Molecular size reforming of undersized and oversized polyoxymethylene dimethyl ethers†
Yanyan Zheng,
Fang Liu,
Liang Guo,
Tiefeng Wang* and
Jinfu Wang*
Beijing Key Laboratory of Green Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China. E-mail: wangtf@tsinghua.edu.cn; wangjfu@tsinghua.edu.cn
First published on 9th August 2016
Abstract
Polyoxymethylene dimethyl ethers (CH3–O–(CH2O)n–CH3, PODEn) are potential environmentally benign coal-based diesel fuel blending compounds. They are synthesized from dimethoxymethane (DMM) and paraformaldehyde (PF). Among the PODEn homologues, PODE3–4 have a good match with diesel as a fuel, while the undersized PODE1–2 and oversized PODEn>4 have unsuitable properties. This work studied the molecular size reforming of undersized PODE1–2 and oversized PODEn>4 by two different methods, namely, self-reforming and reacting with DMM over an acidic ion exchange resin. The molecular size reforming of PODE1–2 and PODEn>4 by self-reforming gave a high concentration of formaldehyde, which shifted the distribution to longer chain PODEn and formed solid PF. In contrast, PODE1–2 and PODEn>4 were mainly converted to PODE3–4 by the reaction with DMM and the high concentration of formaldehyde was also diminished. The equilibrium reorganized molecular size distribution of PODE1–2 and PODEn>4 followed the Schulz–Flory distribution. A proposed kinetic model described well the molecular size reforming pathways of PODE1–2 and PODEn>4. A methanol-to-PODEn close-loop process was proposed to enhance atom-economy by recycling the PODE1–2 and PODEn>4 streams.
1. Introduction
The coal chemical industry is oriented to the development of high efficiency, safety, cleanliness, and optimum utilization. Coal-based compounds as alternative fuels or chemicals are receiving increasing attention for alleviating environmental pollution and reducing dependence on petroleum.1,2 Methanol is a key intermediate chemical in several important coal-to-chemical processes, such as methanol-to-olefins (MTO),3,4 methanol-to-propylene (MTP)5,6 and methanol-to-aromatics (MTA).7,8 Recently, the methanol-to-oxygenated fuel9–12 process has become an important route to covert coal to fuel blending compounds. Among these, polyoxymethylene dimethyl ethers (PODEn) are the most promising green diesel fuel blending compounds because diesel engines can use these without needing to be modified.11–14 Particulate pollutants in the combustion of a mixture of PODEn and diesel are significantly reduced due to the oxygen content in PODEn.15,16 Considering the properties of PODEn-diesel blending oil, especially the density, heating value and combustion emissions, the proposed blending ratio of PODEn in diesel is 10–20%.PODEn refers to the compounds with the formula CH3–O–(CH2O)n–CH3, and PODE1 is dimethoxymethane (DMM, CH3OCH2OCH3). PODEn can be synthesized from an end-group provider (DMM or methanol) and a chain-group provider (trioxane, paraformaldehyde or formaldehyde solution). The potential feedstocks are all important derivatives of methanol. Table 1 gives a comparison of the MTO, MTP and MTA processes and the methanol-to-PODE (MTPODE) process in terms of the reaction temperature, pressure, catalyst, reactor, and feedstock consumption. MTO, MTP and MTA processes are typically operated at a higher temperature than the MTPODE process, thus they have higher energy consumption. The methanol consumption (t methanol/t product) is a key parameter used to measure the economics of a process. The methanol consumption of the MTPODE process is 1.3–1.4 t methanol/t product, which is much lower than that in the MTO, MTP and MTA processes. The methanol consumption was calculated based on a MTPODE process developed by our group11,17–19 and shown in Fig. 1. In the MTPODE process, methanol is oxidized to formaldehyde, which is condensed to paraformaldehyde (PF) or trioxane. Then methanol reacts with formaldehyde to form dimethoxymethane (DMM, CH3OCH2OCH3). Finally, DMM reacts with PF or trioxane to produce the PODEn compounds.
