA content of 0.091 ± 0.001 mg ml−1 corresponded to a 280-fold increase in RA aqueous solubility. The instability of Formulations B and C was probably due to supersaturation of the micelles with RA on the day of preparation, which led to drug precipitation over the first month of storage.22 As can be seen from the DLS results, all micelles presented diameters below 20 nm. The incorporation of RA did not affect the size of the micelle, possibly indicating that RA due to its linear structure could align with the hexPLA moiety of the polymer. Furthermore, the addition of Quinoline Yellow as photostabilizer did not affect micelle size either.
Of the photostabilizers tested, Quinoline Yellow was selected as the optimal excipient given its ability to stabilize TRN. The maximum absorption wavelength of Quinoline Yellow, 420 nm, is the exact wavelength triggering photoisomerization of tretinoin.28 Quinoline Yellow present in the aqueous solution might act as a shield and absorb light at 420 nm before it reaches the drug containing core of the micelle. Moreover, in the event of photoisomerization its presence led to an increased formation of isotretinoin, which is the principal active isomer of TRN.
RA delivery to the skin and targeting of the hair follicle
The absence of transdermal RA permeation from the micelle formulation suggests that systemic exposure to the drug can be avoided. In terms of topical delivery, the optimal micelle formulation contained 0.005% of RA, which was 8-fold less than that in Retin-A® Micro (0.04%), but was able to deliver a similar amount of RA to skin. As mentioned previously, in order to compare delivery from the different formulations, delivery data must be expressed as a percentage of the applied dose; this delivery efficiency is described by eqn (1).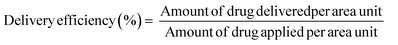 | (1) |
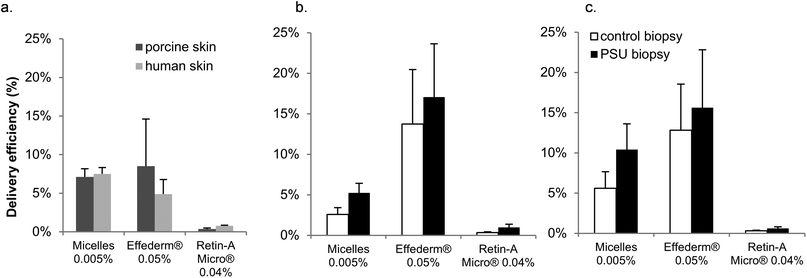
Fig. 9 presents the delivery efficiencies obtained for the different formulations. Delivery to full-thickness porcine and human skins is presented in Fig. 9a. It is obvious that the micelle formulation had a significantly higher delivery efficiency than Retin-A® Micro (7.1 ± 1.1% vs. 0.4 ± 0.1% and 7.5 ± 0.8% vs. 0.8 ± 0.1%; p = 0.005 and p < 0.001 for porcine and human skins, respectively) and matched the delivery efficiency yielded by the 0.05% Effederm® solution (8.5 ± 6.1% and 4.9 ± 1.9% for porcine and human skin, respectively). The high delivery from Effederm® is expected and most probably due to its high ethanol and propylene glycol content. Nanoparticulate topical drug delivery systems are known to be of major interest for targeting the PSU.5 Liposomes, solid lipid nanoparticles and microspheres have been formulated and have shown considerable improvement in skin delivery. Retin-A Micro® is a microsphere based gel that reached the market in 1997.29 It uses the Microsponge® technology developed by Advanced Polymer Systems, Inc. Microsponge® are micron size acrylate polymeric porous microspheres where the drug is adsorbed in the pores.5,30 The RA loaded microspheres have shown efficacy in vivo31 and a favorable irritation profile in comparison to RA cream with similar drug content.3 The increased delivery efficiency was attributed to the ability of the particles to accumulate in the PSU. It was therefore a good comparator formulation.
