Optical properties of rhodoxanthin accumulated in Aloe arborescens Mill. leaves under high-light stress with special reference to its photoprotective function
Mark Merzlyak*a, Alexei Solovchenkoa and Sergei Pogosyanb
aDepartment of Physiology of Microorganisms, Faculty of Biology, Moscow State University, 119992, GSP-2, Moscow, Russia. E-mail: mnm@6.CellImm.bio.msu.ru; m_merzlyak@mail.ru; Fax: +7 095 9393807; Tel: +7 095 9392587
bDepartment of Biophysics, Faculty of Biology, Moscow State University, 119992, GSP-2, Moscow, Russia. E-mail: pogosyan@biophys.msu.ru; Fax: +7 095 9391115; Tel: +7 095 9395150
First published on 25th February 2005
Abstract
In Aloe arborescens Mill. leaves, strong sunlight or its combination with drought induces the accumulation of the red keto-carotenoid, rhodoxanthin. Simultaneously, the transformation of chloroplasts into chromoplasts accompanied by degradation of thylakoid membranes and formation of plastoglobuli, large in size and number, takes place. Depending on stress conditions the build up of rhodoxantin occurred along with the loss of chlorophyll or on the background of relatively high content of the pigment in the leaves. Microspectrophotometrical measurements showed the presence of chlorophyll-free plastids and retention of carotenoids during leaf adaptation to strong sunlight. The plastid spectra contained absorption bands of common for higher plants carotenoids together with those of rhodoxantin, with absorption maxima situated in the blue (440–480 nm) and the green ranges of the spectrum, respectively. The studies of whole-leaf optical properties revealed a broad band of rhodoxanthin absorption in the blue–green range peaking near 540–550 nm. Within this spectral band the accumulation of rhodoxanthin occurring, probably, in plastoglobuli considerably increased light absorption by stressed Aloe leaves. A possible photoprotective function of rhodoxanthin and other carotenoids as an internal light trap analogous to that accomplished by anthocyanins in other plant species is discussed.
Introduction
Excessive quantities of absorbed light quanta which can not be utilised through photochemical reactions of photosynthesis increase the probability of photodamage to plants, especially under unfavourable conditions such as drought, chilling and solarization.1–3 Therefore, mechanisms lowering the amount of light reaching the photosynthetic apparatus play an important role in the acclimation of plants to their environment. Probably, one of the most common defence mechanisms operating in plants is screening and trapping of solar radiation due to the accumulation of photoprotective pigments absorbing solar radiation within specific wavebands in certain structures and cell compartments. Thus, numerous data in the literature indicate that the build up of flavonoids in epidermal cells of many higher plant species provides protection against radiation in the UV4–7 and the shortwave part of the visible spectrum.8–10 Then, anthocyanins, the vacuolar flavonoids, responsible for red coloration of some leaves and fruits protect against radiation in the green range.11–13 Although flavonols are almost ubiquitous in higher plants, anthocyanins are relatively rare and there are species accumulating anthocyanins only in low amounts or lacking them completely.14,15The carotenoids (Car) represent a group of pigments exerting photoprotective effects via different mechanisms. Within thylakoid membranes, these effects are related with deactivation of chlorophyll (Chl) excited states, quenching of singlet oxygen, interception of free radicals and dissipation of the excessive absorbed light energy via xanthophyll cycle.3,16,17 Some lines of evidence suggest that the accumulation of Car, which takes place in plants under high-light stress conditions and in the course of senescence, is related with their ability to trap harmful radiation and thus provide photoprotection in the blue range.18
The Car are represented by xanthophylls and carotenes in the majority of higher plant species, which do not contain keto-groups (referred to subsequently as non-keto Car, NKC) and exhibit characteristic three-peaked absorption spectra between 400 and 480 nm and are yellow-to-orange in colour.19,20 Although the composition of higher plant Car is highly conserved,21 under stressful conditions the accumulation of rhodoxanthin (Rhd, 4′,5′-didehydro-4,5′-retro-β,β-carotene-3,3′-dione) takes place in the leaves of some species. Due to the presence of two conjugated keto-groups rhodoxanthin absorption maximum of this Car is situated at longer wavelengths, the spectrum does not exhibit fine structure and its solutions are red.19,20
The reversible colour changes from green to reddish-brown as a result of Rhd accumulation have been extensively studied in the sun-exposed winter needles of several gymnosperms (Cryptomeria, Metasequoia, Taxodium, Chamaecyparis and Thuja)22–26 and during cold hardening of western red cedar (Thuja plicata) seedlings.28 Histological studies of Cryptomeria japonica and Taxodium distichum showed that the transition of chloroplasts to chromopolasts took place along with changes in coloration of sun leaves and Rhd was found in form of reddish particles inside chromoplasts.23 The measurements of Chl fluorescence induction curves allowed Han et al. to suggest that ‘rhodoxanthin might play an important role in long-term acclimation to cold’25 and its ‘accumulation functions to decrease the light intensity reaching the photosynthetic apparatus’27 and that it might in this way protect the photosynthetic machinery. However, little evidence for such a role of Rhd is available in the literature,25 although recently Han et al.26 estimated that in C. japonica needles Rhd intercepts about 12% of incident light.