| Technology | Temperature/°C | Pressure/MPa | Catalyst | Reactor | Methanol consumption/t methanol per (t product) |
|---|---|---|---|---|---|
| a Methanol-to-formaldehyde section.b Methanol reacts with formaldehyde to DMM section.c DMM reacts with formaldehyde to PODE section. | |||||
| MTO (UOP) | 350–550 | 0.1–0.5 | SAPO-34 | Fluidized-bed | 2.85 |
| MTO (OCP) | 420–480 | 0.276 | SAPO-34 | Fast-fluidized/fixed-bed | 2.54 |
| MTP (Lurgi) | 460–480 | 0.16 | ZSM-5 | Fixed-bed | 3.22–3.52 |
| FMTP | 380–450 | 0.2–0.3 | SAPO-34 | Fluidized-bed | 3.36 |
| MTA (Tsinghua) | 400–500 | 0.1 | ZSM-5 | Fluidized-bed | 2.35 |
| MTPODE (Tsinghua) | 300–350a | 0.05–0.10a | Fe–Moa | Fixed beda,b | 1.30–1.40 |
| 70–90b,c | 0.2–0.3b,c | Solid acidb,c | Fluidized-bedc | ||
 | ||
| Fig. 1 Methanol consumption in the methanol-to-PODE (MTPODE) process (ME: methanol; FA: formaldehyde; DMM: dimethoxymethane; PF: paraformaldehyde). | ||
The fuel properties of the PODEn homologues (n from 1 to 8) are compared with those of commercial diesel in Table 2.20–22 Among the PODEn homologues, PODE3–4 match the diesel fuel properties well except for a higher density. However, PODE1–2 do not meet a security criterion and would cause vapour lock during combustion due to their low flash point. Furthermore, their low viscosity will cause wear of the fuel injection pump. PODEn>4 have poor low temperature fluidity, high melting points and high viscosity, which significantly affect their use in an ambient environment. Our previous work23 showed that the formation of PODEn from DMM and paraformaldehyde followed a sequential propagation mechanism. The molecular size distribution of the PODEn compounds obeyed the Schulz–Flory distribution.23 Zhao et al.24 also verified the application of Schulz–Flory distribution model for molecular size distribution during the synthesis of PODEn. Besides the targeted PODE3–4 compounds, a significant amount of undersized PODE1–2 and oversized PODEn>4 compounds were also produced in the reported processes,22,24–28 as listed in Table 3. Therefore, the molecular size reforming of PODE1–2 and PODEn>4 to give the targeted PODE3–4 compounds by recycling the byproducts is needed in the industrial process, which was also the basis and precondition for the methanol consumption calculation in Fig. 1.
| Diesel (ASTM D 975) | DMM | PODE2 | PODE3 | PODE4 | PODE5 | PODE6 | PODE7 | PODE8 | |
|---|---|---|---|---|---|---|---|---|---|
| a Temperature equivalent to 90% of distilled volume. | |||||||||
| Density at 25 °C/g cm−3 | 0.80–0.86 | 0.86 | 0.96 | 1.02 | 1.07 | 1.10 | 1.13 | 1.16 | 1.20 |
| Melting point/°C | — | −105 | −65 | −41 | −7 | 18.5 | 58 | — | — |
| Boiling point/°C | 288a | 42 | 105 | 156 | 201 | 242 | 280 | — | — |
| Viscosity at 25 °C/mPa s | 1.04–1.92 | 0.58 | 0.64 | 1.05 | 1.75 | 2.79 | 4.14 | 5.81 | 7.80 |
| Cetane number | 40–65 | 29 | 63 | 78 | 90 | 100 | 104 | — | — |
| Oxygen content/wt% | — | 42.1 | 45.3 | 47.1 | 48.2 | 49.0 | 49.6 | 50.0 | 50.3 |
| Ref. | Temperature/K | Feedstocks | Catalyst | Reactor | Molecular size distribution/wt% | ||
|---|---|---|---|---|---|---|---|
| PODE1–2 | PODE3–5 | PODEn>5 | |||||
| 22 | 353 | Tri + DMM(nDMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 1.38 nCH2O = 1.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Amberlyst 36 | Batch autoclave | 75.75 | 22.95 | 1.29 |
| 23 | 373 | PF + DMM(nDMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 2 nCH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
H2SO4 | Batch autoclave | 67.00 | 29.39 | 3.61 |
| 24 | 353 | MeOH + CH2O(nMeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 1 nCH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Acid cation exchange resin | Fixed-bed | 40.84 | 50.22 | 8.94 |
| 25 | 353 | Tri + DMM(nDMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 1.19 nCH2O = 1.19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Amberlyst 46 | Batch autoclave | 65.05 | 30.15 | 4.80 |
| 26 | 403 | MeOH + tri(nMeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 1 nCH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
S/Fe | Batch autoclave | 49.44 | 41.70 | 8.87 |
| 27 | 443 | Tri + DMM(nDMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 1 nCH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Alkanesulfonic acid-functionalized ionic liquids | Batch autoclave | 57.49 | 34.81 | 7.70 |
To recycle undesired PODEn compounds, Burger et al.22,29 proposed to feed the unconverted reactants and the PODEn of undesired chain lengths (n = 1, 2 or >4) back into the reactor in a PODEn production process using DMM and trioxane as feedstock. They supposed that PODE1–2 underwent chain propagation reactions while PODEn>4 were cleaved when they were fed back into the reactor. However, a significant amount of long chain PODEn compounds, even PODEn>10, were formed in the industrial process,29 which indicate that there were different reforming pathways for the PODE1–2 and PODEn>4 compounds.
