Expedient access to unsymmetrical triarylmethanes through N-heterocyclic carbene catalysed 1,6-conjugate addition of 2-naphthols to para-quinone methides†
Panjab Arde and
Ramasamy Vijaya Anand *
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Sector 81, Knowledge City, S. A. S. Nagar, Manauli (PO), Punjab – 140306, India. E-mail: rvijayan@iisermohali.ac.in
First published on 9th August 2016
Abstract
The utility of N-heterocyclic carbene as a Brønsted base catalyst for the synthesis of functionalised unsymmetrical triarylmethanes from 2-naphthol and para-quinone methides is described. This protocol allows the installation of naphthyl substituents on p-quinone methides through 1,6-conjugate addition to deliver the unsymmetrical triarylmethanes in good to excellent yields. Mild reaction conditions and 100% atom economy make this method synthetically advantageous over the other hitherto known methods.
The remarkable contribution of triarylmethanes has been comprehended not only in the dye industry but also in the development of organic functional materials.1 In recent years, another dominant application of triarylmethanes has been reconnoitred in the area of drug discovery as many of the symmetrical as well as unsymmetrical triarylmethanes possess interesting medicinal properties (Fig. 1).2 In addition, the triarylmethane core has been found as an integral part of some naturally occurring molecules.3 Moreover, some specially designed triarylmethane derivatives could be utilised as sensors for detecting metal as well as cyanide ions.4
Although, both symmetrical and unsymmetrical triarylmethanes could be accessed through traditional Friedel–Crafts arylation of arenes with diarylmethanol or its derivatives under Lewis5 or Brønsted acid6 catalysed/mediated conditions, the requirement of electron rich arenes and harsh reaction conditions restrict this method from the synthetic point of view. To address these issues, a few traditional metal catalysed methods have been developed.7 Out of these protocols, palladium8 or nickel9 or other metal10 catalysed coupling of diarylmethane derivatives with suitable aryl coupling partners have become the most popular method to access achiral and/or enantiometrically enriched unsymmetrical triarylmethanes. Very recently, another elegant approach has been disclosed based on rhodium catalysed 1,4-addition of arylboronic acids to o-quinone methides.11 A metal free and base mediated direct coupling between diarylmethanes and aryl fluorides to access triarylmethanes has also been reported very recently.12 Although all the above mentioned strategies show a wide substrate scope and excellent functional group tolerance, none of these methods meet 100% atom economy. Therefore, developing a simple and atom economical approach for the synthesis of triarylmethanes, especially under organocatalytic conditions, would be more striking and highly desirable.
While working in the areas of N-heterocyclic carbene (NHC) catalysis13 and the synthesis of triarylmethanes,14 we anticipated that NHC could act as a Brønsted base to activate 2-naphthols to react with para-quinone methides (p-QMs)15 through 1,6-conjugate addition, which would lead to unsymmetrical triarylmethanes. To our surprise, so far, only a limited number of literature precedents are available, where NHC was employed as a Brønsted base,16 although the nucleophilic nature of NHCs has been explored in many organocatalytic transformations.17 The Brønsted base behavior of NHC has been first uncovered in transesterification reactions.18 Following these seminal reports, 1,4-conjugate addition reactions such as intramolecular19 and intermolecular Michael,20 oxa-Michael,21 aza-Michael22 and phospha-Michael reactions23 have been disclosed based on the utility of NHC as a Brønsted base. NHC has also been used as a base in other transformation such as silyl enol ether formation24 and ring opening of isochromene derivatives.25 Very recently, our group reported 1,6-hydrophosphonylation of p-quinone methides and fuchsones to access diaryl- and triarylmethyl phosphonates, respectively, using NHC as a Brønsted base.26 Herein, we disclose the synthesis of highly functionalised triarylmethane derivatives through 1,6-conjugate addition of 2-naphthols to p-QMs using NHC as a Brønsted base catalyst.