An important information on physiological function of a pigment as an internal light screen and/or light trap could be obtained through the comparative studies of optical properties of tissues from stressed and non-stressed plants, e.g., those taken from sun and shade.10,11,18,29 For such experiments, species with large leaf blades are more suitable than needles of coniferous species.25,26
Many tropical rosette plants, often possessing CAM metabolism, are able to tolerate extremely wide variety of illumination conditions and their rapid changes. In Aloe vera subjected to artificial drought under high irradiance Diaz et al.30 observed remarkable changes in leaf Car content including the build up of significant amounts of Rhd. Aloe arborescens, probably the most widely cultivated species of the genus Aloe in the world,31 also develops reddish leaf coloration under natural light-stress conditions. Thus, during the winter period recently planted aloe cuttings often turn bright red in colour as a result of the action of strong sunlight and/or drought. Establishment of the root system or shading causes the plants to revert to green coloration (Professor Y. Gutterman, personal communication, Fig. 1).
 | ||
| Fig. 1 The appearance of intact (left) and stressed (right) Aloe arborescens plants and their leaf cross-sections (lower plates). | ||
In this paper we examined influence of natural light-stress conditions on A. arborescens with special reference to possible photoprotective function of Rhd. The main goal of the study was to estimate the effect(s) of Rhd on optical properties of both whole leaves and plastids in situ. We also investigated the ultrastructural changes of A. arborescens plastids occurring in the course of the plant adaptation to strong solar radiation.
Experimental
Plant material
Aloe arborescens Mill. (Liliaceae), the multi-branched shrub growing in Sede-Boker Campus (the J. Blaustein Institute for Desert Research, BIDR, Ben-Gurion University of the Negev, Israel) in the Negev Desert (34° 46′ E 30° 51′ N, 460 m a.s.l.) were used. The experiments with whole leaves (n = 16) were performed in January, 2003, with plants provided by Professor Y. Gutterman from the Introduction Garden when they developed red pigmentation as a result of solarization and water deficiency during establishment of their root system and on the same plants in April 2004. In the latter case leaves also frequently developed red colouration due to high solar radiation; the green leaf samples were collected from plants shaded by covering with a black net. For experiments with isolated adaxial chlorenchyma (AC) tissues performed in April, 2004 plants with leaf colours from green to red (n = 16) grown in the park of Sede-Boker Campus have been selected.Pigment extraction and assay
The procedure by Folch et al.32 allowing separation of Chl and Car from polar light-absorbing compounds including flavonols and anthocyanins10 was used for pigment extraction. The pieces of leaves were homogenized with a mortar and pestle in 10 ml of chloroform–methanol (2 ∶ 1, vol/vol) mixture and the homogenate was passed through a paper filter. Then, distilled water was added to the amount of 0.2 of the extract volume, and the diluted filtrate was centrifuged in glass test tubes for 10 min at 3000 g to complete the separation of chloroform and water–methanol phases. The chloroform phase was taken for further pigment analysis. Leaf Chl and NKC (for the latter molecular weight of 570 was accepted) content was quantified using absorption coefficients reported by Wellburn.33 An arbitrary Car absorption coefficient19E1%1cm of 2500 at 507 nm in chloroform was accepted for Rhd, close to that reported in n-hexane.19,20Treatment of the extract spectra
The absorption spectra of leaf extracts, Chl a and b (both from Fluka, Switzerland) and Rhd were recorded with a Cary-50 (Varian, USA) spectrophotometer in chloroform. The equivalent absorbance of leaf extract was calculated as a product AVS−1l−1, where A, V, S and l are absorbance, volume of chloroform extract (ml), AC area (cm2) taken for pigment extraction and cuvette pathlength (cm), respectively.The pigment composition in aloe was studied with the spectral reconstruction method (simulation of the absorption spectrum of an extract under examination by a linear combination of the absorption spectra of its constituents) previously developed, in particular, for analysing Car in higher plant leaf and algae extracts.34 In contrast to these systems, within aloe in addition to Chl one should expect a significant contribution of Rhd to extract absorption in the green–orange range.30 Therefore, aloe extract spectra were fitted using the least squares approach to finding the optimum values of the fitting parameters for fiducial pigments (i.e. Chl a, Chl b and Rhd) between 530 and 700 nm. Then the spectral curves for these pigments were extrapolated to shorter wavelengths and spectra for NKC and Rhd were calculated.
