An improved miniprotein host for fluorogenic supramolecular assembly on the surface of living cells†
Bi Xu,
Xinqi Zhou and
Cliff I. Stains*
Department of Chemistry, University of Nebraska – Lincoln, Lincoln, NE 68588, USA. E-mail: cstains2@unl.edu
First published on 11th February 2016
Abstract
An improved miniprotein host capable of fluorogenic supramolecular assembly with a sI-Pht guest is reported. This improved host, termed 6.2.22, displays significant enhancements in both the EC50 for complexation and fluorescence activation of the sI-Pht guest, allowing for improved resolution of supramolecular complexation on the surface of living yeast.
Supramolecular assembly harnesses noncovalent interactions between a host–guest pair to allow for the controlled self-assembly of macromolecular complexes.1,2 This strategy has been employed in a wide variety of applications including the assembly of functional materials with tunable properties3–8 as well as the construction of sensors.9,10 However, the design of host–guest pairs that are compatible with living systems remains challenging.11 As a consequence, native protein-based hosts capable of rapamycin-,12 abscisic acid-,13 or gibberellic acid-induced14 assembly are commonly used in biological systems. In addition, rationally designed host–guest systems using naturally occurring small-molecule binding proteins have been used to control biological responses.15 While these systems provide a powerful means to manipulate biological functions,16–22 more work is needed in order to define multiple host–guest systems to allow for the control of multiple biological functions in an orthogonal manner. One potential solution to this problem would be the development of design principles that could afford non-native host–guest systems compatible with living cells. Elegant work along these lines has shown that the β-cyclodextrin23 and cucurbit[n]uril24–30 hosts can be employed to induce the supramolecular assembly of proteinaceous guests. Importantly, these non-native host–guest systems may avoid potential off-target effects associated with native host–guest systems.31 However, facile delivery of these reagents into living cells represents a potential barrier to their routine use.
Inspired by this work, we have recently described an approach capable of affording miniprotein hosts that undergo supramolecular assembly with user-defined small-molecule guests.32 As our first example, we reported the evolution of a consensus mutant based on the tenth type III domain of fibronectin (Fn3),33–35 termed 6.2.18, capable of supramolecular assembly with an environment-sensitive dye known as sI-Pht36 to afford an approximately 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6.2.18
1, 6.2.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sI-Pht complex and a concurrent 570-fold increase in fluorescence. We hypothesized that selection via fluorescence-activated cell sorting (FACS) for increased fluorescence during successive rounds of yeast surface display37 yielded mutants capable of undergoing supramolecular assembly with sI-Pht. Leveraging the genetically encodable nature of the host molecule, we demonstrated the ability to visualize supramolecular complexation of 6.2.18 with sI-Pht on the surface of living yeast without the need for washing. Herein, we provide further support for the utility of our design approach by disclosing the characterization of an improved Fn3 mutant termed 6.2.22. Importantly, this mutant undergoes supramolecular complexation in response to addition of sI-Pht, forming an approximately 5
sI-Pht complex and a concurrent 570-fold increase in fluorescence. We hypothesized that selection via fluorescence-activated cell sorting (FACS) for increased fluorescence during successive rounds of yeast surface display37 yielded mutants capable of undergoing supramolecular assembly with sI-Pht. Leveraging the genetically encodable nature of the host molecule, we demonstrated the ability to visualize supramolecular complexation of 6.2.18 with sI-Pht on the surface of living yeast without the need for washing. Herein, we provide further support for the utility of our design approach by disclosing the characterization of an improved Fn3 mutant termed 6.2.22. Importantly, this mutant undergoes supramolecular complexation in response to addition of sI-Pht, forming an approximately 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6.2.22
1, 6.2.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sI-Pht complex. In addition, 6.2.22 displays a 4.1-fold improvement in the EC50 of complex formation compared to 6.2.18 and yields a 750-fold increase in fluorescence upon complex formation. These properties allow for improved imaging of supramolecular assembly on the surface of living yeast. Taken together, these results indicate that our new design paradigm could provide a general approach for identifying non-native host–guest pairs that are compatible with biological systems.
sI-Pht complex. In addition, 6.2.22 displays a 4.1-fold improvement in the EC50 of complex formation compared to 6.2.18 and yields a 750-fold increase in fluorescence upon complex formation. These properties allow for improved imaging of supramolecular assembly on the surface of living yeast. Taken together, these results indicate that our new design paradigm could provide a general approach for identifying non-native host–guest pairs that are compatible with biological systems.
