Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electroorganic synthesis of nitriles via a halogen-free domino oxidation–reduction sequence†
Marius F.
Hartmer
and
Siegfried R.
Waldvogel
*
Institut für Organische Chemie, Johannes Gutenberg-Universität, Duesbergweg 10-14, D-55128 Mainz, Germany. E-mail: waldvogel@uni-mainz.de
First published on 17th September 2015
Abstract
A direct electroorganic sequence yielding nitriles from oximes in undivided cells is reported. Despite the fact that intermediate nitrile oxides might be formed, the method is viable to prepare benzonitriles without substituents ortho to the aldoxime moiety. This constant current method is easy to perform for a broad scope of substrates and employs common electrodes, such as graphite and lead.
In the past, the formation of nitriles from oximes has been realized by the use of harsh dehydration agents such as phosphorous pentoxide, thionyl chloride, benzenesulfonyl chloride, acetic anhydride, and many others.1,2 Unfortunately, these strongly corrosive agents have to be used stoichiometrically or even in excess. This results in unattractive waste generation and considerable safety issues upon work up. Recently, catalytic approaches have emerged which reduce the waste generation significantly.3 However, recycling of the expensive and toxic catalysts still remains an issue. Consequently, new methods for the aldoxime dehydration are highly desired.
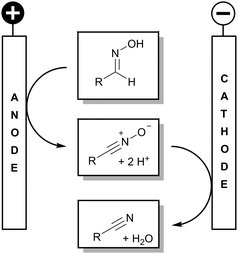
Electrochemistry has become an attractive alternative to conventional methods in organic synthesis, since only electricity is applied which might even originate from renewable resources, and therefore no reagent waste is produced.4 Shono et al. reported an electrochemical protocol to generate nitriles from oximes with halogenides as a mediator.5 However, such halogen species cause corrosion at the precious platinum electrodes.6,7 Additionally, low current yields make this protocol in particular unattractive. We report a direct, halogen-free procedure to generate nitriles from oximes by the application of inexpensive and easily available electrode materials.
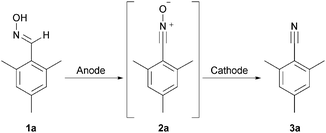
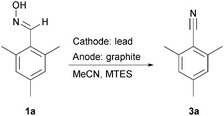
We chose mesitylaldoxime (1a) as test substrate. Within the course of transformation we observe stable nitrile oxide as intermediate (2a, Scheme 1). Initially, we investigated the anodic reaction using methyltriethyl ammonium methylsulfate (MTES) in acetonitrile as electrolyte. Graphite and glassy carbon were tested as carbon anode materials at room temperature. Glassy carbon served as cathode material in both cases. Graphite turned out to be an excellent anode leading to almost quantitative consumption of the starting material upon application of 2.5 F (Fig. 1).
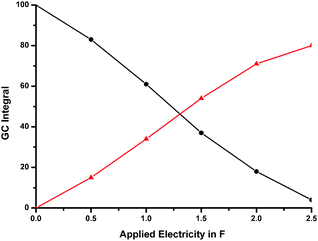 | ||
| Fig. 1 Transformation of mesitylaldoxime (black curve) at graphite to intermediate nitrile oxide 2a (red curve) in the course of electrolysis. | ||
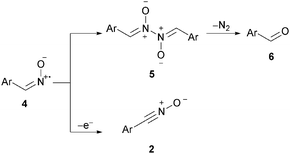
However, the conversion at glassy carbon electrodes was poor. Only the intermediate nitrile oxide was formed, indicating a possible deactivation of the cathodic surface. It turned out that the deoxygenation is not promoted. As outlined, the intermediate was observed and identified via GC-MS. The anodic conversion leads to nitrile oxide which is the same intermediate as in the halogenide-mediated electrolysis by Shono et al. Hence, we consider the transformation as a domino oxidation–reduction sequence with respect to the definition of domino reactions, since a stable intermediate is formed which in turn reacts without manipulating the reaction conditions to the final product (Scheme 2).8
Nevertheless, the key for the desired reaction sequence seemed to be the deoxygenation of nitrile oxide formed. Having some recent experience with lead electrodes and variations thereof,9 we tested lead, stainless steel (A5), nickel, platinum, and boron-doped diamond as cathode materials. Indeed, lead turned out within the screening (see ESI† for GC-MS data) as an excellent cathode material, whereas the deoxygenation was less favored at the other electrode materials. Surprisingly, platinum showed a very poor performance although it is known as the cathode material of choice for this particular deoxygenation reaction.5
Subsequently, the influence of temperature and current density was studied (Table 1). Performing the electrolysis at 22 °C proved to be an optimum concerning the temperature, whereas rising (50 °C) or lowering (0 °C) the temperature led to a drop in yield (entries 1 and 5). Investigating the current density rendered the best yields with 10 mA cm−2 (entry 3), whereas lower (5 mA cm−2) or higher (20 mA cm−2) current densities resulted in decreased yields and selectivity, respectively (entries 2 and 4).