Chromatography
Rhodoxanthin was purified from extracts of red A. arborescens leaves with TLC on silica gel plates (Merck, Germany) using chloroform ∶ acetone (13 ∶ 7, vol/vol) mixture as a mobile phase. The apparatus used for HPLC analysis of pigments comprised a Merck–Hitachi L-7100 pump, a 200 × 4.5 mm Nucleosil RP C-18 column fitted with a guard column (Merck, Germany) and a Waters 481 UV detector (Waters, USA). The chromatograms were recorded using the Clarity software (DataApex, Czech Republic). The modified solvent system by Pintea et al.35 was used for elution of pigments: (A) acetonitrile ∶ water ∶ triethyl amine (85 ∶ 12 ∶ 0.25, by vol) and (B) ethylacetate ∶ triethyl amine (100 ∶ 0.25, by vol). A flow rate of 1 ml min−1 and a two-step linear solvent gradient from 0 to 30% B (18 min), then from 30 to 100% B (6 min), with a 6 min hold at the final concentration was used. The Car were identified using standard solutions of neoxanthin, violaxanthin and antheraxanthin (Sigma, USA) and Rhd. The eluted components were monitored at 450 nm for Car and Chl and at 510 nm for Rhd.Leaf spectral measurements
The reflection, R(λ), and transmission, T(λ), spectra were recorded with a LI-1800 portable spectroradiometer equipped with an 1800-12 external integrating sphere (Li-Cor Inc., USA). The reflection spectra were taken from adaxial surfaces of whole aloe leaves and adaxial chlorenchyma (AC) tissue preparations. Since whole-leaf transmittance measurements of aloe leaves are complicated by their thickness, curved shape and the presence of water-storing parenchyma (gel), preparations of AC were used. The AC samples were prepared by cutting the leaves longitudinally, taking the adaxial halves and careful scratching off the water-storage parenchyma with subsequent washing with distilled water. Then the AC preparations were slightly dried with filtering paper and measured immediately. The leaf transmission was corrected for non-complete collection of light by an integrating sphere as described elsewhere.36 The absorptance and absorbance spectra were calculated as 1 − R(λ) − T(λ) and −log [T(λ)/(1 − R(λ))], respectively.Microscopy
The light microphotographs of hand-made cross-sections of aloe leaves were taken with a Zeiss Axioscope microscope fitted with a digital camera (Carl Zeiss, Germany).The absorption spectra of single plastids were measured in leaf cross-sections with customized Leitz MPV2 system (Ernst Leitz Wetzlar GMBH, Germany) equipped with a 150 W high-pressure xenon lamp as a light source and a USB 2000 (grating #2) spectrometer (Ocean Optics, USA); the microscope was set to trans-illumination mode. To equalise the spectral energy output of the light source with spectral sensitivity of the spectrometer two 5 mm thick purple glass FS-2 filters (Krasnogorsk, Russia) were placed between the light source and the sample. The cross-sections were enclosed between a slide and a cover slip in 66 mM phosphate buffer (pH 7.0). The measured area was selected by an iris diaphragm adjusted to a single plastid size. The light passed through the sample was directed by the microscope optics to the spectrometer and the spectra were recorded between 400 and 800 nm. The absorption of the sample was calculated as A(λ) = −log(E1(λ)/E2(λ)), where E1(λ) and E2(λ) are irradiances of a sample and reference (adjacent to the sample area), respectively.
For electron microscopy the fixation either in glutaraldehyde–OsO4 or in glutaraldehyde–KMnO4 (all from Sigma, USA) was used. The ultrathin sections were prepared with LKB-8800 ultratom (LKB, Sweden) and examined under a JEM-100 B transmission electron microscope (JEOL, Japan).
Results
Light and electron microscopy of leaves
Under both high-light and drought/high-light stresses the A. arborescens leaves acquired red coloration whereas after the establishing of the root system or shading they reverted to the original green leaf colour. In particular, Fig. 1 (panel A) demonstrates the effect of drought and strong sunlight on plants used in the experiments carried out in January, 2003. The micrographs of cross-sections through the adaxial epidermis and subepidermal tissues of A. arborescens are presented in Fig. 1 (Panel B). The Aloe leaves featured a thick cuticle, with well developed wax layer. The content of the epidermal cells was transparent in green leaves but vacuoles of the red leaves were filled with pale yellow sap and occasionally contained raphids. Both types of leaves had large rounded or oblong mesophyll cells with spacious vacuoles and large lens-shaped plastids evenly distributed in the parietal layer of cytoplasm. Most of the plastids of green leaves possessed the characteristic green colour but some of them were green-to-yellowish. Red-coloured plastids were observed in reddish-to-red leaves; the overall number of plastids was close in the green and red leaves (Fig. 1, Panel B).The ultrastructural investigation showed that in the green leaves the plastids possessed the shape and inner organisation characteristic of sun leaf chloroplasts (Fig. 2A), containing well developed granae and stromal thylakoids with readily discernible membranes and one or two large starch granules laying in the centre of the organelle. The onset of chromoplast formation brought about progressive degeneration of the chloroplast membrane system and a loss of granal structure (Fig. 2). The membranes of stroma thylakoids progressively blurred and eventually disappeared during chloroplast–chromoplast transition. Thylakoid membranes of granae also became less distinct until the individual thylakoids became indiscernible, the regions occupied by granae turned to a homogenous electrondense round-shaped loci. Most striking features of plastids from the red leaves included the absence of thylakoids and appearance of globular structures with sharp edges possessing higher electron density than adjacent stroma. The starch granules in such plastids were preserved or even increased in size. In the red leaves the osmiophylic globules vastly increased in size and number and occupied a considerable part of plastid volume (Fig. 2C).