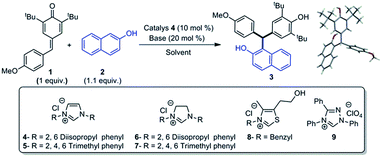
The optimisation studies were carried out with p-QM 1 and 2-naphthol under various reaction conditions using different NHC precursors (4–9), and the results are shown in Table 1. When the reaction was performed using 4 as a NHC precursor and NaH as a base in THF, the expected product 3 was obtained in 60% yield in 24 h (entry 1). The structure of 3 was indisputably confirmed by spectroscopic methods as well as X-ray analysis (Table 1). Prompted by this result, we elaborated the optimisation studies in different solvents (entries 2–7). Out of several solvents screened, dichloromethane was found to be the most suitable solvent for this methodology, as 3 was obtained almost in quantitative yield in 18 h under this condition (entry 3). Further experiments were carried out using other NHC precursors (5–9) in CH2Cl2. However, in all those cases (entries 8–12), the product 3 was obtained in relatively less yield when compared to entry 3. The effectiveness of other bases such as K3PO4 and Cs2CO3 for this transformation were found to be inferior when compared to NaH (entries 13 & 14). To confirm the role of NHC in this transformation, a couple of experiments were performed only in the presence of base without NHC precursors (entries 15 & 16), but in both the cases, the product was obtained in <10% yield. Surprisingly, when the reaction was carried out with DBU as a base without NHC precursor, 3 was isolated in 67% yield though the reaction was not completed even after 48 h (entry 17). In another experiment, the reaction between 1 and 2 was carried out with IPr (free NHC) in the absence of base and, in this case, 3 was obtained in 87% yield after 24 h. This observation clearly indicates that the NHC is actually acting as a catalyst.
| Entry | Catalyst | Base | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: all reactions were carried out with 0.062 mmol of 1 in 0.3 mL of solvent at room temperature.b The reaction was carried out with free IPr NHC. | |||||
| 1 | 4 | NaH | THF | 24 | 60 |
| 2 | 4 | NaH | Et2O | 24 | 80 |
| 3 | 4 | NaH | CH2Cl2 | 18 | 99 |
| 4 | 4 | NaH | DCE | 24 | 91 |
| 5 | 4 | NaH | PhMe | 21 | 93 |
| 6 | 4 | NaH | DMSO | 24 | 90 |
| 7 | 4 | NaH | MeCN | 24 | 20 |
| 8 | 5 | NaH | DCE | 22 | 93 |
| 9 | 6 | NaH | DCE | 24 | 78 |
| 10 | 7 | NaH | CH2Cl2 | 24 | 80 |
| 11 | 8 | NaH | CH2Cl2 | 24 | 60 |
| 12 | 9 | NaH | CH2Cl2 | 24 | 84 |
| 13 | 4 | K3PO4 | CH2Cl2 | 24 | 90 |
| 14 | 4 | Cs2CO3 | CH2Cl2 | 24 | 94 |
| 15 | — | NaH | CH2Cl2 | 24 | 7 |
| 16 | — | Cs2CO3 | CH2Cl2 | 24 | 5 |
| 17 | — | DBU | CH2Cl2 | 48 | 67 |
| 18b | IPr | — | CH2Cl2 | 24 | 87 |
Having found the optimal reaction conditions (entry 3, Table 1), we went on to study the scope and limitations of this transformation using 2-naphthol and a diverse set of p-quinone methides, and the results are shown in Table 2. Electronic effects of the aryl substituents in p-QMs were found to have minimal influence in the reaction as the p-QMs derived from both electron-poor and electron-rich aromatic aldehydes underwent smooth conversion to their corresponding triarylmethanes in very high yields. In general, this methodology worked extremely well in the cases of p-QMs (1a–h) derived from electron-rich aromatic aldehydes as the expected triarylmethanes (3a–h) were obtained in excellent isolated yields (89–99%). Other p-QMs (1i–m) derived from benzaldehyde or arylated benzaldehydes also underwent 1,6-conjugate addition and provided the respective triarylmethanes (3i–m) in good to excellent yields (75–92%). This method was found to be robust for the p-QMs derived from halogen substituted aromatic aldehydes. For example, p-QMs 1n and 1o reacted with 2-naphthol under the optimal conditions and gave the corresponding products 3n and 3o respectively in very high yields. In the cases of electron-poor aryl substituted p-QMs (1p–q), the products 3p and 3q were isolated in 86 and 92% yields correspondingly. The heteroaryl-containing triarylmethanes 3r and 3s were obtained in moderate yields from the p-QMs (1r and 1s), prepared from furan-2-carboxaldehyde and thiophene-2-carboxaldehyde respectively. The ferrocene containing triarylmethane 3t was obtained from 1t in 74% yield under the standard conditions. The efficacy of this protocol was also examined with p-QM 1u, derived from 2,6-diisopropyl phenol, and in this case, the product 3u was obtained in 75% isolated yield.