Targeted delivery into the hair follicle has become an area of major interest in recent years and a number of techniques have been used to identify and to quantify the contribution of follicular delivery (Table 5).32,33
| Technique | Advantages | Disadvantages |
|---|---|---|
| Comparison of delivery to normal skin and follicle-free scar skin | • Possible to use in animal models in vitro and in vivo | • Scarred tissue presents major differences compared to normal skin, including increased collagen content |
| • Possible to use in humans in vivo | ||
| • Possible to study the effect of follicular pathway on transdermal delivery | ||
| Selective sealing of follicular orifices with polymeric material or wax | • Possible to use in animal models in vitro and in vivo | • Risk of accidentally sealing follicle-free skin areas |
| • Possible to use in humans in vivo | • Possible leakage of drug through follicular seal | |
| • Possible to study the contribution of the follicular pathway on transdermal delivery | ||
| Differential stripping: tape stripping of stratum corneum and cyanoacrylate follicle biopsies followed by drug extraction | • Direct quantification of drug present in the biopsy | • No possibility to study the contribution of follicular pathway on transdermal delivery |
| • Possible to use in animal models in vitro and in vivo | • Cyanoacrylate follicle biopsy may contain only the hair shaft, leaving the remaining PSU components in the skin34 | |
| • Possible to use in humans in vivo | ||
| Confocal imaging | • Visualization of drug/model dye in skin and follicle | • Use of “model” fluorescent dyes potentially different from the drug. |
| • No quantitative data |
Techniques using rodent skin models were not considered here as these animals are known to have major differences in skin structure, and more importantly in hair follicle structure and number in comparison with human skin.35
Selective sealing and differential stripping are two of the most widely used techniques.8,34,36–38 The first consists in blocking the PSU with a wax or polymer in order to determine the contribution of the transfollicular pathway to the (trans)dermal delivery of a drug, whereas the second relies on the harvesting of follicular casts using tape stripping and cyanoacrylate cast biopsies and focuses on the quantification of drug retained in the hair follicle. For the treatment of acne, the hair follicle is the target site. However, we were reluctant to use the differential stripping method to quantify the amount of RA retained in the hair follicle. Indeed, one of the main drawbacks of this technique is that the biopsy obtained usually only contains the hair shaft whilst leaving structures like the sebaceous gland and epithelial cells of the PSU in the skin.34
In order to avoid this disadvantage, we have developed an innovative method to quantify drug delivery to the whole PSU. The method is inspired by the follicular unit extraction technique used in the treatment of alopecia by hair transplantation.39 This technique consists in harvesting the PSU with a 1 mm punch. The PSU is harvested entirely, so as to remain viable, and then reimplanted in the diseased region. In our study this technique enables quantification of the drug in the entire PSU. As can be seen from Fig. 2, the punch biopsies obtained contain the PSU surrounded by intact skin. Therefore, all data have been compared to control biopsies where no PSU was present.
It was evident from the present study that for colloidal formulations (micelles and Retin-A Micro®) the PSU containing biopsies in both human and porcine skin showed a significantly increased RA deposition in comparison to the control samples, indicating a preferential deposition of RA in the PSU structure. This phenomenon was not observed with Effederm® solution where deposition was comparable in both groups emphasizing that only micro- and nanoparticulate formulations can yield targeted follicular delivery.