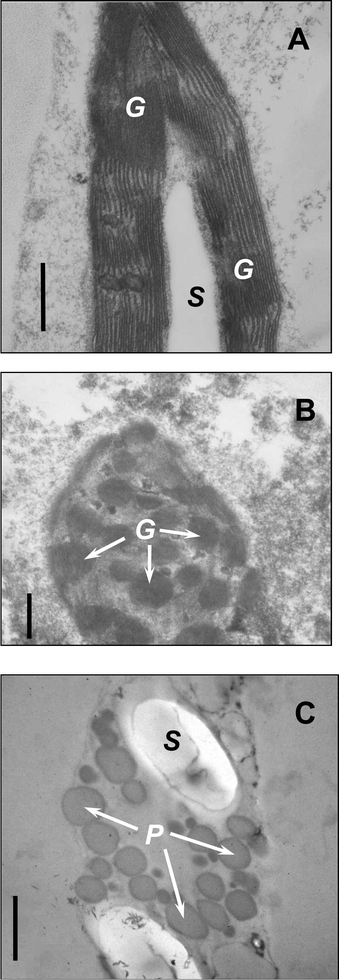 | ||
| Fig. 2 Ultrastructure of plastids of green (A), reddish-green (B), and red (C) leaves of A. arborescens. G—granae, S—starch grain, P—plastoglobuli. Bar = 0.5 µm. A, B—fixation with glutaraldehyde and KMnO4, C—fixation with glutaraldehyde and OsO4. | ||
Microspectrophotometry of plastids
The representative absorption spectra of plastids from green-to red aloe leaves are shown in Fig. 3. It should be noted that the spectral curves presented reproduce, in fact, the shape of absorption spectra of the plastids since their optical density could hardly be determined precisely, due to uncertainties in the positioning of the measuring beam and the selection of the reference area during the measurement. The plastids from green leaves contained resolved Chl absorption features in the orange–red range and a distinct maximum near 440 nm in the blue. The reddening of the leaves was accompanied by a decrease in magnitude of Chl peaks and an increase in absorption between 480 and 600 nm with shoulders 520 and 550 nm in plastid spectra (curves 2 and 3). At the advanced stages of the process plastids devoid of Chl with characteristic spectral properties in the blue–green region appeared and increased in number (curves 3–6). Sometimes a weakly resolved band centred near 500 nm were observed (curves 4 and 5). Plastid absorption in the leaves with intense red coloration was higher and did not possess remarkable spectral details in the band 450–550 nm. In this case a region of high absorption extended to wavelengths over 550 nm (curve 6).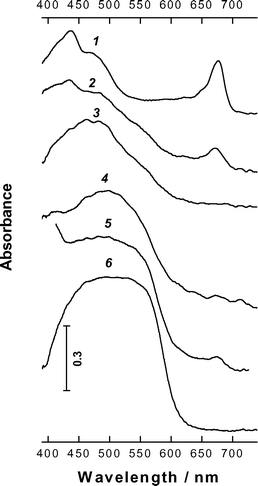 | ||
| Fig. 3 Typical absorption spectra of aloe plastids of green leaves (1) to red leaves (6). | ||
Pigments and leaf optical properties
Optical spectra of whole aloe leaves as well as AC preparations are shown in Figs. 4–6. In addition, Fig. 6 (inserts) demonstrates the results of HPLC separation and quantitative pigment analysis in green and red leaves.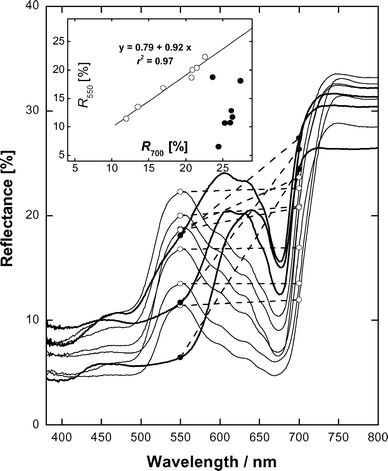 | ||
| Fig. 4 Representative reflection spectra of whole aloe leaves. Reflectances at 500 and 700 nm are shown as symbols and connected by dashed lines to show difference between them. Insert: Relationship between reflectances at 500 and 700 nm for visually green (open symbols) and reddish to red (closed symbols) leaves. | ||
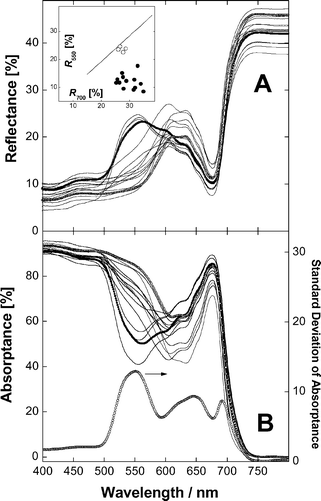 | ||
| Fig. 5 Reflection (A) and absorption (B) spectra of aloe adaxial chlorenchyma tissues. Panel A, insert: Relationship between reflectances at 550 and 700 nm for visually green (open symbols) and reddish to red (closed symbols) leaves. A line represents a linear fit for R550vs.R700 for whole green leaves (see Fig. 4, insert). Panel B. The lower curve is the trace of the standard deviation of absorptance (right scale) for all samples. The spectra of green and red specimens with close chlorophyll absorption near 678 nm shown as symbols were used for comparison and further analysis in Fig. 6. | ||
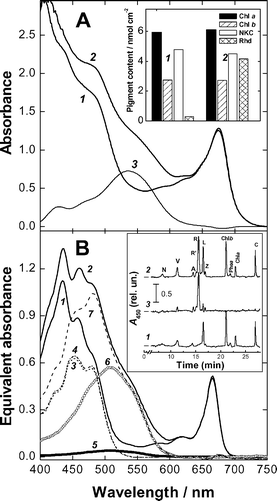 | ||
| Fig. 6 Absorption spectra and pigment analysis of green and red aloe adaxial chlorenchyma (AC) tissues (see also Fig. 5). Panel A. Absorbance spectra of AC from green (1) and red (2) leaves; (3) difference spectrum ‘red − green’. Insert: AC chlorophyll, non-ketocarotenoid (NKC) and rhodoxanthin (Rhd) content. Panel B. Absorbance spectra of pigments from green (1, 3 and 5) and red (2, 4, 6 and 7) AC (see panel A). 1 and 2 are the spectra of total extracts; spectra 3–7 are results of spectral reconstruction analysis for NKC (3 and 4) and Rhd (5 and 6); 7–spectrum of total carotenoids (NKC + Rhd) from the red AC. Insert: HPLC of aloe pigments form green (1) and red (2) AC. Chromatograms are normalised to the magnitude of chlorophyll b peak. 3–difference between chromatograms 2 and 1. N—neoxanthin, V—violaxanthin, A—antheraxanthin, R′—rhodoxanthin derivative, R—rhodoxanthin, L—lutein, Z—zeaxanthin, Phea—phaeophytin a, and C—β-carotene. | ||
To characterise pigment composition aloe leaves were extracted with the method allowing separation of water- and liposoluble components.10 The water–methanol phase of extracts from both green and red aloe leaves obtained was pale-yellow in colour. The addition of hydrochloric acid (final concentration 0.1%) did not result in the appearance of characteristic absorption near 530 nm indicating the absence of anthocyanin pigments. Chl and Car were recovered in chloroform phase; the HPLC (Fig. 6B, insert, curves 1 and 2) and TLC (not shown) analyses revealed the presence of the NKC (neoxanthin, violaxanthin, anteraxanthin, zeaxanthin and β-carotene) common for majority of higher plant leaves.21,37 The light-stressed A. arborescens leaves contained significant amounts of the red carotenoid, Rhd. The presence of small amounts of a Rhd derivative (probably, its isomer24) with lower retention time should be also mentioned (Fig. 6B, insert). For both green and red aloe leaves no evidence has been obtained for the accumulation of Car esters eluted in this HPLC system between Chl a and carotene(s).