To elaborate the substrate scope further, 1 was subjected to 1,6-conjugate addition reaction with various 2-naphthol derivatives under optimised reaction conditions and the results are summarised in Table 3. It is evident from Table 3 that this protocol worked efficiently for all the 2-naphthol derivatives tried. For instance, the reaction of 1 with 6-methoxy-2-naphthol provided the desired triarylmethane 10a in 92% yield in 12 h. Other naphthol derivatives such as 6-bromo-2-naphthol and 6-phenyl-2-naphthol reacted smoothly with 1 to give the products 10b and 10c respectively in 95% yield. Under the reaction conditions, 6-hydroxyquinoline gave the corresponding product 10d in 86% isolated yield. However, in the case of relatively electron-poor 2-naphthol derivative 2e, the triarylmethane 10e was obtained only in 64% yield. Unfortunately, simple phenol failed to react with 1 under the optimal reaction conditions.
| a Reaction conditions: all reactions were carried out with 0.062 mmol of 1 in 0.3 mL of solvent at room temperature. |
|---|
 |
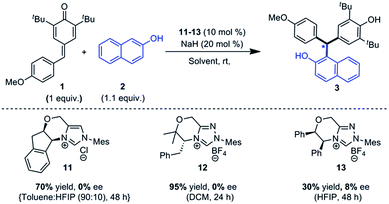
An enantioselective version of this reaction was also attempted. It is known in the literature that the enantioselective version of similar type of reactions is very challenging due to the potential reversibility of the reaction.21 Many different reaction conditions were employed for this transformation using a variety of chiral NHC pre-catalysts (11–13). When 11 and 12 were used as a catalyst for the reaction between 1 and 2, the product 3 was isolated in 70% and 95% yields respectively (Scheme 1). However, in both the cases, the product 3 was obtained as a racemic mixture at room temperature. Lowering the reaction temperature to sub-zero resulted in substantial lowering of the yield of 3 without any improvement in the enantioselectivity. When the reaction was carried out using 13 as a precatalyst in hexafluoroisopropanol (HFIP) as a solvent,27 3 was obtained in 30% yield with a marginal improvement in the enantioselectivity (8% ee).
A plausible mechanism for this reaction has been proposed based on the outcome of the reaction as well as the literature reports for similar kind of transformations (Scheme 2). In the initial step, NHC (I) abstracts the phenolic proton of 2-naphthol to generate the 2-naphthoxide anion, which immediately adds to the p-QM II in an 1,6-fasion to generate the intermediate III. Aromatization of the intermediate III followed by proton abstraction from IV leads to the formation of the product with the release of NHC I.
Conclusions
In conclusion, we have disclosed here an atom-economical method for the synthesis of highly functionalised unsymmetrical triarylmethane derivatives through 1,6-conjugate addition of 2-naphthols to p-QMs using NHC as a Brønsted base catalyst. Further studies toward enhancing the asymmetric induction in this transformation are currently ongoing.Acknowledgements
We are grateful to the Department of Science and Technology (EMR/2015/001759), New Delhi for financial support and IISER Mohali for providing infrastructure. PA thanks the CSIR New Delhi for a research fellowship. We also thank Mr Siddheshwar K. Bankar (IISER Mohali) for his help in solving the crystal structure. The NMR and HRMS facilities at IISER Mohali are gratefully acknowledged. XtaLabmini single crystal X-ray facility of the Department of Chemical Sciences at IISER Mohali is acknowledged for the data collections.Notes and references
- (a) D. F. Duxbury, Chem. Rev., 1993, 93, 381 CrossRef CAS; (b) V. Nair, S. Thomas, S. C. Mathew and K. G. Abhilash, Tetrahedron, 2006, 62, 6731 CrossRef CAS; (c) M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250 CrossRef CAS PubMed.