However, expression of the data in terms of delivery efficiencies (Fig. 9b and 9c) reveals that the micelle formulation delivers RA to PSU much more efficiently than the microspheres: 5- and 17-fold greater in porcine and human skin, respectively, (5.2 ± 1.2% vs. 1.0 ± 0.4% and 10.4 ± 3.2% vs. 0.6 ± 0.2%, respectively). This superiority may be explained by the nanometer scale size of the micelles as compared to the micron-sized microspheres and more importantly by their structure. Indeed microspheres are solid state porous beads that adsorb RA. The rate of drug release from the beads when in contact with skin is unknown and may be governed by the relative affinity of RA for the skin and the bead. Previous studies into polymeric micelle mediated skin delivery could not elucidate the mechanism of the micelle interaction with skin. Two hypotheses were proposed (i) either the micelle remains intact upon skin contact and as the glass transition temperature of the polymer is low its core enables better interaction with skin and better partitioning of the drug from the micelle into the skin or ii) the micelle can disassemble upon skin contact and release its contents. Moreover, the single polymer chain may also act as a penetration enhancer.22 The selective and preferential accumulation of micelles in the PSU has been visualized and inferred from CLSM studies in previous reports22 and confirmed quantitatively in the present study. In previous work, we quantified the biodistribution of tacrolimus as a function of position in the epidermis and dermis.22 By analogy, it would be extremely interesting to perform a similar biodistribution study on single punch biopsies to elucidate the exact amount of RA as a function of position in the different skin layers in PSU-free and PSU-presenting samples.
Given these promising results, novel RA loaded micelles should undergo testing in acne patients since delivery to diseased skin may be in many ways different from delivery to healthy skin. Indeed, in acne the majority of the follicular ducts are obstructed due to the physiopathology of the condition and micelle interaction with the PSU may be hindered. In conclusion, the results presented here indicate that not only do the micelles act as nanosized vehicles for RA and allow an increased and targeted transport of the drug to the PSU, they also enable a reduction in drug content, which might lead to an improved clinical management of Acne vulgaris and a better skin irritation profile.
Conclusions
Previous work on polymeric micelle mediated topical skin delivery has suggested that interaction with the PSU is one of the preferential drug penetration pathways into skin. This hypothesis has been confirmed quantitatively in the present study using an innovative punch biopsy technique to harvest the PSU. The successful formulation of RA into polymeric micelles and the increased delivery efficiency provided by them to the target site may lead to a more selective and safer topical treatment of Acne vulgaris.Acknowledgements
We thank the University of Geneva for a teaching assistantship for ML and for providing financial support for the purchase of the Waters Xevo® TQ-MS detector and the Fondation Ernst and Lucie Schmidheiny and the Société Académique de Genève for providing equipment grants. We would also like to express our gratitude to Prof. Brigitte Pittet-Cuénod and her colleagues from the Department of Plastic, Aesthetic and Reconstructive Surgery, Geneva University Hospital (Geneva, Switzerland) for providing human skin samples. Finally, we would like to thank Rémi Lafaix for his contribution to the experimental work and Dr Van Nguyen for precious advice.References
- H. Gollnick, Current concepts of the pathogenesis of acne: implications for drug treatment, Drugs, 2003, 63, 1579–1596 CrossRef CAS.
- E. Contassot and L. E. French, New Insights into Acne Pathogenesis: Propionibacterium Acnes Activates the Inflammasome, J. Invest. Dermatol., 2014, 134, 310–313 CrossRef CAS PubMed.
- G. F. Webster, Topical tretinoin in acne therapy, J. Am. Acad. Dermatol., 1998, 39, S38–44 CrossRef CAS.
- K. Sardana and V. N. Sehgal, Retinoids: fascinating up-and-coming scenario, J. Dermatol., 2003, 30, 355–380 CrossRef CAS PubMed.
- G. A. Castro and L. A. Ferreira, Novel vesicular and particulate drug delivery systems for topical treatment of acne, Expert Opin. Drug Delivery, 2008, 5, 665–679 CrossRef CAS PubMed.
- D. Papakostas, F. Rancan, W. Sterry, U. Blume-Peytavi and A. Vogt, Nanoparticles in dermatology, Arch. Dermatol. Res., 2011, 303, 533–550 CrossRef CAS PubMed.
- U. Blume-Peytavi and A. Vogt, Human hair follicle: reservoir function and selective targeting, Br. J. Dermatol., 2011, 165(Suppl 2), 13–17 CrossRef CAS PubMed.