Typically for higher plants,36,38,39 whole-leaf and AC reflectance was low in the blue and high in the NIR (750–800 nm) and pronounced band of Chl absorption peaking near 678 nm was present in the red range of the spectrum (Figs. 4 and 5). Reflection spectral features of whole leaves and AC were similar but in the former case reflectance in the NIR did not exceed 30–34%. Characteristic of green leaves29,38,39 relatively high reflectance was recorded within the range 550–600 nm and R550 and R700 were close and highly correlated in a wide range of their changes (Fig. 4 and 5, inserts). Both in whole leaves and AC the development of red coloration was accompanied by a decrease in reflectance between 500 and 600 nm, moving the green edge of reflectance towards longer wavelengths and a strong decrease of R550 as compared with R700 (Figs. 4 and 5, inserts). In red leaves as well as in their AC R550 nm was low and reached 6.5 and 8.5%, respectively.
In spite of relatively low Chl content (<10 nmol cm−2, see Fig. 6A) AC from aloe leaves possessed high absorption (Figs. 5 and 6). Throughout visible range leaf absorption significantly exceeded equivalent absorption of pigments recovered in chloroform phase. Thus, in the red band leaf absorbance was more than two-fold higher. The difference between leaf and extract absorption was especially high near 550 nm in green but not in red leaves (cf. curves 1 and 2 in Fig. 6A and B). AC absorption spectra in Figs. 5 and 6 demonstrate that the development of red pigmentation is associated with an increase of absorption in the green range, which exceeded 80% near 550 nm. The standard deviation (STD) spectrum calculated for the entire data set revealed a strong peak of absorption variation near 550 nm as well as a band 620–700 nm with a gap around 678 nm (Fig. 5B). At wavelengths shorter than 500 nm absorption possessed a low variation especially below 410–420 nm.
To estimate Rhd spectral properties in aloe leaves, two AC samples obtained from green and red leaves with close Chl content and absorption in the red range of the spectrum were compared (Fig. 6). The difference ‘red–green’ absorbance spectrum of AC showed a broad band 450–600 nm bearing a peak centred at 535–540 nm with a small shoulder near 460–470 nm (Fig. 6A).
In accordance with optical measurements, both samples possessed close Chl content (Fig. 6A, insert) and equivalent absorption of the extracts in the red bands of Chl a and b (Fig. 6B). Furthermore, the spectral reconstruction analysis of the extracts showed very close content and spectral properties of NKC in the red and green tissues. Red AC accumulated Rhd in amounts close to that of total NKC. Then, Rhd absorption in its maximum (510 nm) was comparable with that of NKC (at 452 nm) (Fig. 6). HPLC analysis (Fig. 6B, insert) showed the presence and close relative content of Chl a, Chl b as well as individual NKC in both AC samples and remarkably high amount of Rhd in the red sample. The presence of small amounts of Rhd in chloroform extract from visually green AC should be also mentioned. The difference between the chromatograms normalised to the amplitude of the Chl b peak (Fig. 6B, insert, curve 3) revealed only a small increase in content of some xanthophylls in red leaves. Thus, the spectral difference between absorption of red and green AC (Fig. 6A) could be to a large extent attributed to Rhd.
Discussion
The development of red pigmentation is frequently observed in higher plants subjected to high sunlight and other stressful conditions. In most cases this response involves the accumulation of anthocyanins, the flavonoid pigments with vacuolar localisation.11–14,29 However in some plant species, seemingly devoid of anthocyanins, stress-induced coloration of leaves is related with accumulation of the red plastidic ketocarotenoid Rhd that is in accord with a general concept on protective role of carotenogenesis triggered by redox signal(s) induced by (photo)oxidative stress.40 The stress-induced Rhd accumulation was described in some conifers23–28 as well as in the Aloe genus.30 In the line with a general photoprotective role of red pigments as screens for solar radiation this work was devoted the study of A. arborescens plants accumulating significant amounts of Rhd in the leaves under strong-light/drought conditions.Ultrastructural observations showed that the adaptation A. arborescens to the stressful conditions is accompanied by a deep rearrangement of chloroplasts including degradation of thylakoids and accumulation of globular electron-dense structures (probably of lipidic nature) resembling the osmyophylic globules encountered in plastids of senescing leaves.41–44 Taking into account the (ultra)structural details observed (Figs. 1 and 2), the plastids of the red aloe leaves can be regarded as chromoplasts and, more specifically, carotenoidoplasts.