- (a) S. D. Cho, K. Yoon, S. Chintharlapalli, M. Abdelrahim, P. Lei, S. Hamilton, S. Khan, S. K. Ramaiah and S. Safe, Cancer Res., 2007, 67, 674 CrossRef CAS PubMed; (b) P. M. Wood, L. L. Woo, J. R. Labrosse, M. N. Trusselle, S. Abbate, G. Longhi, E. Castiglioni, F. Lebon, A. Purohit and M. J. Reed, J. Med. Chem., 2008, 51, 4226 CrossRef CAS PubMed; (c) S. Mondal and G. Panda, RSC Adv., 2014, 4, 28317 RSC.
- (a) S. Antus, E. Schindlbeck, S. Ahmad, O. Seligmann, V. M. Chari and H. Wagner, Tetrahedron, 1982, 38, 133 CrossRef CAS; (b) K. Baba, K. Maeda, W. Tabata, M. Doi and M. Kozawa, Chem. Pharm. Bull., 1988, 36, 2977 CrossRef CAS.
- (a) L. Yu, D. Chen, J. Li and P. G. Wang, J. Org. Chem., 1997, 62, 3575 CrossRef CAS; (b) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS PubMed; (c) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, J. Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed; (d) P. Kaur, D. Sareen, S. Kaur and K. Singh, Inorg. Chem. Commun., 2009, 12, 272 CrossRef CAS.
- For selected examples, see (a) S. J. Ji, M. F. Zhou, D. G. Gu, Z. Q. Jiang and T. P. Loh, Eur. J. Org. Chem., 2004, 1584 CrossRef CAS; (b) V. Nair, K. G. Abhilash and N. Vidya, Org. Lett., 2005, 7, 5857 CrossRef CAS PubMed; (c) C. R. Liu, M. B. Li, C. F. Yang and S. K. Tian, Chem. Commun., 2008, 1249 RSC; (d) G. K. S. Prakash, C. Panja, A. Shakhmin, E. Shah, T. Mathew and G. A. Olah, J. Org. Chem., 2009, 74, 8659 CrossRef CAS PubMed; (e) R. F. A. Gomes, J. A. Coelho, R. F. M. Frade, A. F. Trindade and C. A. M. Afonso, J. Org. Chem., 2015, 80, 10404 CrossRef CAS PubMed; (f) G. Pallikonda and M. Chakravarty, J. Org. Chem., 2016, 81, 2135 CrossRef CAS PubMed.
- (a) S. Shirakawa and S. Kobayashi, Org. Lett., 2006, 8, 4939 CrossRef CAS PubMed; (b) M. Wilsdorf, D. Leichnitz and H. U. Reissig, Org. Lett., 2013, 15, 2494 CrossRef CAS PubMed; (c) M. H. Zhou, Y. J. Jiang, Y. S. Fan, Y. Gao, S. Liu and S. Zhang, Org. Lett., 2014, 16, 1096 CrossRef PubMed; (d) W. Zhao, Z. Wang, B. Chu and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 1461 CrossRef PubMed; (e) Z. Wang, Y. F. Wong and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 13711 CrossRef CAS PubMed; (f) S. Saha, S. K. Alamsetti and C. Schneider, Chem. Commun., 2015, 51, 1461 RSC; (g) M. L. Li, D. F. Chen, S. W. Luo and X. Wu, Tetrahedron: Asymmetry, 2015, 26, 219 CrossRef CAS; (h) Y. F. Wong, Z. Wang and J. Sun, Org. Biomol. Chem., 2016, 14, 5751 RSC.
- M. Nambo and C. M. Crudden, ACS Catal., 2015, 5, 4734 CrossRef CAS.