The present work is to develop an effective method for recycling the PODE1–2 and PODEn>4 compounds produced in the MTPODE process. The molecular size reforming of the undersized PODE1–2 and oversized PODEn>4 compounds by the two different reactions of self-reforming and reaction with DMM over an acidic ion exchange resin was investigated. A previously developed kinetic model was used for the description of the molecular size reforming pathway. The equilibrium reorganized molecular size distribution of PODE1–2 and PODEn>4 was found to follow the Schulz–Flory distribution. Based on the experimental results, a MTPODE close-loop process was proposed for recycling the undersized PODE1–2 and oversized PODEn>4 compounds.
2. Experimental
2.1 Materials
The PODE1–2 and PODEn>4 samples were obtained from a 10kt/a PODEn demonstration plant using the technology developed by our group in Shandong Yuhuang Chemical (Group) Co., Ltd. in China. Previous works19,27 showed that water is a compound that causes side reactions when producing PODEn. Therefore, anhydrous feedstocks of DMM and PF were used in the 10kt/a PODEn demonstration plant.DMM (analytic reagent grade, >99 wt%) was purchased from Alfa Aesar-Johnson Matthey. PF (polymer grade, >96 wt%) was purchased from Sinopharm Chemical Reagent Co., Ltd. The NKC-9 ion exchange resin of H+ type was provided by Tianjin Bohong Resin Technology Co., Ltd.
2.2 Experimental
The experiments were conducted in a batch stirred autoclave to study the molecular size reforming of PODE1–2 and PODEn>4 by self-reforming or reacting with DMM. A detailed description of the experimental setup was reported elsewhere.11,23 The NKC-9 ion exchange resin was used as the catalyst because it had high activity and good stability for PODEn production from PF and DMM.11,23For each experiment, the feedstocks (PODE1–2, PODEn>4 and DMM) were first loaded into the reactor. Then, the reactor was sealed and heated. When the reactor temperature reached the desired value, the catalyst was released from a glass bottle and uniformly dispersed by stirring at 500 rpm. This time was used as the initial reaction time. The products were sampled at different reaction times and quantitatively analysed by gas chromatography-mass spectrometry (GC-MS). A detailed description of the GC-MS analysis method was reported elsewhere.11,19,23,30 In brief, the method was established based on mass conservation without standard PODEn samples.30 The relative mass response factor of PODEn compounds could be described as f(PODEn) = 1.140n−1 with fPODE1 = 1.30 The carbon balance was within ±3%. Detailed carbon balance data were provided in the ESI.† The concentration of formaldehyde was determined by the sodium sulfite titration method described in ASTM D2194-02 (2012). More detailed description of calibration and good reproducibility of the sodium sulphite titration method in this system was reported elsewhere.19,31,32
3. Results and discussion
3.1 Reactions and molecular size distribution
In the reaction scheme for the formation of PODEn from DMM and PF catalysed by NKC-9 ion exchange resin, PF depolymerizes to monomeric formaldehyde and dissolves in the liquid phase due to the acidic catalyst (reaction (1)). PODEn reacts with monomeric formaldehyde to form longer chain molecules PODEn+1 (reaction (2)). This scheme was supported by the experimental results that PODE2, PODE3, PODE4, etc. appeared one by one in the system.11,15 Burger22,27 proposed a similar scheme for the formation of PODEn from DMM and trioxane.
 | (1) |
 | (2) |
The sequential propagation mechanism leads to a Schulz–Flory equilibrium molecular size distribution described by eqn (3).23
 | (3) |
 | (4) |
The molecular size distribution of PODEn is determined by the reaction temperature and effective molar ratio of DMM/CH2O. An equivalent DMM/CH2O molar ratio Meq. is defined as:
 | (5) |
In principle, the propagation of PODEn by insertion of formaldehyde is similar after formaldehyde has been produced in the same concentration from decomposition of trioxane or paraformaldehyde. However, the decomposition rate and equilibrium of trioxane and paraformaldehyde are different, which result in different formaldehyde concentration in solution. The decomposition of formaldehyde may depend on its polymerization degree. Xia et al.33 set the degree of paraformaldehyde polymerization as 3 to reduce the computational cost. However, the paraformaldehyde used in this work had a polymerization degree of 8–100. In addition, Xia et al.33 used sulfonic acid-functionalized ionic liquids as catalyst while this work used cation exchange resin. Due to these factors, the sequential formation of PODEn was observed in this work, different from the mechanism suggested by Xia et al.33
According to the reaction mechanism, the production of PODE1–2 and PODEn>4 is unavoidable in a single pass PODEn synthesis system. Fig. 2 shows a block flow diagram of the first stage PODEn demonstration process using DMM and PF as feedstock. In this process, DMM and PF were fed into a fluidized bed reactor to produce PODEn using the strong acid ion exchange resin as catalyst. After reaction, the reaction mixture was fed into a rectifying sequence and separated into different fractions. The DMM stream was separated as the overhead fraction in the pre-distillation tower and was directly fed back into the reactor. The side withdrawal mainly contained PODE2 and a portion of the DMM (PODE1), thus it was named as the undersized PODE1–2 stream. A portion of unreacted formaldehyde was dissolved in the DMM stream and PODE1–2 stream. The remaining formaldehyde from the reaction mixture was separated by extractive distillation using water as the extractant. Formaldehyde and water formed azeotrope at top of the extractive distillation tower. The extracted formaldehyde solution was used as feedstock to produce DMM. The desired PODE3–4 stream and oversized PODEn>4 stream were separated in a vacuum distillation tower.