In the progress of chloroplast-to-chromoplast transformation the plastids gradually lost Chl, turned reddish in colour and spectral features of Car absorption appeared in their spectra (Fig. 3) strongly suggesting retention of Car over Chl. At advanced stages of Chl-loss two types of Car absorption became apparent in plastid spectra. The comparison of plastid spectra with reconstructed absorption spectra of Car in leaf extracts (Fig. 6B) makes it possible to distinguish two main types of Car absorption in plastids: attributable to NKC (in the range 460–500 nm) and to Rhd at longer wavelengths appearing as shoulders in the region 520–550 nm (Fig. 3). Such a large shift of Rhd absorption maximum compared to solutions (Fig. 6B, see19,20,29) may involve aggregation of the pigment due to its high local concentration, presumably in lipid globules.
The contributions of NKC and Rhd into absorption of individual plastids were different and frequently the spectral features of Rhd appeared on NKC background (Fig. 3, curves 3–6). In dark red plastids a strong absorption in a broad unresolved band of 450–550 nm was observed (Fig. 3, curve 6). Thus, the spectral analysis of plastids in situ suggests that under high light/high light–drought stress in aloe leaves selective and significant destruction of Chl takes place along with retention of Car and/or activation of their biosynthesis. Then, it appears that in aloe plastids the build up of Rhd occurs in addition to that of NKC. The increase in the content of violaxanthin, zeaxanthin and lutein together with the accumulation of Rhd has been documented in A. vera plants subjected to drought under high light conditions.30 In winter-hardening sun-exposed needles of Cr. japonica, the increase in Rhd occurring along with Chl breakdown was also accompanied by an increase in xanthophylls of xanthophyll cycle pool.26,27 Nevertheless, it was concluded that Rhd might play a more important role than the volaxanthin cycle Car in protecting the photosynthetic apparatus from photodamage in winter.26 To the best of our knowledge, no evidence has been obtained on the involvement of Rhd in photoprotection within thylakoid membranes. It was reported that light-harvesting chlorophyll-protein complex of Cr. japonica does not retain Rhd.25 In contrast to some other non-native xanthophylls Rhd did not facilitate the reassembly of monomeric recombinant LHCIIb complex.37 Taking into account the changes of ultrastructure observed it is likely that the main depot of Rhd in aloe plastids is situated out of thylakoid membranes in plastoglobuli as it occurs in the course of leaf senescence.42 This is in consistence with the proposed role of osmiophylic globules as a depot for plastidic lipids, prenyl quinons and Car during dismantling of photosynthetic machinery43 as well as with photoprotective function of plastidic Car.18
Strong light stress induced remarkable changes in spectral light reflection and absorption by aloe leaves and AC (Figs. 4–6). Interestingly, all specimens investigated possessed characteristic Chl spectral features in the red, though microspectrophotometry revealed Chl-free plastids in stressed leaves (Fig. 3, curves 4–6). This indicates that aloe leaf optical properties are determined by proportion of plastids with different types of absorption and their distribution within tissue. The accumulation of Rhd under stress conditions brought about a decrease of reflectance along with increase of aloe leaf and AC absorption in the green range of the visible spectrum. The trace of the STD of absorption for AC obtained for leaves of colours from green to red (Fig. 5B) showed a significant variation in the orange–red range with a minimum near 678 nm, due to the saturation of the absorption at high content of the pigment,39 which indicates considerable changes in leaf Chl content. At the same time, high and almost invariable absorption was recorded below 500 nm in the spectral region governed by the combined absorption of Chl and NKC.38 A strong maximum of the STD of absorption peaking near 550 nm was situated in the band of 520–600 nm (Fig. 5B). The comparison of absorption spectra of selected red and green aloe leaves exhibiting close optical properties in the red as well as Chl and NKC content also strongly suggests that Rhd absorption in vivo occurs as a band in the blue–green range. According to these measurements the maximum of Rhd absorption in aloe leaves is located near 540 nm (Fig. 6).
The revealed spectral properties of Rhd in aloe leaves closely resemble those of anthocyanins in plant species accumulating these pigments.11,38 It was shown that quantitative analysis of anthocyanins could be performed using relationships between reflectances at 700 and 550 nm.38 Similarly to the leaves of anthocyanin-free species green aloe leaves possessed close R550 and R700 in a wide range of their changes. The reddening of the leaves manifested itself as the lowering of R550 compared with R700 (Fig. 4 and 5B, inserts). In winter, for aloe plants suffering from combined stress induced by light and drought, the decrease of R550 occurred at higher R700 values (Fig. 4). This suggests that under these conditions the adaptation of aloe involved a considerable decrease in Chl content, probably to reduce the amount of light absorbed by the photosynthetic apparatus. Another response has been observed in mature plants with established root systems. The relationship ‘R550vs.R700’ in Fig. 5, leaf absorption spectra and the data of pigment chemical analysis in Fig. 6 indicate that the accumulation of Rhd frequently took place in leaves with relatively high Chl content and even green leaves contained noticeable amounts of Rhd. It is tempting to speculate that in this case the level of protection provided by the build up of Rhd and, probably, by other mechanism(s) was sufficient to prevent a dramatic decrease in Chl content.
Collectively, the results of spectral measurements indicate that accumulation of Rhd in chromoplasts of light-stressed aloe leaves is able to provide a considerable attenuation of light absorbed by plant tissue in the green range of the visible spectrum. These findings are consistent with the proposed photoprotective function of Rhd,25–28 which is accomplished via efficient internal light trapping aimed to diminish the amount of radiation absorbed by Chl of photosynthetic apparatus under stressful conditions. In addition, it is possible to suggest that in plastids devoid of Chl, the combination of Rhd and NKC is able to protect lipids reserved in osmyophylic globules from the deleterious effects of irradiation over a broad spectral band.