- A. Patzelt and J. Lademann, Drug delivery to hair follicles, Expert Opin. Drug Delivery, 2013, 10, 787–797 CrossRef CAS PubMed.
- A. Patzelt, H. Richter, F. Knorr, U. Schafer, C. M. Lehr, L. Dahne, W. Sterry and J. Lademann, Selective follicular targeting by modification of the particle sizes, J. Controlled Release, 2011, 150, 45–48 CrossRef CAS PubMed.
- M. Morgen, G. W. Lu, D. Du, R. Stehle, F. Lembke, J. Cervantes, S. Ciotti, R. Haskell, D. Smithey, K. Haley and C. Fan, Targeted delivery of a poorly water-soluble compound to hair follicles using polymeric nanoparticle suspensions, Int. J. Pharm., 2011, 416, 314–322 CAS.
- H. Wosicka and K. Cal, Targeting to the hair follicles: current status and potential, J. Dermatol. Sci., 2010, 57, 83–89 CrossRef CAS PubMed.
- R. Chourasia and S. K. Jain, Drug targeting through pilosebaceous route, Curr. Drug Targets, 2009, 10, 950–967 CrossRef CAS.
- R. H. Neubert, Potentials of new nanocarriers for dermal and transdermal drug delivery, Eur. J. Pharm. Biopharm., 2011, 77, 1–2 CrossRef CAS PubMed.
- M. J. F. Contreras, M. M. J. Soriano and A. R. Dieguez, In vitro percutaneous absorption of all-trans retinoic acid applied in free form or encapsulated in stratum corneum lipid liposomes, Int. J. Pharm., 2005, 297, 134–145 CrossRef PubMed.
- S. Kitagawa and M. Kasamaki, Enhanced delivery of retinoic acid to skin by cationic liposomes, Chem. Pharm. Bull., 2006, 54, 242–244 CrossRef CAS.
- C. Sinico, M. Manconi, M. Peppi, F. Lai, D. Valenti and A. M. Fadda, Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle-skin interaction, J. Controlled Release, 2005, 103, 123–136 CrossRef CAS PubMed.
- K. A. Shah, A. A. Date, M. D. Joshi and V. B. Patravale, Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery, Int. J. Pharm., 2007, 345, 163–171 CrossRef CAS PubMed.
- J. Nyirady, M. Nighland, G. Payonk, J. Pote, S. Phillips and R. Grossman, A comparative evaluation of tretinoin gel microsphere, 0.1%, versus tretinoin cream, 0.025%, in reducing facial shine, Cutis, 2000, 66, 153–156 CAS.
- T. Trimaille, R. Gurny and M. Moller, Poly(hexyl-substituted lactides): Novel injectable hydrophobic drug delivery systems, J. Biomed. Mater. Res., Part B, 2007, 80A, 55–65 CrossRef CAS PubMed.
- K. Mondon, M. Zeisser-Labouebe, R. Gurny and M. Moller, Novel cyclosporin A formulations using MPEG-hexyl-substituted polylactide micelles: a suitability study, Eur. J. Pharm. Biopharm., 2011, 77, 56–65 CrossRef CAS PubMed.
- Y. G. Bachhav, K. Mondon, Y. N. Kalia, R. Gurny and M. Moller, Novel micelle formulations to increase cutaneous bioavailability of azole antifungals, J. Controlled Release, 2011, 153, 126–132 CrossRef CAS PubMed.
- M. Lapteva, K. Mondon, M. Moller, R. Gurny and Y. N. Kalia, Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis, Mol. Pharm., 2014, 11, 2989–3001 CrossRef CAS PubMed.
- M. Lapteva, V. Santer, K. Mondon, I. Patmanidis, G. Chiriano, L. Scapozza, R. Gurny, M. Moller and Y. N. Kalia, Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways, J. Controlled Release, 2014, 196, 9–18 CrossRef CAS PubMed.