In our first stage PODEn demonstration process, the yield of PODE1–2 and PODEn>4 stream were 33.15 wt% and 1.68 wt% under the typical conditions of feeding ratio nDMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nCH2O = 2
nCH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reaction temperature T = 80 °C. As listed in Table 4, the PODE1–2 stream contained 13.68 wt% PODE1, 73.14 wt% PODE2, 2.30 wt% PODE3 and 10.88 wt% formaldehyde, and the PODEn>4 stream contained 73.48 wt% PODE5 and 26.52 wt% PODE6.
1 and reaction temperature T = 80 °C. As listed in Table 4, the PODE1–2 stream contained 13.68 wt% PODE1, 73.14 wt% PODE2, 2.30 wt% PODE3 and 10.88 wt% formaldehyde, and the PODEn>4 stream contained 73.48 wt% PODE5 and 26.52 wt% PODE6.
| Stream | Formaldehyde/% | PODE1/% | PODE2/% | PODE3/% | PODE4/% | PODE5/% | PODE6/% |
|---|---|---|---|---|---|---|---|
| PODE1–2 | 10.88 | 13.68 | 73.14 | 2.30 | 0.00 | 0.00 | 0.00 |
| PODEn>4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 73.48 | 26.52 |
3.2 Molecular size self-reforming of PODE1–2 and PODEn>4
To recycle the PODE1–2 and PODEn>4 streams, the chain propagation of PODE1–2 and depolymerization of PODEn>4 towards PODE3–4 are the desired reaction pathways. The self-reforming experiments were conducted in an autoclave reactor at 80 °C with 5 wt% NKC-9 catalyst for both the PODE1–2 and PODEn>4 streams. The equivalent DMM/CH2O molar ratio Meq. was 0.82 and 0.24 for the PODE1–2 and PODEn>4 streams, respectively.The molecular size distribution of the products from PODE1–2 self-reforming is shown in Fig. 3. Fig. 3(A) shows the mass fractions of PODE1–6 and formaldehyde as a function of reaction time. The main species, PODE2, was quickly consumed and its fraction decreased from 72.85 wt% to 24.39 wt% in the first 10 min. On the one hand, PODE2 decomposed into DMM and formaldehyde; on the other hand, PODE2 reacted with formaldehyde forming PODE3. The concentration of formaldehyde increased from 10.88 wt% to 18.26 wt% in 10 min, indicating that the depolymerization reaction was faster than the chain propagation reaction during this period. The concentration of PODE3 showed a peak value of 9.84 wt% at 5 min. After 5 min, the amount of PODE3 decreased and correspondingly, there was a sequential increase of PODE4–6. Note that the amount of PODEn>3 was 2.51 wt% at 10 min due to a low initial amount. Overall, the self-reforming of the PODE1–2 stream mainly proceeded in the depolymerization direction, which formed large amounts of DMM and formaldehyde. Due to the formation of formaldehyde, the molecular size distribution shifted to longer chain PODEn compounds.27
Fig. 3(B) shows the relationship between ln(wn/(n + 1.533)) and n at different reaction times, where wn is the mass fraction of PODEn. The measured equilibrium molecular size distribution followed the Schulz–Flory distribution with a = 0.537 in eqn (3). In this system, the factor a was determined by the reaction temperature and equivalent DMM/CH2O molar ratio. During the molecular size self-reforming process, the data points of ln(wn/(n + 1.533)) gradually shifted to the equilibrium line with a = 0.537.