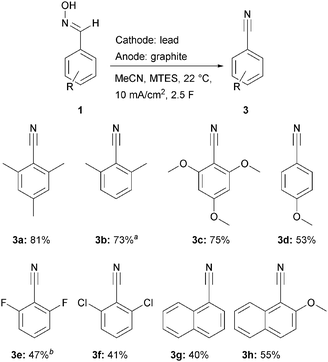
With the optimized electrolysis parameters in hand, we applied the method to a range of substrates in order to elucidate the scope of the reaction (Scheme 3). These substrates vary in stability for the intermediately formed nitrile oxides as well as their preference for dimerization. Stabilization of reactive intermediates such as nitrile oxides can be achieved by bulky groups ortho to the aldoxime moiety.
The best results were obtained with mesitylaldoxime (1a, 81%), closely followed by 2,4,6-trimethoxybenzaldoxime (1c, 75%) and 2,6-dimethylbenzaldoxime (1b, 73%). The unexpectedly low yield of the stabilized 2,6-dichlorobenzaldoxime (1f, 41%) can be attributed to the competing dechlorination reaction (see ESI† for GC-MS data). The analogue debromination reaction was even more dominant and disadvantageous for the nitrile formation, whereas 2,6-difluorobenzonitrile (3e, 47%) was isolated in a higher yield due to the higher C,F bond strength (Ar–F: 526 kJ mol−1; Ar–Cl: 400 kJ mol−1; Ar–Br: 336 kJ mol−1).10
The stabilized 2-methoxy-1-naphthaldoxime (1h) resulted in a yield of 55%, whereas the partially stabilized naphthylaldoxime (1g) led to a yield of 40%. Surprisingly the non-stabilized 4-methoxybenzonitrile (3c) was synthesized in an acceptable yield of 53%.
In general, stabilized substrates result in higher yields than non-stabilized congeners. However, the corresponding aldehyde was detected in all electrolyses by reaction monitoring.
It is noteworthy, that the aldehyde formation was significantly higher for the non-stabilized substrates. It is known that aldoximes are oxidized in a divided cell to iminoxyl radicals which dimerize to aldazine bis-N-oxides (5, Scheme 4).11
These aldazine bis-N-oxides react further to aldehydes by loss of nitrogen. We anticipate that the dimerization takes place prior to the deoxygenation of the intermediate. In contrast, only small amounts of aldehyde have been observed for stabilized substrates. We anticipate that the stability of the iminoxyl radical formed is based on the sterical hindrance and therefore, no or very little dimerization occurs. Hence the formation of nitrile oxide is favored which then undergoes deoxygenation at the cathode leading to the nitrile in high yield.
In conclusion, we developed a direct, halogen-free protocol for the synthesis of nitriles from oximes with yields up to 81% by applying inexpensive and readily available electrode materials such as lead12 and graphite. This protocol is applicable for a range of substrates under constant current and ambient conditions. Furthermore, some insight into the mechanistic scenery can be deduced confirming a domino oxidation–reduction sequence.
We wish to acknowledge financial support from the Federal Ministry of Education and Research (CARLOTTA, FKZ 01DM14005).
Notes and references
-
(a) S. Gabriel and R. Meyer, Ber. Dtsch. Chem. Ges., 1881, 14, 2332 CrossRef CAS PubMed
; (b) C. Moureau, Bull. Soc. Chim. Fr., 1894, 11, 1067 Search PubMed
; (c) R. Scholl, Monatsh. Chem., 1918, 39, 231 CrossRef CAS
; (d) A. Werner and A. Piguet, Ber. Dtsch. Chem. Ges., 1904, 37, 4295 CrossRef CAS PubMed
; (e) L. Claisen and R. Stock, Ber. Dtsch. Chem. Ges., 1891, 24, 130 CrossRef CAS PubMed
; (f) O. L. Brady and G. P. McHugh, J. Chem. Soc., Trans., 1923, 123, 1190 RSC
; (g) L. Claisen and O. Manasse, Ber. Dtsch. Chem. Ges., 1887, 20, 2194 CrossRef CAS PubMed
; (h) H. Metzger, in Methoden der organischen Chemie, ed. E. Müller, Thieme Verlag, Stuttgart, 4th edn, 1968, vol. X/4 Search PubMed
; (i) D. T. Mowry, Chem. Rev., 1948, 42, 189 CrossRef CAS
.