- For selected examples, see (a) G. A. Molander and M. D. Elia, J. Org. Chem., 2006, 71, 9198 CrossRef CAS PubMed; (b) T. Niwa, H. Yorimitsu and K. Oshima, Org. Lett., 2007, 9, 2373 CrossRef CAS PubMed; (c) J. Y. Yu and R. Kuwano, Org. Lett., 2008, 10, 973 CrossRef CAS PubMed; (d) J. Zhang, A. Bellomo, A. D. Creamer, S. D. Dreher and P. J. Walsh, J. Am. Chem. Soc., 2012, 134, 13765 CrossRef CAS PubMed; (e) A. Bellomo, J. Zhang, N. Trongsiriwat and P. J. Walsh, Chem. Sci., 2013, 4, 849 RSC; (f) S. C. Matthew, B. W. Glasspoole, P. Eisenberger and C. M. Crudden, J. Am. Chem. Soc., 2014, 136, 5828 CrossRef CAS PubMed; (g) J. Zhang, A. Bellomo, N. Trongsiriwat, T. Jia, P. J. Carroll, S. D. Dreher, M. T. Tudge, H. Yin, J. R. Robinson, E. J. Schelter and P. J. Walsh, J. Am. Chem. Soc., 2014, 136, 6276 CrossRef CAS PubMed; (h) M. Nambo and C. M. Crudden, Angew. Chem., Int. Ed., 2014, 53, 742 CrossRef CAS PubMed; (i) M. Nambo, M. Yar, J. D. Smith and C. M. Crudden, Org. Lett., 2015, 17, 50 CrossRef CAS PubMed.
- (a) B. L. H. Taylor, M. R. Harris and E. R. Jarvo, Angew. Chem., Int. Ed., 2012, 51, 7790 CrossRef CAS PubMed; (b) M. R. Harris, L. E. Hanna, M. A. Greene, C. E. Moore and E. R. Jarvo, J. Am. Chem. Soc., 2013, 135, 3303 CrossRef CAS PubMed; (c) Q. Zhou, H. D. Srinivas, S. Dasgupta and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 3307 CrossRef CAS PubMed.
- (a) X. Zhao, G. Wu, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2011, 133, 3296 CrossRef CAS PubMed; (b) Y. Y. Sun, J. Yi, X. Lu, Z. Q. Zhang, B. Xiao and Y. Fu, Chem. Commun., 2014, 50, 11060 RSC.
- Y. Huang and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 7556 CrossRef CAS PubMed.
- X. Ji, T. Huang, W. Wu, F. Liang and S. Cao, Org. Lett., 2015, 17, 5096 CrossRef CAS PubMed.
- (a) P. Arde, B. T. Ramanjaneyulu, V. Reddy, A. Saxena and R. V. Anand, Org. Biomol. Chem., 2012, 10, 848 RSC; (b) P. Arde, V. Reddy and R. V. Anand, RSC Adv., 2014, 4, 49775 RSC; (c) B. T. Ramanjaneyulu, S. Mahesh and R. V. Anand, Org. Lett., 2015, 17, 6 CrossRef CAS PubMed; (d) B. T. Ramanjaneyulu, S. Mahesh and R. V. Anand, Org. Lett., 2015, 17, 3952 CrossRef CAS PubMed.
- V. Reddy and R. V. Anand, Org. Lett., 2015, 17, 3390 CrossRef CAS PubMed.
- For selected recent examples, where p-QMs has been utilised as an electrophile, see: (a) W. D. Chu, L. F. Zhang, X. Bao, X. H. Zhao, C. Zeng, J. Y. Du, G. B. Zhang, F. X. Wang, X. Y. Ma and C. A. Fan, Angew. Chem., Int. Ed., 2013, 52, 9229 CrossRef CAS PubMed; (b) L. Caruana, F. Kniep, T. K. Johansen, P. H. Poulsen and K. A. Jørgensen, J. Am. Chem. Soc., 2014, 136, 15929 CrossRef CAS PubMed; (c) K. Gai, X. Fang, X. Li, A. Lin and H. Yao, Chem. Commun., 2015, 51, 15831 RSC; (d) A. Lopez, A. Parra, C. Jarava-Barrera and M. Tortosa, Chem. Commun., 2015, 51, 17684 RSC; (e) Z. Yuan, X. Fang, X. Li, J. Wu, H. Yao and A. Lin, J. Org. Chem., 2015, 80, 11123 CrossRef CAS PubMed; (f) A. Parra and M. Tortosa, ChemCatChem, 2015, 7, 1521 CrossRef; (g) Y. Lou, P. Cao, T. Jia, Y. Zhang, M. Wang and J. Liao, Angew. Chem., Int. Ed., 2015, 54, 12134 CrossRef CAS PubMed; (h) L. Caruana, M. Fochi and L. Bernardi, Molecules, 2015, 20, 11733 CrossRef CAS PubMed; (i) F. S. He, J. H. Jin, Z. T. Yang, X. Yu, J. S. Fossey and W. P. Deng, ACS Catal., 2016, 6, 652 CrossRef CAS; (j) K. Zhao, Y. Zhi, A. Wang and D. Enders, ACS Catal., 2016, 6, 657 CrossRef CAS.