A kinetic model was proposed in our previous work19 based on the sequential propagation mechanism of PODEn, and assuming second order kinetics for propagation and first order kinetics for depolymerization. The rate constants for propagation (kp) and depolymerization (kd) and the reaction equilibrium constants (Kn) were found to be independent of molecular size. The pre-exponential factors Ap for propagation and Ad for depolymerization were 1.84 × 104 L mol−1 min−1 and 5.36 × 106 min−1, respectively. The activation energy Ep for propagation and Ed for depolymerization were 39.52 kJ mol−1 and 52.01 kJ mol−1, respectively. The concentration of PODE1–6 at different reaction times were calculated from the initial reactant concentrations using the kinetic model. During model calculation, the concentration of formaldehyde in homogenous solution (CF) was used as an input parameter, as illustrated in our previous work.19 In most cases the CF become constant within 10 min in this work, and the calculated molecular size distribution was found insensitive to the CF values, therefore the CF was regarded as constant during the kinetic calculations. The CF data used in the calculations were annotated below the corresponding figures. Good agreement was obtained between the experimental and calculated weight fractions of PODE1–6, as shown in Fig. 3(C) and (D). While a similar pseudo-homogeneous model was not capable to describe the formation of PODEn from DMM and trioxane, indicating the differences between trioxane and paraformaldehyde when reacting with DMM.19
It is difficult to analyse the molecular size distribution during the PODEn>4 self-reforming process, because the reaction mixture became a slurry in several minutes due to the formation of a large amount of CH2O. In this case, the kinetic model was used to predict the molecular size distribution, as shown in Fig. 4. Fig. 4(A) shows the mass fractions of PODE1–8 and formaldehyde as a function of reaction time. PODE5 and PODE6 were quickly consumed and their concentrations decreased from 74.48 wt% and 25.52 wt% to 31.39 wt% and 19.41 wt% in 10 min, respectively. Meanwhile the mass fraction of formaldehyde increased from zero to 9.10 wt%. The maximum concentrations of PODE4 (21.23 wt%) and PODE7 (6.61 wt%) appeared at 10 min. The polymerization of formaldehyde produced some solid PF powder, which were suspended in the reaction solution and increased the viscosity of the suspension. PODE3 and PODE8 showed their maximum concentrations at 30 min. At equilibrium, the mass fractions of DMM and PODE2 increased to 21.32 wt% and 12.73 wt%, respectively, and the total mass fraction of formaldehyde, including both the dissolved formaldehyde and solid PF, increased to 51.33 wt%. As a result, the molecular size self-reforming of the PODEn>4 stream formed a large amount of PODE1–2 and formaldehyde and a small amount of PODE7–8 (0.76 wt%). Fig. 4(B) shows the variation of ln(wn/(n + 1.533)) as a function of n at different reaction times. The equilibrium molecular size distribution agreed well with the Schulz–Flory model with a = 0.801. The calculated mass fractions of PODE1–8 during the self-reforming of PODEn>4 is shown in Fig. 4(C).
The direct recycling of PODE1–2 and PODEn>4 stream to the reactor decreased the value of Meq., thus effecting a change from the equilibrium molecular size distribution. A high concentration of formaldehyde shifted the distribution to longer chain PODEn with n even larger than 10,27,29 and also prevented the depolymerization of fresh PF feed. This accounts for the results reported by Burger et al.29 that a significant amount of long chain PODEn compounds, even PODEn>10, were formed in a process that recycled the PODE1–2 and PODEn>4 streams to the reactor. The resulting high concentration of solid PF would deteriorate the fluidization quality when a fluidized bed reactor is used.
3.3 Molecular size reforming by reacting with DMM
Based on the self-reforming results discussed in Section 3.2, we proposed a new approach to change the molecular size distribution by reacting PODE1–2 or PODEn>4 with DMM. In this approach, the amount of added fresh DMM was the most important parameter. In this work, the PODE1–2 and PODEn>4 streams were respectively mixed with DMM to form new streams with Meq. = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reaction temperature was 80 °C and the NKC-9 catalyst loading was 5 wt%.