Utilizing yeast surface display, we have previously described the evolution of a consensus Fn3 domain, termed 6.2.18 (Fig. 1a and b), capable of supramolecular assembly with sI-Pht (Fig. 1c).32 This consensus mutant was shown to be capable of producing a 570-fold enhancement in fluorescence upon sI-Pht binding and displayed an EC50 of 14 μM for supramolecular complexation. However, while screening one hundred and five positive colonies for binding to sI-Pht we identified an additional mutant, termed 6.2.22, which yielded a 3.7-fold increase in sI-Pht fluorescence on the surface of yeast compared to 6.2.18 (Fig. 1b and d). Intrigued by this result, we set out to characterize the binding between 6.2.22 and sI-Pht.
 | ||
| Fig. 1 (a) Structure of the wild-type Fn3 domain with the randomized BC, DE, and FG loops highlighted in red (PDB: 1TTG). (b) Amino acid sequences for loop regions in wild-type Fn3 (wtFn3), 6.2.18, and 6.2.22. (c) The structure of sI-Pht. (d) Fluorescence of yeast cells (107) displaying the indicated mutant. Cells were incubated with 300 nM sI-Pht for 30 min. Data are normalized to cells displaying wtFn3. | ||
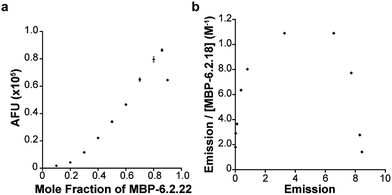
The mutant Fn3 domain, 6.2.22, and wild-type Fn3 (wtFn3) were expressed as C-terminal fusions to the maltose binding protein (MBP, Fig. S1†). Fusion proteins were purified to homogeneity (Fig. S2†). The CD spectrum of MBP-6.2.22 and MBP-wtFn3 could be overlaid (Fig. 2a). However, it should be noted that since we could not obtain sufficient quantities of untagged protein, we cannot definitively rule out differences between the structure of 6.2.22 and wtFn3. Nonetheless, taken together with the bias of yeast surface display for folded proteins,38 this experiment indicates that 6.2.22 likely maintains a folded structure. Titration of sI-Pht with increasing concentrations of MBP-6.2.22 yielded an EC50 of 3.4 μM (Fig. 2b). Importantly, this represents a 4.1-fold improvement in EC50 compared to 6.2.18. From a practical perspective, this decrease in EC50 lowers the amount of sI-Pht needed in order to obtain a fluorescence signal. The resulting complex displayed an excitation maximum at 591 nm and an emission maximum at 613 nm (Fig. S3†). The quantum yield and molar extinction coefficient of the complex was determined to be 0.31 and 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, respectively. This represents a 1.6-fold increase in brightness compared to the 6.2.18–sI-Pht complex. Thus, we attribute the increased fluorescence of the 6.2.22–sI-Pht complex on the surface of yeast (Fig. 1d) to a combination of the enhanced fluorescence of the 6.2.22–sI-Pht complex as well as increased affinity for sI-Pht. Interestingly, the transition between unbound and bound sI-Pht occurred over a relatively narrow concentration range of 6.2.22 (Hill slope = 28, Fig. 2b). To further investigate the nature of 6.2.22–sI-Pht binding, we performed a Job's plot analysis (Fig. 3a). These data clearly indicated that binding was not 1
000 M−1 cm−1, respectively. This represents a 1.6-fold increase in brightness compared to the 6.2.18–sI-Pht complex. Thus, we attribute the increased fluorescence of the 6.2.22–sI-Pht complex on the surface of yeast (Fig. 1d) to a combination of the enhanced fluorescence of the 6.2.22–sI-Pht complex as well as increased affinity for sI-Pht. Interestingly, the transition between unbound and bound sI-Pht occurred over a relatively narrow concentration range of 6.2.22 (Hill slope = 28, Fig. 2b). To further investigate the nature of 6.2.22–sI-Pht binding, we performed a Job's plot analysis (Fig. 3a). These data clearly indicated that binding was not 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and instead pointed to a binding stoichiometry in which excess protein was bound to sI-Pht. Further analysis using a Scatchard plot demonstrated positive cooperativity during the formation of the 6.2.22–sI-Pht complex (Fig. 3b).