- M. G. Motto, K. L. Facchine, P. F. Hamburg, D. J. Burinsky, R. Dunphy, A. R. Oyler and M. L. Cotter, Separation and Identification of Retinoic Acid Photoisomers, J. Chromatogr. A, 1989, 481, 255–262 CrossRef CAS.
- D. J. Elbaum, Comparison of the stability of topical isotretinoin and topical tretinoin and their efficacy in acne, J. Am. Acad. Dermatol., 1988, 19, 486–491 CrossRef CAS.
- P. A. Lehman and A. M. Malany, Evidence for percutaneous absorption of isotretinoin from the photo-isomerization of topical tretinoin, J. Invest. Dermatol., 1989, 93, 595–599 CAS.
- K. Mondon, M. Zeisser-Labouebe, R. Gurny and M. Moller, MPEG-hexPLA micelles as novel carriers for hypericin, a fluorescent marker for use in cancer diagnostics, Photochem. Photobiol., 2011, 87, 399–407 CrossRef CAS PubMed.
- M. Brisaert and J. Plaizier-Vercammen, Investigation on the photostability of a tretinoin lotion and stabilization with additives, Int. J. Pharm., 2000, 199, 49–57 CrossRef CAS.
- Retin-A Microsphere approval, http://www.accessdata.fda.gov/drugsatfda_docs/nda/97/020475ap.pdf, 23.02.2015.
- S. Smith, V. Morhenn and G. Webster, The characteristics and utility of solid phase porous microspheres: a review, J. Drugs Dermatol., 2006, 5, 969–974 Search PubMed.
- R. Berger, A. Barba, A. Fleischer, J. J. Leyden, A. Lucky, D. Pariser, E. Rafal, D. Thiboutot, D. Wilson, R. Grossman and M. Nighland, A double-blinded, randomized, vehicle-controlled, multicenter, parallel-group study to assess the safety and efficacy of tretinoin gel microsphere 0.04% in the treatment of acne vulgaris in adults, Cutis, 2007, 80, 152–157 Search PubMed.
- V. M. Meidan, Methods for quantifying intrafollicular drug delivery: a critical appraisal, Expert Opin. Drug Delivery, 2010, 7, 1095–1108 CrossRef CAS PubMed.
- E. Touitou, V. M. Meidan and E. Horwitz, Methods for quantitative determination of drug localized in the skin, J. Controlled Release, 1998, 56, 7–21 CrossRef CAS.
- A. Teichmann, U. Jacobi, M. Ossadnik, H. Richter, S. Koch, W. Sterry and J. Lademann, Differential stripping: determination of the amount of topically applied substances penetrated into the hair follicles, J. Invest. Dermatol., 2005, 125, 264–269 CAS.
- OECD guidance notes on dermal absorption, http://www.oecd.org/chemicalsafety/testing/46257610.pdf, 23.02.2015.
- A. Teichmann, N. Otberg, U. Jacobi, W. Sterry and J. Lademann, Follicular penetration: development of a method to block the follicles selectively against the penetration of topically applied substances, Skin Pharmacol. Physiol., 2006, 19, 216–223 CrossRef CAS PubMed.
- B. N. Matos, T. A. Reis, T. Gratieri and G. M. Gelfuso, Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles, Int. J. Biol. Macromol., 2015, 75, 225–229 CrossRef CAS PubMed.
- A. Patzelt, H. Richter, R. Buettemeyer, H. J. Huber, U. Blume-Peytavi, W. Sterry and J. Lademann, Differential stripping demonstrates a significant reduction of the hair follicle reservoir in vitro compared to in vivo, Eur. J. Pharm. Biopharm., 2008, 70, 234–238 CrossRef CAS PubMed.
- L. M. Bicknell, N. Kash, C. Kavouspour and R. M. Rashid, Follicular unit extraction hair transplant harvest: a review of current recommendations and future considerations, Dermatol. Online J., 2014, 20 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr04770f |
| This journal is © The Royal Society of Chemistry 2015 |