1. The reaction temperature was 80 °C and the NKC-9 catalyst loading was 5 wt%.
The molecular size distributions of the products of PODE1–2 reforming with DMM are shown in Fig. 5. Fig. 5(A) shows the mass fractions of PODE1–6 and formaldehyde as a function of reaction time. The concentration of PODE2 quickly decreased from 36.80 wt% to 26.11 wt% in 10 min, and the desired conversion, namely, to PODE3, was dominant. The concentration of DMM slightly increased first and showed its peak value of 60.19 wt% at 10 min. Meanwhile the concentration of formaldehyde slightly increased from 5.56 wt% to 6.06 wt%, indicating that the chain propagation reactions were slower than the reversed depolymerization reactions during this period. After 10 min, the concentration of DMM gradually decreased to its initial level. At equilibrium, almost all converted PODE2 compound underwent chain propagation to PODE3–6, among which PODE3–4 had a selectivity of 87.05 wt%. Finally, the concentration of formaldehyde decreased to 1.91 wt%. Fig. 5(B) shows the plots of ln(wn/(n + 1.533)) as a function of n at different reaction times. The equilibrium molecular size distribution data at Meq. = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 agreed well with the Schulz–Flory model with ae = 0.438. This was very close to the experimental value ae = 0.444 with DMM and PF at the DMM/CH2O molar ratio 2
1 agreed well with the Schulz–Flory model with ae = 0.438. This was very close to the experimental value ae = 0.444 with DMM and PF at the DMM/CH2O molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reported in our previous work.19 This indicated that the factor ae is determined by Meq. of a stream and is independent of the stream composition at a specific temperature. The comparison of the experimental and calculated molecular size distribution of PODE1–6 and the corresponding parity plot were shown in Fig. 5(C) and (D), respectively. Good agreement was obtained. Compared to the molecular size distribution of the products of PODE1–2 self-reforming (Fig. 3), the reaction of the PODE1–2 stream with DMM at Meq. = 2
1 reported in our previous work.19 This indicated that the factor ae is determined by Meq. of a stream and is independent of the stream composition at a specific temperature. The comparison of the experimental and calculated molecular size distribution of PODE1–6 and the corresponding parity plot were shown in Fig. 5(C) and (D), respectively. Good agreement was obtained. Compared to the molecular size distribution of the products of PODE1–2 self-reforming (Fig. 3), the reaction of the PODE1–2 stream with DMM at Meq. = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mainly converted PODE2 to the desired products PODE3–4, and the high concentration of formaldehyde was also diminished.
1 mainly converted PODE2 to the desired products PODE3–4, and the high concentration of formaldehyde was also diminished.
Fig. 6 illustrates the experimental results of the reforming of the PODEn>4 stream when reacted with DMM. The mass fractions of PODE1–8 and formaldehyde at different reaction times are shown in Fig. 6(A). PODE5 and PODE6 were quickly consumed and were mainly converted to the desired PODE2–4. In the first 10 min, the mass fraction of DMM decreased from 73.72 wt% to 58.44 wt%, and the concentration of formaldehyde slightly increased from zero to 1.90 wt%. The concentration of PODE7–8 showed a small peak value at 20–30 min and decreased to the equilibrium value of 0.37 wt%, indicating an effective control of chain propagation to long chain PODEn. The final concentration of formaldehyde was 2.48 wt%. For a further analysis of the molecular size distribution, ln(wn/(n + 1.533)) was plotted as a function of n at different reaction times in Fig. 6(B). The equilibrium molecular size distribution data at Meq. = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were well described by eqn (3) with ae = 0.441, which was very close to the experimental value ae = 0.444 at the same conditions.19 Good agreement between the experimental data and the proposed kinetic model19 are shown in Fig. 6(C) and (D). Different from the self-reforming of PODEn>4 stream (Fig. 4), the reforming of the PODEn>4 stream by reaction with DMM at Meq. 2
1 were well described by eqn (3) with ae = 0.441, which was very close to the experimental value ae = 0.444 at the same conditions.19 Good agreement between the experimental data and the proposed kinetic model19 are shown in Fig. 6(C) and (D). Different from the self-reforming of PODEn>4 stream (Fig. 4), the reforming of the PODEn>4 stream by reaction with DMM at Meq. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mainly converted PODE5–6 to the desired product PODE2–4.
1 mainly converted PODE5–6 to the desired product PODE2–4.