1, and instead pointed to a binding stoichiometry in which excess protein was bound to sI-Pht. Further analysis using a Scatchard plot demonstrated positive cooperativity during the formation of the 6.2.22–sI-Pht complex (Fig. 3b).
Encouraged by the above results, we asked whether 6.2.22 was capable of selectively recognizing sI-Pht. As potential off-target dyes we chose 4DMN39–41 and NBD42,43 (Fig. 4a). Importantly, both dyes are capable of achieving dramatic increases in fluorescence upon changes in the local solvent environment. For example, 4DMN undergoes a 1200-fold increase in fluorescence in dioxane compared to water, accompanied by a blue-shift in emission maximum from 554 to 512 nm.40 Exposure of MBP-6.2.22 to sI-Pht resulted in a 750-fold increase in fluorescence, while no observable increase in fluorescence was seen in the presence of NBD (Fig. 4b). In the presence of 4DMN, a modest 32-fold increase in fluorescence was observed coupled with a shift in the emission maximum to 535 nm. We attribute this increase in 4DMN fluorescence to weak, nonspecific interactions between the uncharged 4DMN dye and the relatively hydrophobic 6.2.22 mutant (Fig. 1b).32 These results demonstrate the ability of 6.2.22 to selectivity activate the fluorescence of the sI-Pht fluorophore.
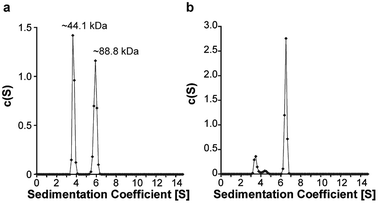
Satisfied that 6.2.22 was capable of selectively recognizing the sI-Pht dye, we further investigated 6.2.22–sI-Pht complexation using sedimentation velocity to directly visualize protein species before and after addition of dye (Fig. 5). Prior to the addition of dye, both monomeric (∼44.1 kDa, 48%) and dimeric (∼88.8 kDa, 51%) MBP-6.2.22 were observed. However, in the presence of sI-Pht, we clearly observed the formation of a new, larger molecular weight complex (∼227 kDa). This new complex could be directly visualized by monitoring at the dye absorbance (Fig. S4†). To further characterize the binding between 6.2.22 and sI-Pht, we performed isothermal titration calorimetry (ITC) experiments (Fig. 6). These data demonstrated that complex formation was enthalpically favoured and entropically disfavoured, as expected for supramolecular assembly. Taken together, these experiments provide clear evidence for supramolecular complexation of 6.2.22 upon addition of sI-Pht. Based on this data, we estimate a binding stoichiometry of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6.2.22
1, 6.2.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sI-Pht. The precise determination of the molecular structure and binding stoichiometry of the complex awaits further high resolution structural studies.
sI-Pht. The precise determination of the molecular structure and binding stoichiometry of the complex awaits further high resolution structural studies.
 | ||
| Fig. 6 ITC data obtained from titrating MBP-6.2.22 (80 μM) with sI-Pht (400 μM). Fit values are: N = 0.2, EC50 = 790 nM, ΔH = −17.9 kcal mol−1, ΔS = −32 cal mol−1 deg−1. The difference between the EC50 measured in Fig. 2b may be due to the difference in the physical parameters measured by these techniques. | ||
Taking advantage of the genetically encodable nature of the 6.2.22 host, we expressed 6.2.22 on the surface of living yeast utilizing the covalent interaction of Aga1 and Aga2 (Fig. 7a).37 In addition, we expressed 6.2.18 and wtFn3 as controls. Labelling each population of cells with an Alexa Fluor 488 conjugated anti-c-myc antibody and subsequent imaging using confocal microscopy, demonstrated expression of all proteins on the yeast cell surface (Fig. 7b). Additional labelling with sI-Pht, without washing, only produced observable fluorescence on the cells expressing 6.2.18 and 6.2.22. Moreover, fluorescence images obtained from the 6.2.22 population were brighter than those obtained from the 6.2.18 population under the same conditions. These results further demonstrate the selectivity of the sI-Pht guest, as no appreciable red fluorescence is observed on cells expressing wtFn3 (Fig. 7b).