- (a) Y. J. Kim and A. Streitwieser, J. Am. Chem. Soc., 2002, 124, 5757 CrossRef CAS PubMed; (b) T. L. Amyes, S. T. Diver, J. P. Richard, F. M. Rivas and K. Toth, J. Am. Chem. Soc., 2004, 126, 4366 CrossRef CAS PubMed; (c) R. S. Massey, C. J. Collett, A. G. Lindsay, A. D. Smith and A. C. O'Donoghue, J. Am. Chem. Soc., 2012, 134, 20421 CrossRef CAS PubMed.
- For selected reviews, see: (a) D. Enders, O. Niemeier and A. Hanseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (b) E. M. Phillips, A. Chan and K. A. Scheidt, Aldrichimica Acta, 2009, 42, 55 CAS; (c) P. C. Chiang and J. W. Bode, TCI MAIL, 2011, 149, 2 Search PubMed; (d) A. T. Biju, N. Kuhl and F. Glorius, Acc. Chem. Res., 2011, 44, 1182 CrossRef CAS PubMed; (e) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336 RSC; (f) C. D. Campbell, K. B. Ling and A. D. Smith, in N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis, ed. C. S. J. Cazin, Springer, Dortrecht, 2011, vol. 32, p. 262 Search PubMed; (g) S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906 RSC; (h) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed; (i) D. M. Flanigan, R. Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed.
- (a) G. A. Grasa, R. M. Kissling and S. P. Nolan, Org. Lett., 2002, 4, 3583 CrossRef CAS PubMed; (b) T. Kano, K. Sasaki and K. Maruoka, Org. Lett., 2005, 7, 1347 CrossRef CAS PubMed; (c) M. Movassaghi and K. A. Schmidt, Org. Lett., 2005, 7, 2453 CrossRef CAS PubMed.
- T. Boddaert, Y. Coquerel and J. Rodriquez, Adv. Synth. Catal., 2009, 351, 1744 CrossRef CAS.
- J. Chen and Y. Huang, Nat. Commun., 2014, 5, 4437 Search PubMed.
- E. M. Phillips, M. Reidrich and K. Scheidt, J. Am. Chem. Soc., 2010, 132, 13179 CrossRef CAS PubMed.
- Q. Kang and Y. Zhang, Org. Biomol. Chem., 2011, 9, 6715 CAS.
- M. Hans, L. Delaude, J. Rodriquez and Y. Coquerel, J. Org. Chem., 2014, 79, 2758 CrossRef CAS PubMed.
- J. J. Song, Z. Tan, J. T. Reeves, D. R. Fandrick, N. K. Yee and C. H. Senanayake, Org. Lett., 2008, 10, 877 CrossRef CAS PubMed.
- X. W. Fan and Y. Cheng, Org. Biomol. Chem., 2012, 10, 9079 CAS.
- P. Arde and R. V. Anand, Org. Biomol. Chem., 2016, 14, 5550 CAS.
- It has been reported in the literature that HFIP helps in improving the enantioselectivity in some specific cases. Although the exact function of HFIP is not well understood, it is believed that HFIP stabilises the transition state through a strong hydrogen bond. For examples, see: (a) A. Berkessel, J. A. Adrio, D. Hüttenhain and J. M. Neudörfl, J. Am. Chem. Soc., 2006, 128, 8421 CrossRef CAS PubMed; (b) I. A. Shuklov, N. V. Dubrovina and A. Börner, Synthesis, 2007, 2925 CrossRef CAS; (c) B. Satpathi and S. S. V. Ramasastry, Angew. Chem., Int. Ed., 2016, 55, 1777 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, spectra. CCDC 1452070. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11116e |
| This journal is © The Royal Society of Chemistry 2016 |