3.4 Conceptual close-loop MTPODE process
Based on the experimental results discussed in Sections 3.2 and 3.3, different PODEn streams with the same Meq. gave the same equilibrium molecular size distribution after reaction. Therefore, after the reforming of PODE1–2 and PODEn>4 by reaction with DMM at Meq. = 2, these byproducts can be recycled by feeding the equilibrium products directly into the rectifying sequence without disturbing the operation stability of the system. Compared with the process in Fig. 2, an improved process of close-loop MTPODE is proposed, as shown in Fig. 7. In this process, methanol is the sole raw material. First, methanol is oxidized to formaldehyde (in the solution state) which is then condensed to paraformaldehyde powder (PF). Then methanol reacts with formaldehyde in an acetalation reactor (for example a catalytic distillation tower) to produce DMM. The DMM and PF are fed into the PODEn synthesis reactor I. The PODEn products and unreacted feedstocks are fed into a rectifying sequence to separate the DMM, PODE1–2, FA, PODE3–4 and PODEn>4 streams, similar to that described in Fig. 2. The FA solution from the FA separation tower is fed back to the acetalation reactor and the PODE1–2 and PODEn>4 streams are fed into the PODEn synthesis reactors II and III, respectively. The products from the PODEn synthesis reactors II and III are fed into the rectifying sequence for separation. This process is a closed cycle because the desired PODE3–4 is the only product. Compared to the first stage PODEn process in Fig. 2, the close-loop MTPODE process in Fig. 7 would achieve the recycling of PODE1–2 and PODEn>4 and give PODE3–4 as the only product. This would give an advantage to the MTPODE process in terms of methanol consumption as shown in Fig. 1. The close-loop MTPODE process in Fig. 7 had been designed for a 300kt/a PODEn plant in China.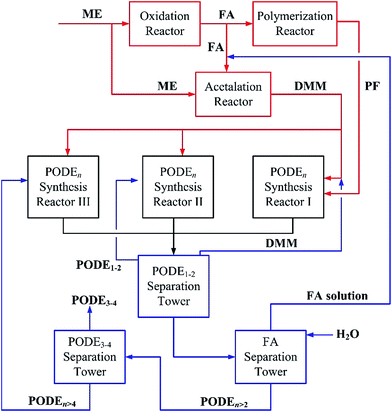 | ||
| Fig. 7 Schematic of the proposed methanol-to-PODE recycling process. (ME: methanol, FA: formaldehyde, PF: paraformaldehyde, DMM: dimethoxymethane, PODE: polyoxymethylene dimethyl ethers). | ||
4. Conclusions
The molecular size reforming of undersized PODE1–2 and oversized PODEn>4 compounds by the two reactions of self-reforming and reaction with DMM over an acidic ion exchange resin was studied. Molecular size self-reforming of the PODE1–2 and PODEn>4 streams resulted in a high concentration of formaldehyde, which shifted the distribution to longer chain PODEn and formed solid PF. Molecular size reforming of the PODE1–2 and PODEn>4 streams by reaction with DMM with Meq. at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gave the desired reactions, i.e., chain propagation of PODE1–2 and depolymerization of PODEn>4 to give PODE3–4. The use of the byproducts of a MTPODE process by recycling the PODE1–2 and PODEn>4 streams to react with DMM in independent reactors and then feeding the equilibrium products directly into the rectifying sequence was proposed. The proposed kinetic model gave good descriptions of the molecular size distribution of the production of PODE1–2 and PODEn>4 reforming.
1 gave the desired reactions, i.e., chain propagation of PODE1–2 and depolymerization of PODEn>4 to give PODE3–4. The use of the byproducts of a MTPODE process by recycling the PODE1–2 and PODEn>4 streams to react with DMM in independent reactors and then feeding the equilibrium products directly into the rectifying sequence was proposed. The proposed kinetic model gave good descriptions of the molecular size distribution of the production of PODE1–2 and PODEn>4 reforming.
Conflict of interest
The authors declare no competing financial interest.Acknowledgements
We acknowledge the help of professor Dezheng Wang and the financial support by the China Postdoctoral Science Foundation funded project (2015M570113) and the National Natural Science Foundation of China (No. 21476122).References
- G. Liu and E. D. Larson, Energy Procedia, 2014, 63, 7315–7329 CrossRef CAS.
- L. M. Balster, E. Corporan, M. J. DeWitt, J. T. Edwards, J. S. Ervin, J. L. Graham, S.-Y. Lee, S. Pal, D. K. Phelps and L. R. Rudnick, Fuel Process. Technol., 2008, 89, 364–378 CrossRef CAS.
- X. Aihua, W. Li and S. Yulin, Energy Fuels, 2014, 28, 3339–3344 CrossRef.
- J. Lefevere, S. Mullens, V. Meynen and J. Van Noyen, Chem. Pap., 2014, 68, 1143–1153 CAS.
- B. Jiang, X. Feng, L. Yan, Y. Jiang, Z. Liao, J. Wang and Y. Yang, Ind. Eng. Chem. Res., 2014, 53, 4623–4632 CrossRef CAS.
- A. K. Jamil, O. Muraza, M. Yoshioka, A. M. Al-Amer, Z. Yamani and T. Yokoi, Ind. Eng. Chem. Res., 2014, 53(50), 19498–19505 CrossRef CAS.
- H. Ji, Q. Zhang, B. Wang, C. Li and H. Shan, Catal. Lett., 2014, 144, 1860–1867 CrossRef CAS.
- G. Q. Zhang, T. Bai, T. F. Chen, W. T. Fan and X. Zhang, Ind. Eng. Chem. Res., 2014, 53, 14932–14940 CrossRef CAS.
- Q. Tang, H. Xu, Y. Zheng, J. Wang, H. Li and J. Zhang, Appl. Catal., A, 2012, 413, 36–42 CrossRef.