Conclusions
In conclusion, we have characterized an improved host, 6.2.22, capable of fluorogenic supramolecular assembly with the sI-Pht guest. This host–guest pair displays marked improvements in the EC50 for supramolecular assembly as well as fluorescence enhancement upon complex formation. In comparison to our previously described system, these advantages enable improved visualization of host–guest complexation on the surface of living yeast. In a broader context, these results further support our design paradigm, in which selection for fluorescence enhancement of environment-sensitive dyes can be utilized to bias directed evolution of miniprotein hosts towards supramolecular assembly. Our laboratory is currently investigating the use of this system to control the proximity of membrane-bound signaling enzymes in cells. In addition, we envision the ability to isolate hosts that selectively recognize spectrally orthogonal guest molecules, enabling potential multiplexing applications. We are also pursuing strategies to modulate the stoichiometry of host–guest assembly during directed evolution.Acknowledgements
We acknowledge Prof. K. Dane Wittrup for providing the Fn3 domain library as well as the EBY100 yeast strain, the Morrison Miscopy Facility, the Biophysical Core, the Research Instrumentation/NMR facility, the Nebraska Center for Mass Spectrometry, and helpful technical discussions with D. Rajasekhar Reddy. This work was supported by a Nebraska EPSCoR First Award and the University of Nebraska – Lincoln.Notes and references
- J. M. Lehn, Science, 1985, 227, 849–856 CAS.
- J. M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4763–4768 CrossRef CAS PubMed.
- V. Hernandez-Gordillo and J. Chmielewski, Biomaterials, 2014, 35, 7363–7373 CrossRef CAS PubMed.
- R. A. Miller, A. D. Presley and M. B. Francis, J. Am. Chem. Soc., 2007, 129, 3104–3109 CrossRef CAS PubMed.
- D. E. Przybyla, C. M. Rubert Perez, J. Gleaton, V. Nandwana and J. Chmielewski, J. Am. Chem. Soc., 2013, 135, 3418–3422 CrossRef CAS PubMed.
- F. G. Klarner, B. Kahlert, A. Nellesen, J. Zienau, C. Ochsenfeld and T. Schrader, J. Am. Chem. Soc., 2006, 128, 4831–4841 CrossRef PubMed.
- C. Renner, J. Piehler and T. Schrader, J. Am. Chem. Soc., 2006, 128, 620–628 CrossRef CAS PubMed.
- E. R. Kay and D. A. Leigh, Angew. Chem., 2015, 54, 10080–10088 CrossRef CAS PubMed.
- E. V. Anslyn, J. Org. Chem., 2007, 72, 687–699 CrossRef CAS PubMed.
- L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS PubMed.
- D. A. Uhlenheuer, K. Petkau and L. Brunsveld, Chem. Soc. Rev., 2010, 39, 2817–2826 RSC.
- S. N. Ho, S. R. Biggar, D. M. Spencer, S. L. Schreiber and G. R. Crabtree, Nature, 1996, 382, 822–826 CrossRef CAS PubMed.
- F. S. Liang, W. Q. Ho and G. R. Crabtree, Sci. Signaling, 2011, 4, rs2 CrossRef PubMed.
- T. Miyamoto, R. DeRose, A. Suarez, T. Ueno, M. Chen, T. P. Sun, M. J. Wolfgang, C. Mukherjee, D. J. Meyers and T. Inoue, Nat. Chem. Biol., 2012, 8, 465–470 CrossRef CAS PubMed.
- H. N. Lin, W. M. Abida, R. T. Sauer and V. W. Cornish, J. Am. Chem. Soc., 2000, 122, 4247–4248 CrossRef CAS.
- E. R. Ballister, C. Aonbangkhen, A. M. Mayo, M. A. Lampson and D. M. Chenoweth, Nat. Commun., 2014, 5, 5475 CrossRef PubMed.
- E. R. Ballister, S. Ayloo, D. M. Chenoweth, M. A. Lampson and E. L. Holzbaur, Curr. Biol., 2015, 25, R407–R408 CrossRef CAS PubMed.
- K. A. Brown, Y. Zou, D. Shirvanyants, J. Zhang, S. Samanta, P. K. Mantravadi, N. V. Dokholyan and A. Deiters, Chem. Commun., 2015, 51, 5702–5705 RSC.