As it has been already mentioned, the effect of plastidic Rhd on leaf light absorption closely resembles that of vacuolar anthocyanins. It appears that both pigments are able to serve as effective broad-band internal traps for radiation in the green range exactly in the gap between the bands of strong Chl and Car absorption in which light penetrates deeply into leaf tissues.11,38 Remarkably, anthocyanins and Rhd, the pigments disparate in terms of their biosynthesis, (photo)chemistry and subcellular localisation but with similar in vivo optical properties are relied upon by different plant species for the purposes of long-term adaptation to and protection against strong solar irradiation in the visible range.
Acknowledgements
The authors are grateful to Professor Y. Gutterman for generous supply of aloe leaves, Professor A. Karnieli, Professor Z. Cohen and Dr I. Khozin-Goldberg (all from BIDR) for the possibility of using spectroradiometric and chromatographic devices and to Ms N. P. Buzulukova (Faculty of Biology, Moscow State University) for her help in preparation of samples for electron microscopy. This work was supported in part by fellowships from BIDR to MM and AS.References
- K. Asada, Production and action of active oxygen species in photosynthetic tissues in Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants, ed. C. H. Foyer and P. M. Mullineaux, CRC Press, Boca Raton, FL, 1994, 77–104 Search PubMed.
- C. H. Foyer, M. Lelandais and K. J. Kunert, Photooxidative stress in plants, Physiol. Plant., 1994, 92, 696–717 CrossRef CAS.
- K. Niyogi, Photoprotection revisited: genetic and molecular approaches, Annu. Rev. Plant Physiol. Mol. Biol., 1999, 50, 333–359 CrossRef CAS.
- J. F. Bornman and T. C. Vogelmann, Effect of UV-B radiation on leaf optical properties measured with fiber optics, J. Exp. Bot., 1991, 42, 547–554 Search PubMed.
- C. S. Cockell and J. Knowland, Ultraviolet radiation screening compounds, Biol. Rev., 1999, 74, 311–345 CrossRef CAS.
- D. C. Close and C. McArthur, Rethinking the role of many plant phenolics–protection from photodamage not herbivores?, Oikos, 2002, 99, 166–172 CrossRef CAS.
- A. Solovchenko and M. Merzlyak, Optical properties and contribution of cuticle to UV protection in plants: experiments with apple fruit, Photochem. Photobiol. Sci., 2003, 8, 861–866 RSC.
- M. Havaux and K. Kloppstech, The protective functions of carotenoids and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants, Planta, 2001, 213, 953–966 CrossRef CAS.
- A. Solovchenko and M. Schmitz-Eiberger, Significance of skin flavonoids for UV-B protection in apple fruits, J. Exp. Bot., 2003, 54, 1977–1984 Search PubMed.
- A. E. Solovchenko, O. B. Chivkunova, M. N. Merzlyak and I. V. Reshetnikova, A spectrophotometric analysis of pigments in apples, Russ. J. Plant Physiol., 2001, 48, 693–700 CrossRef CAS.
- M. N. Merzlyak and O. B. Chivkunova, Light stress induced pigment changes and evidence for anthocyanin photoprotection in apple fruit, J. Photochem. Photobiol., B, 2000, 55, 154–162 CrossRef.
- W. A. Hoch, E. L. Zeldin and B. H. McCown, Physiological significance of anthocyanins during autumnal leaf senescence, Tree Physiol., 2001, 21, 1–8 Search PubMed.
- W. J. Stein, S. J. E. Wand, D. M. Holcroft and G. Jacobs, Anthocyanins in vegetative tissues: a proposed unified function in photoprotection, New Phytol., 2002, 155, 349–361 Search PubMed.
- D. Strack and V. Wray, Anthocyanins in Methods in Plant Biochemistry, ed. J. B. Harborne and P. M. Dey, Academic Press, Inc., London, 1989, vol. 1, pp. 326–352 Search PubMed.
- J. B. Harborne and C. A. Williams, Advances in flavonoid research since 1992, Phytochem., 2000, 55, 481–504 Search PubMed.
- B. Demmig-Adams, A. M. Gilmore and W. W. Adams, In vivo functions of carotenoids in higher plants, FASEB J., 1996, 10, 403–413 Search PubMed.
- A. J. Young and H. A. Frank, Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence, J. Photochem. Photobiol., B, 1996, 36, 3–15 CrossRef CAS.
- M. N. Merzlyak and A. E. Solovchenko, Photostability of pigments in ripening apple fruit: Possible photoprotective role of carotenoids during plant senescence, Plant Sci., 2002, 163, 881–888 CrossRef CAS.
- G. Britton, General carotenoid methods, in ‘Steroids and Isoprenoids’, ed. J. H. Law and H. C. Rilling, Methods Enzymol. Part B, 1985, 111, 113–147 Search PubMed.
- G. Britton, UV/Visible spectroscopy, in Carotenoids, ed. G. Britton, S. Liaaen-Jensen and H. Pfander, Birkhäuser Verlag, Basel, 1995, vol. 1B, pp. 13–59 Search PubMed.
- A. J. Young, Occurrence and distribution of carotenoids in photosynthetic systems, in Carotenoids in Photosynthesis, ed A. J. Young and G. Britton, Chapman and Hall, London, 1993, pp. 16–71 Search PubMed.