-
(a) D. L. J. Clive, J. Chem. Soc. D, 1970, 1014 RSC
; (b) H. G. Foley and D. R. Dalton, J. Chem. Soc., Chem. Commun., 1973, 628 RSC
; (c) V. P. Kukhar and V. I. Pasternak, Synthesis, 1974, 563 CrossRef CAS
; (d) M. M. Rogic, J. F. Van Peppen, K. P. Klein and T. R. Demmin, J. Org. Chem., 1974, 39, 3424 CrossRef CAS
; (e) A. R. Katritzky, G.-F. Zhang and W.-Q. Fan, Org. Prep. Proced. Int., 1993, 25, 315 CrossRef CAS PubMed
.
-
(a) S. H. Yang and S. Chang, Org. Lett., 2001, 3, 4209 CrossRef CAS PubMed
; (b) K. Ishihara, Y. Furuya and H. Yamamoto, Angew. Chem., Int. Ed., 2002, 41, 2983 ( Angew. Chem. , 2002 , 114 , 3109 ) CrossRef CAS
; (c) L. Yu, H. Li, X. Zhang, J. Ye, J. Liu, Q. Xu and M. Lautens, Org. Lett., 2014, 16, 1346 CrossRef CAS PubMed
.
-
(a)
Organic electrochemistry, ed. H. Lund and O. Hammerich, M. Dekker, New York, NY, 4th edn, 2001 Search PubMed
; (b) Organic electrochemistry, ed. H. J. Schäfer, A. J. Bard and M. Stratmann, Wiley-VCH, Weinheim, 2004, vol. 8 Search PubMed
; (c) E. Steckhan, T. Arns, W. R. Heineman, G. Hilt, D. Hoormann, J. Jörissen, L. Kröner, B. Lewall and H. Pütter, Chemosphere, 2001, 43, 63 CrossRef CAS
; (d) S. R. Waldvogel and B. Elsler, Electrochim. Acta, 2012, 82, 434 CrossRef CAS PubMed
; (e) R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492 RSC
; (f) B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099 RSC
; (g) H. J. Schäfer, C. R. Chim., 2011, 14, 745 CrossRef PubMed
.
- T. Shono, Y. Matsumura, K. Tsubata, T. Kamada and K. Kishi, J. Org. Chem., 1989, 54, 2249 CrossRef CAS
.
- Average pricing at London Metal Exchange (May 2015): 36716 $ per kg.
-
(a) J. A. Bittles and E. L. Littauer, Corros. Sci., 1970, 10, 29 CrossRef CAS
; (b) E. Doná, M. Cordin, C. Deisl, E. Bertel, C. Franchini, R. Zucca and J. Redinger, J. Am. Chem. Soc., 2009, 131, 2827 CrossRef PubMed
.
-
(a) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed. Engl., 1993, 32, 131 CrossRef PubMed
; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed
; (c) L.-F. Tietze, G. Brasche and K. M. Gericke, Domino reactions in organic synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed
.
-
(a) C. Gütz, M. Selt, M. Bänziger, C. Bucher, C. Römelt, N. Hecken, F. Gallou, T. R. Galvão and S. R. Waldvogel, Chem. – Eur. J., 2015, 21, 13878 CrossRef PubMed
; (b) C. Edinger, J. Kulisch and S. R. Waldvogel, Beilstein J. Org. Chem., 2015, 11, 294 CrossRef CAS PubMed
; (c) C. Edinger and S. R. Waldvogel, Eur. J. Org. Chem., 2014, 5144 CrossRef CAS PubMed
; (d) C. Edinger, V. Grimaudo, P. Broekmann and S. R. Waldvogel, ChemElectroChem, 2014, 1, 1018 CrossRef CAS PubMed
; J. Kulisch, M. Nieger, F. Stecker, A. Fischer and S. R. Waldvogel, Angew. Chem., Int. Ed., 2011, 50, 5564 ( Angew. Chem. , 2011 , 123 , 5678 ) Search PubMed
.
-
D. R. Lide, CRC handbook of chemistry and physics, 2006–2007, CRC Press, Boca Raton, FL, 87th edn, 2006 Search PubMed
.
-
(a) V. A. Petrosyan, M. E. Niyazymbetov and E. V. Ul'yanova, Russ. Chem. Bull., 1989, 38, 1548 CrossRef
; (b) V. A. Petrosyan, M. E. Niyazymbetov and E. V. Ul'yanova, Russ. Chem. Bull., 1990, 39, 546 CrossRef
.
- Average pricing at London Metal Exchange (May 2015): 2 $ per kg.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc06437f |
| This journal is © The Royal Society of Chemistry 2015 |