- A. V. Karginov, Y. Zou, D. Shirvanyants, P. Kota, N. V. Dokholyan, D. D. Young, K. M. Hahn and A. Deiters, J. Am. Chem. Soc., 2011, 133, 420–423 CrossRef CAS PubMed.
- K. M. Schelkle, T. Griesbaum, D. Ollech, S. Becht, T. Buckup, M. Hamburger and R. Wombacher, Angew. Chem., 2015, 54, 2825–2829 CrossRef CAS PubMed.
- L. A. Banaszynski and T. J. Wandless, Chem. Biol., 2006, 13, 11–21 CrossRef CAS PubMed.
- M. Putyrski and C. Schultz, FEBS Lett., 2012, 586, 2097–2105 CrossRef CAS PubMed.
- L. Zhang, Y. Wu and L. Brunsveld, Angew. Chem., 2007, 46, 1798–1802 CrossRef CAS PubMed.
- D. T. Dang, H. D. Nguyen, M. Merkx and L. Brunsveld, Angew. Chem., 2013, 52, 2915–2919 CrossRef CAS PubMed.
- D. T. Dang, J. Schill and L. Brunsveld, Chem. Sci., 2012, 3, 2679–2684 RSC.
- L. M. Heitmann, A. B. Taylor, P. J. Hart and A. R. Urbach, J. Am. Chem. Soc., 2006, 128, 12574–12581 CrossRef CAS PubMed.
- L. A. Logsdon, C. L. Schardon, V. Ramalingam, S. K. Kwee and A. R. Urbach, J. Am. Chem. Soc., 2011, 133, 17087–17092 CrossRef CAS PubMed.
- H. D. Nguyen, D. T. Dang, J. L. van Dongen and L. Brunsveld, Angew. Chem., 2010, 49, 895–898 CrossRef CAS PubMed.
- L. C. Smith, D. G. Leach, B. E. Blaylock, O. A. Ali and A. R. Urbach, J. Am. Chem. Soc., 2015, 137, 3663–3669 CrossRef CAS PubMed.
- D. A. Uhlenheuer, J. F. Young, H. D. Nguyen, M. Scheepstra and L. Brunsveld, Chem. Commun., 2011, 47, 6798–6800 RSC.
- S. D. Liberles, S. T. Diver, D. J. Austin and S. L. Schreiber, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 7825–7830 CrossRef CAS.
- B. Xu, X. Zhou and C. I. Stains, J. Am. Chem. Soc., 2015, 137, 14252–14255 CrossRef CAS PubMed.
- T. F. Chen, S. de Picciotto, B. J. Hackel and K. D. Wittrup, Methods Enzymol., 2013, 523, 303–326 CAS.
- B. J. Hackel, A. Kapila and K. D. Wittrup, J. Mol. Biol., 2008, 381, 1238–1252 CrossRef CAS PubMed.
- A. Koide, C. W. Bailey, X. L. Huang and S. Koide, J. Mol. Biol., 1998, 284, 1141–1151 CrossRef CAS PubMed.
- C. J. MacNevin, D. Gremyachinskiy, C. W. Hsu, L. Li, M. Rougie, T. T. Davis and K. M. Hahn, Bioconjugate Chem., 2013, 24, 215–223 CrossRef CAS PubMed.
- E. T. Boder and K. D. Wittrup, Nat. Biotechnol., 1997, 15, 553–557 CrossRef CAS PubMed.
- D. W. Colby, B. A. Kellogg, C. P. Graff, Y. A. Yeung, J. S. Swers and K. D. Wittrup, Methods Enzymol., 2004, 388, 348–358 CAS.
- J. Kollar, P. Hrdlovic, S. Chmela, M. Sarakha and G. Guyot, J. Photochem. Photobiol., A, 2005, 170, 151–159 CrossRef CAS.
- G. Loving and B. Imperiali, J. Am. Chem. Soc., 2008, 130, 13630–13638 CrossRef CAS PubMed.
- G. Loving and B. Imperiali, Bioconjugate Chem., 2009, 20, 2133–2141 CrossRef CAS PubMed.
- P. B. Ghosh and M. W. Whitehouse, Biochem. J., 1968, 108, 155–156 CrossRef CAS PubMed.
- D. Lancet and I. Pecht, Biochemistry, 1977, 16, 5150–5157 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figures, characterization data, and experimental details. See DOI: 10.1039/c6ra01215a |
| This journal is © The Royal Society of Chemistry 2016 |