- B. Czeczuga, Different rhodoxanthin contents in the leaves of gymnosperms grown under various light intensities, Biochem. Syst. Ecol., 1987, 15, 531–533 CrossRef CAS.
- K. Ida, Eco-physiological studies on the response of taxodiaceous conifers to shading, with special reference to the behaviour of leaf pigments. I. Distribution of carotenoids in green and autumnal reddish brown leaves of gymnosperms, Bot. Magaz. (Tokyo), 1981, 94, 41–54 Search PubMed.
- K. Ida, F. Saito and S. Takeda, Isomers of rhodoxanthin in reddish brown leaves of gymnosperms and effect of daylight intensity on the contents of pigments during autumnal coloration, Bot. Magaz. (Tokyo), 1991, 104, 157–170 Search PubMed.
- Q. Han, K. Shinohara, Y. Kakubari and Y. Mukai, Photoprotective role of rhodoxanthin during cold acclimation in Cryptomeria japonica, Plant, Cell Environ., 2003, 26, 715–723 Search PubMed.
- Q. Han, S. Katahata, Y. Kakubari and Y. Mukai, Seasonal changes in the xanthophyll cycle and antioxidants in sun-exposed and shaded parts of the crown of Cryptomeria japonica in relation to rhodoxanthin accumulation during cold acclimation, Tree Physiol., 2004, 24, 609–616 Search PubMed.
- M. N. Merzlyak, A. E. Solovchenko, A. I. Smagin and A. A. Gitelson, Apple flavonols during fruit adaptation to solar radiation: spectral features and technique for non-destructive assessment, J. Plant Physiol., 2004, 162, 151–160.
- H. G. Weger, S. N. Silim and R. D. Guy, Photosynthetic acclimation to low temperature by western red cedar seedlings, Plant, Cell Environ., 1993, 16, 711–717 CAS.
- A. A. Gitelson, M. N. Merzlyak and O. B. Chivkunova, Optical properties and non-destructive estimation of anthocyanin content in plant leaves, Photochem. Photobiol., 2001, 74, 38–45 CrossRef CAS.
- M. Diaz, E. Ball and U. Lüttge, Stress-induced accumulation of the xanthophyl rhodoxanthin in leaves of Aloe vera, Plant Physiol. Biochem., 1990, 28, 679–682 CAS.
- Y. Gutterman and E. Chauser-Volfson, The distribution of the phenolic metabolites barbaloin, aloeresin and aloenin as a peripheral defense strategy in the succulent leaf parts of Aloe arborescens, Biochem. Syst. Ecol., 2000, 28, 825–838 CrossRef CAS.
- J. Folch, M. Lees and G. H. Sloane-Stanley, A simple method for the isolation and purification of total lipids from animal tissues, J. Biol. Chem., 1957, 226, 497–509 CAS.
- A. R. Wellburn, The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution, J. Plant Physiol., 1994, 144, 307–313 CAS.
- K. R. Naqvi, T. Hj. Hassan and Y. A. Naqvi, Expeditious implementation of two new methods for analysing the pigment composition of photosynthetic specimens, Spectrochim. Acta, Part A, 2004, 60, 2783–2791 CrossRef.
- A. Pintea, C. Bele, S. Andrei and C. Socaciu, HPLC analysis of carotenoids in four varieties of Calendula officinalis L. flowers, Acta Biol. Szeged., 2003, 47, 37–40 Search PubMed.
- M. N. Merzlyak, O. B. Chivkunova, T. B. Melø and K. R. Naqvi, Does a leaf absorb radiation in the Near Infra-Red (780–900 nm)? A new approach to quantifying optical reflection, absorption and transmission of leaves, Photosynth. Res., 2002, 72, 263–270 CrossRef CAS.
- S. Phillip, H. Hobe Paulsen, P. Molnar, H. Hashimoto and A. J. Young, The binding of xanthophylls to the bulk light-harvesting complex of photosystem II of higher plants. A specific requirement for carotenoids with a 3-hydroxy-end group, J. Biol. Chem., 2002, 277, 25160–25169 CrossRef.
- A. A. Gitelson, Y. Zur, O. B. Chivkunova and M. N. Merzlyak, Assessing carotenoid content in plant leaves with reflectance spectroscopy, Photochem. Photobiol., 2002, 75, 272–281 CrossRef CAS.
- A. Gitelson, U. Gritz and M. N. Merzlyak, Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves, J. Plant Physiol., 2003, 160, 271–282 CAS.
- F. Bouvier, R. A. Backhaus and B. Camara, Induction and control of chromoplast-specific trebouxiophyceae gene by oxidative stress, J. Biol. Chem., 1998, 273, 30651–30659 CrossRef CAS.
- J. Hudák, Plastid senescence, 1. Changes of chloroplast structure during natural senescence in cotyledons of Sinapis alba L., Photosynthetica, 1981, 15, 174–178.
- D. Steinmüller and M. Tevini, Composition and function of plastoglobuli. I. Isolation and purification from chloroplasts and chromoplasts, Planta, 1985, 163, 201–207.
- M. Tevini and D. Steinmüller, Composition and function of plastoglobuli. II. Lipid composition of leaves and plastoglobuli during senescence, Planta, 1985, 163, 91–96 CrossRef CAS.
- M. Vishnevetsky, M. Ovadis and A. Vainstein, Carotenoid sequestration in plants: the role of carotenoid-associated proteins, Trends Plant Sci., 1999, 4, 1360–1385.
| This journal is © The Royal Society of Chemistry and Owner Societies 2005 |