- K. D. Vertin, J. M. Ohi, D. W. Naegeli, K. H. Childress, G. P. Hagen, C. I. McCarthy, A. S. Cheng and R. W. Dibble, Methylal and methylal-diesel blended fuels for use in compression-ignition engines, SAE Technical Paper, 1999 Search PubMed.
- Y. Zheng, Q. Tang, T. Wang, Y. Liao and J. Wang, Chem. Eng. Technol., 2013, 36, 1951–1956 CrossRef CAS.
- M. Marchionna, R. Patrini, D. Sanfilippo, A. Paggini, F. Giavazzi and L. Pellegrini, Stud. Surf. Sci. Catal., 2001, 136, 489–494 CrossRef CAS.
- L. Pellegrini, M. Marchionna, R. Patrini, C. Beatrice, N. Del Giacomo and C. Guido, Combustion Behaviour and Emission Performance of Neat and Blended Polyoxymethylene Dimethyl Ethers in a Light-Duty Diesel Engine, SAE Technical Paper, 2012 Search PubMed.
- G. J. An, X. D. Wang, C. B. Lu, C. H. Xiong, Y. J. Zhou, Y. W. Liu and H. Y. Shang, Appl. Mech. Mater., 2014, 672–674, 676–679 CrossRef.
- Y. Ren, Z. Huang, H. Miao, Y. Di, D. Jiang, K. Zeng, B. Liu and X. Wang, Fuel, 2008, 87, 2691–2697 CrossRef CAS.
- M. Natarajan, E. A. Frame, D. W. Naegeli, T. Asmus, W. Clark, J. Garbak, A. Manuel, D. Gonzalez, E. Liney and W. Piel, Oxygenates for advanced petroleum-based diesel fuels: Part 1. Screening and selection methodology for the oxygenates, SAE Technical Paper, 2001 Search PubMed.
- J. Wang, Q. Tang, S. Wang, T. Wang, S. Chen and Y. Wang, Fluidized Bed Reactor and Method for Preparing Polyoxymethylene Dimethyl Ethers from Dimethoxymethane and Paraformaldehyde, US Pat., No. 20,150,273,426, 1, October 2015.
- J. Wang, Y. Zheng, S. Wang, T. Wang, S. Chen and C. Zhu, Method for Producing Polyoxymethylene Dimethyl Ethers, US Pat., 20,150,291,722[P], October 15 2015.
- Y. Zheng, Q. Tang, T. Wang and J. Wang, Chem. Eng. Sci., 2015, 134, 758–766 CrossRef CAS.
- R. H. Boyd, J. Polym. Sci., 1961, 50, 133–141 CrossRef CAS.
- J. Wu, Z. Xu, Z. Liu and B. Wang, J. Chem. Eng. Data, 2005, 50, 966–968 CrossRef CAS.
- J. Burger, M. Siegert, E. Ströfer and H. Hasse, Fuel, 2010, 89, 3315–3319 CrossRef CAS.
- Y. Zheng, Q. Tang, T. Wang and J. Wang, Chem. Eng. J., 2015, 278, 183–189 CrossRef CAS.
- Y. Zhao, Z. Xu, H. Chen, Y. Fu and J. Shen, J. Energy Chem., 2013, 22, 833–836 CrossRef CAS.
- J. Zhang, M. Shi, D. Fang and D. Liu, React. Kinet., Mech. Catal., 2014, 113, 459–470 CrossRef CAS.
- Q. Wu, M. Wang, Y. Hao, H. Li, Y. Zhao and Q. Jiao, Ind. Eng. Chem. Res., 2014, 53, 16254–16260 CrossRef CAS.
- J. Burger, E. Ströfer and H. Hasse, Ind. Eng. Chem. Res., 2012, 51, 12751–12761 CrossRef CAS.
- H. Li, H. Song, L. Chen and C. Xia, Appl. Catal., B, 2015, 165, 466–476 CrossRef CAS.
- J. Burger, E. Ströfer and H. Hasse, Chem. Eng. Res. Des., 2013, 91, 2648–2662 CrossRef CAS.
- Y. Zheng, Q. Tang, T. Wang and J. Wang, J. Chem. Eng. Chin. Univ., 2015, 29, 505–509 CAS.
- G. Maurer, AIChE J., 1986, 32, 932–948 CrossRef CAS.
- J. Kiernan, Microsc. Today, 2000, 1, 8–12 Search PubMed.
- F. Wang, G. Zhu, Z. Li, F. Zhao, C. Xia and J. Chen, J. Mol. Catal. A: Chem., 2015, 408, 228–236 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08255f |
| This journal is © The Royal Society of Chemistry 2016 |





